The Significant Roles of Mg/Ca Ratio, Cl− and SO42− in Carbonate Mineral Precipitation by the Halophile Staphylococcus epidermis Y2
Abstract
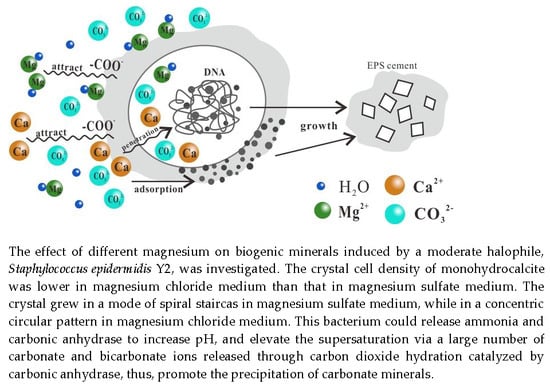
:1. Introduction
2. Materials and Methods
2.1. Isolation and Preservation of S. epidermidis Y2 Bacteria
2.2. 16S rDNA Identification of S. epidermidis Y2 Bacteria
2.3. Qualitative Test of Ammonia Released by S. epidermidis Y2 Bacteria
2.4. Growth Curve of S. epidermidis Y2 Bacteria and pH Changes in the Culture Medium
2.5. Activity of CA Released by S. epidermidis Y2 Bacteria
2.6. Determination of the Concentration of CO32− and HCO3− Ions
2.7. Amino Acid Composition of EPS
2.8. Carbonate Minerals Induced by S. epidermidis Y2 Bacteria with Different Sources of Magnesium
2.9. Analysis of Carbonate Minerals Induced by S. epidermidis Y2 in the MgSO4 Group and the MgCl2 Group
2.10. Analysis of the Nanometer Spheres and Element Distribution Outside and Inside of S. epidermidis Y2 Cells
3. Results
3.1. Identification of S. epidermidis Y2 Bacteria
3.2. Qualitative Ammonia Experiment
3.3. Growth Curve and pH Curve of S. epidermidis Y2
3.4. Determination of CA Activity
3.5. Determination of Carbonate and Bicarbonate Ions in the Liquid Culture Medium
3.6. Amino Acid Compositions of EPS
3.7. XRD Analysis of Minerals in the Culture Medium with Different Sources of Magnesium
3.8. SEM and EDS Analyses of Minerals Induced by S. epidermidis Y2 in the MgCl2 Group and the MgSO4 Group
3.8.1. SEM and EDS Analyses of Minerals in the MgSO4 Medium
3.8.2. SEM and EDS Analyses of Minerals in the MgCl2 Medium
3.9. HRTEM and SAED Analyses of S. epidermidis Y2 Bacterial Superthin Slices
3.10. STEM and EDS Analyses of the Ultrathin Slices of S. epidermidis Y2
4. Discussion
4.1. Mechanisms of Biomineralization Induced by S. epidermidis Y2 Bacteria
4.1.1. pH Increase
4.1.2. CA Activity
4.2. The Role of Mg2+ Ions on Monohydrocalcite
4.3. Effects of Different Sources of Mg2+ on Monohydrocalcite Crystal Formation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bakhshi:, E.; Torab, F.M. Determining Wettability of Fractured Carbonate Reservoirs. Nat. Resour. Res. 2016, 25, 211–225. [Google Scholar] [CrossRef]
- Correia, M.G.; Maschio, C.; Schiozer, D.J. Integration of multiscale carbonate reservoir heterogeneities in reservoir simulation. J. Petrol. Sci. Eng. 2015, 131, 34–50. [Google Scholar] [CrossRef]
- Chen, J.T.; Van Loon, A.; Han, Z.Z.; Chough, S.K. Funnel-shaped, breccia-filled clastic dykes in the Late Cambrian Chaomidian Formation (Shandong Province, China). Sediment. Geol. 2009, 221, 1–6. [Google Scholar] [CrossRef]
- Han, Z.Z.; Meng, R.R.; Yan, H.X.; Zhao, H.; Han, M.; Zhao, Y.Y.; Sun, B.; Sun, Y.B.; Wang, J.; Zhuang, D.X. Calcium carbonate precipitation by Synechocystis sp. PCC6803 at different Mg/Ca molar ratios under the laboratory condition. Carbonates Evaporites 2016, 32, 561–575. [Google Scholar] [CrossRef]
- Bose, S.; Chakrabarti, G.; Shome, D. Evolution of Palaeo-Mesoproterozoic open coast ramp-clues from stromatolite-bearing carbonate facies of Vempalle Formation, Cuddapah Basin, India. Arab. J. Geosci. 2017, 10. [Google Scholar] [CrossRef]
- Siahi, M.; Hofmann, A.; Hegner, E.; Master, S. Sedimentology and facies analysis of Mesoarchaean stromatolitic carbonate rocks of the Pongola Supergroup, South Africa. Precambrian Res. 2016, 10, 244–264. [Google Scholar] [CrossRef]
- Bull, A.T. Microbial Diversity & Bioprospecting; ASM Press: Washington, DC, USA, 2004. [Google Scholar]
- Banfield, J.F.; Cervini-Silva, J.; Nealson, K.H. Molecular Geomicrobiology; Mineralogical Society of America: Chantilly, VA, USA, 2005. [Google Scholar]
- Dong, H.L. Mineral-microbe interactions: A review. Front. Earth Sci. China 2010, 4, 127–147. [Google Scholar] [CrossRef]
- Cassarino, G.; Olzi, E.; Rizzo, R. Bacterially induced mineralization of calcium carbonate in terrestrial environments: The role of exopolysaccharides and amino acids. J. Sediment. Res. 2003, 73, 485–490. [Google Scholar] [CrossRef]
- Yoshimura, T.; Tamenori, Y.; Suzuki, A.; Kawahata, H.; Iwasaki, N.; Hasegawa, H.; Luan, T.N.; Kuroyanagi, A.; Yamazaki, T.; Kuroda, J. Altervalent substitution of sodium for calcium in biogenic calcite and aragonite. Geochim. Cosmochim. Acta 2017, 202, 21–38. [Google Scholar] [CrossRef]
- Menadakis, M.; Maroulis, G.; Koutsoukos, P.G. Incorporation of Mg2+, Sr2+, Ba2+ and Zn2+ into aragonite and comparison with calcite. J. Math. Chem. 2009, 46, 484–491. [Google Scholar] [CrossRef]
- Weiner, S. On Biomineralization; Oxford University Press: Oxford, UK, 1989; pp. 119–130. [Google Scholar]
- Han, Z.Z.; Zhuang, D.X.; Yan, H.X.; Zhao, H.; Sun, B.; Li, D.; Sun, Y.W.; Hu, W.Y.; Xuan, Q.Z.; Chen, J.A. Thermogravimetric and kinetic analysis of thermal decomposition characteristics of microbial calcites induced by cyanobacteria Synechocystis sp. PCC6803. J. Therm. Anal. Calorim. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Benzerara, K.; Skouripanet, F.; Li, J.; Férard, C.; Gugger, M.; Laurent, T.; Couradeau, E.; Ragon, M.; Cosmidis, J.; Menguy, N. Intracellular Ca-carbonate biomineralization is widespread in cyanobacteria. Proc. Natl. Acad. Sci. USA 2014, 111, 10933–10938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, H. Biomineralization of Carbonate Minerals Induced by the Halophilic Chromohalobacter israelensis under High Salt Concentrations: Implications for Natural Environments. Minerals 2017, 7. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Rivadeneyra, M.A.; Vasconcelos, C.; McKenzie, J.A. Biomineralization of carbonate and phosphate by moderately halophilic bacteria. FEMS Microbiol. Ecol. 2007, 61, 273–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, F.F.; Xu, H.F.; Shelobolina, E.S.; Converse, B.; Converse, B.; Shen, Z.Z.; Roden, E.E. The catalytic effect of bound extracellular polymeric substances excreted by anaerobic microorganisms on Ca-Mg carbonate precipitation: Implications for the “dolomite problem”. Am. Mineral. 2015, 100, 483–494. [Google Scholar] [CrossRef]
- Han, Z.Z.; Zhao, Y.Y.; Yan, H.X.; Zhao, H.; Han, M.; Sun, B.; Sun, X.Y.; Hou, F.F.; Sun, H.; Han, L. Struvite Precipitation Induced by a Novel Sulfate-Reducing Bacterium Acinetobacter calcoaceticus SRB4 Isolated from River Sediment. Geomicrobiol. J. 2015, 32, 868–877. [Google Scholar] [CrossRef]
- Kenward, P.A.; Goldstein, R.H.; González, L.A.; Roberts, J.A. Precipitation of low-temperature dolomite from an anaerobic microbial consortium: The role of methanogenic Archaea. Geobiology 2009, 7, 556–565. [Google Scholar] [CrossRef] [PubMed]
- Barnes, R.; Goldberg, E. Methane production and consumption in anoxic marine sediments. Geology 1976, 4, 297–300. [Google Scholar] [CrossRef]
- Douglas, S.; Yang, H.X. Mineral biosignatures in evaporites: Presence of rosickyite in an endoevaporitic microbial community from Death Valley, California. Geology 2002, 30, 1075–1078. [Google Scholar] [CrossRef]
- Knauth, L.P. Salinity history of the Earth’s early ocean. Nature 1998, 395, 554–555. [Google Scholar] [CrossRef]
- Chen, J.T.; Chough, S.K.; Lee, J.H.; Han, Z.Z. Sequence-stratigraphic comparison of the upper Cambrian Series 3 to Furongian succession between the Shandong region, China and the Taebaek area, Korea: High variability of bounding surfaces in an epeiric platform. Geosci. J. 2012, 16, 357–379. [Google Scholar] [CrossRef]
- Han, Z.Z.; Zhang, X.L.; Chi, N.J.; Han, M.; Woo, J.; Lee, H.S.; Chen, J.T. Cambrian oncoids and other microbial-related grains on the North China Platform. Carbonates Evaporites 2015, 30, 373–386. [Google Scholar] [CrossRef]
- Han, Z.Z.; Yan, H.X.; Zhou, S.X.; Zhao, H.; Zhang, Y.; Zhang, N.N.; Yao, C.K.; Zhao, L.; Han, C.Y. Precipitation of calcite induced by Synechocystis sp. PCC6803. World J. Microbiol. Biotechnol. 2013, 29, 1801–1811. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.Z.; Yan, H.X.; Zhao, H.; Zhou, S.X.; Han, M.; Meng, X.Q. Bio-precipitation of Calcite with Preferential Orientation Induced by sp. PCC6803. Geomicrobiol. J. 2014, 31, 884–899. [Google Scholar] [CrossRef]
- Rivadeneyra, M.A.; Delgado, G.; Soriano, M.; Ramos-Cormenzana, A.; Delgado, R. Precipitation of carbonates by Nesterenkonia halobia in liquid media. Chemosphere 2000, 41, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Rivadeneyra, M.A.; Delgado, R.; Moral, A.D.; Ferrer, M.R.; Ramos-Cormenzana, A. Precipatation of calcium carbonate by Vibrio spp. from an inland saltern. FEMS Microbiol. Ecol. 1994, 13, 197–204. [Google Scholar] [CrossRef]
- Parraga, J.; Rivadeneyra, M.A.; Delgado, R.; Iniguez, J.; Soriano, M.; Delgado, G. Study of biomineral formation by bacteria from soil solution equilibria. React. Funct. Polym. 1998, 36, 265–271. [Google Scholar] [CrossRef]
- Ferrer, M.R.; Quevedo-Sarmiento, J.; Bejar, V.; Delgado, R.; Ramos-Cormenzana, A.; Rivadeneyra, M.A. Calcium carbonate formation by Deleya halophila: Effect of salt concentration and incubation temperature. Geomicrobiol. J. 1988, 6, 49–57. [Google Scholar] [CrossRef]
- Qiu, X.; Wang, H.M.; Yao, Y.C.; Duan, Y. High salinity facilitates dolomite precipitation mediated by Haloferax volcanii DS52. Earth Planet. Sci. Lett. 2017, 472, 197–205. [Google Scholar] [CrossRef]
- Qiu, X.; Yao, Y.C.; Wang, H.M.; Duan, Y. Live microbial cells adsorb Mg2+ more effectively than lifeless organic matter. Front. Earth Sci. 2018, 12, 160–169. [Google Scholar] [CrossRef]
- Deng, S.C.; Dong, H.L.; Lv, G.; Jiang, H.C.; Yu, B.S.; Bishop, M.E. Microbial dolomite precipitation using sulfate reducing and halophilic bacteria: Results from Qinghai Lake, Tibetan Plateau, NW China. Chem. Geol. 2010, 278, 151–159. [Google Scholar] [CrossRef]
- Grasby, S. Naturally precipitating vaterite (μ-CaCO3) spheres: Unusual carbonates formed in an extreme environment. Geochim. Cosmochim. Acta 2003, 67, 1659–1666. [Google Scholar] [CrossRef]
- Wei, S.P.; Cui, H.P.; Jiang, Z.L.; Liu, H.; He, H.; Fang, N.Q. Biomineralization processes of calcite induced by bacteria isolated from marine sediments. Braz. J. Microbiol. 2015, 46, 455–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuang, D.X.; Yan, H.X.; Tucker, M.E.; Zhao, H.; Han, Z.Z.; Zhao, Y.H.; Sun, B.; Li, D.; Pan, J.T.; Zhao, Y.Y.; et al. Calcite precipitation induced by Bacillus cereus MRR2 cultured at different Ca2+ concentrations: Further insights into biotic and abiotic calcite. Chem. Geol. 2018. [Google Scholar] [CrossRef]
- Han, Z.Z.; Zhao, Y.Y.; Yan, H.X.; Zhao, H.; Han, M.; Sun, B.; Meng, R.R.; Zhuang, D.X.; Li, D.; Gao, W.J. The Characterization of Intracellular and Extracellular Biomineralization Induced by Synechocystis sp. PCC6803 Cultured under Low Mg/Ca Ratios Conditions. Geomicrobiol. J. 2017, 34, 362–373. [Google Scholar] [CrossRef]
- Thatcher, B.; Doherty, A.; Orvisky, E.; Martin, B.; Henkin, R. Gustin from human parotid saliva is carbonic anhydrase VI. Biochem. Biophys. Res. Commun. 1998, 250, 635–641. [Google Scholar] [CrossRef]
- Lindskog, S. Structure and mechanism of carbonic anhydrase. Pharmacol. Ther. 1997, 74, 1–20. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.P.; Chen, W.S.; Yu, L.J.; Li, W.B.; Yu, H.Z. Calcium carbonate precipitation and crystal morphology induced by microbial carbonic anhydrase and other biological factors. Process Biochem. 2010, 45, 1017–1021. [Google Scholar] [CrossRef]
- Favre, N.; Christ, M.L.; Pierre, A.C. Biocatalytic capture of CO2 with carbonic anhydrase and its transformation to solid carbonate. J. Mol. Catal. B-Enzym. 2009, 60, 163–170. [Google Scholar] [CrossRef]
- Sánchez-Román, M.; Romanek, C.S.; Fernández-Remolar, D.C.; Sánchez-Navas, A.; Mckenzie, J.A.; Pibernat, R.A.; Vasconcelos, C. Aerobic biomineralization of Mg-rich carbonates: Implications for natural environments. Chem. Geol. 2011, 281, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Perri, E.; Tucker, M.E.; Słowakiewicz, M.; Whitaker, F.; Bowen, L.; Perrotta, I.D. Carbonate and silicate biomineralization in a hypersaline microbial mat (Mesaieed sabkha, Qatar): Roles of bacteria, extracellular polymeric substances and viruses. Sedimentology 2018, 65, 1213–1245. [Google Scholar] [CrossRef]
- Morgan, J.W.; Forster, C.F.; Evison, L. A comparative study of the nature of biopolymers extracted from anaerobic and activated sludges. Water Res. 1990, 24, 743–750. [Google Scholar] [CrossRef]
- Chen, Y.; Niu, M.; Yuan, S.; Teng, H. Durable antimicrobial finishing of cellulose with QSA silicone by supercritical adsorption. Appl. Surf. Sci. 2013, 264, 171–175. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Zhu, J.Q.; Sun, T.; Zhou, H.Y.; Shao, Q.; Li, G.J.; Liu, X.D.; Yin, Y.S. Porphyrin nanotubes composed of highly ordered molecular arrays prepared by anodic aluminum template method. RSC Adv. 2013, 3, 2765–2769. [Google Scholar] [CrossRef]
- Zhou, S.X.; Chen, H.P.; Ding, C.; Niu, H.L.; Zhang, T.H.; Wang, N.F.; Zhang, Q.Q.; Liu, D.; Han, S.N.; Yu, H.G. Effectiveness of crystallitic carbon from coal as milling aid and for hydrogen storage during milling with magnesium. Fuel 2013, 109, 68–75. [Google Scholar] [CrossRef]
- Zhou, S.X.; Chen, H.P.; Ran, W.X.; Wang, N.F.; Han, Z.Y.; Zhang, Q.Q.; Zhang, X.L.; Niu, H.L.; Yu, H.; Liu, D. Effect of carbon from anthracite coal on decomposition kinetics of magnesium hydride. J. Alloys Compd. 2014, 592, 231–237. [Google Scholar] [CrossRef]
- Chen, Y.; Meng, Q.S.; Wu, M.Y.; Wang, S.G.; Xu, P.F.; Chen, H.R.; Li, Y.R.; Zhang, L.X.; Wang, L.X.; Shi, J.L. Hollow mesoporous organosilica nanoparticles: A generic intelligent framework-hybridization approach for biomedicine. J. Am. Chem. Soc. 2014, 136, 16326–16334. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.Q.; Zhao, Q.; Wang, Y.H.; Ge, S.S.; Shao, Q. A New Layered Six-Connected Network Based on Tetranuclear Copper(II) Cores. J. Chem. Crystallogr. 2012, 42, 706–710. [Google Scholar] [CrossRef]
- Zhao, B.Q.; Shao, Q.; Hao, L.H. Yeast-template synthesized Fe-doped cerium oxide hollow microspheres for visible photodegradation of acid orange 7. J. Colloid Interface Sci. 2018, 511, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.N.; Cao, Y.H.; Shao, Q.; Peng, X.F.; Guo, Z.H. Magnetic Nanocarbon Adsorbents with Enhanced Hexavalent Chromium Removal: Morphology Dependence of Fibrillar vs Particulate Structures. Ind. Eng. Chem. Res. 2017, 56, 10689–10701. [Google Scholar] [CrossRef]
- Wu, Z.J.; Gao, S.; Chen, L.; Jiang, D.W.; Shao, Q.; Zhang, B.; Zhai, Z.H.; Wang, C.; Zhao, M.; Ma, Y.Y. Electrically Insulated Epoxy Nanocomposites Reinforced with Synergistic Core–Shell SiO2@MWCNTs and Montmorillonite Bifillers. Macromol. Chem. Phys. 2017, 218, 1700357. [Google Scholar] [CrossRef]
- Chen, Y.; Han, Q.X.; Wang, Y.L.; Zhang, Q.; Qiao, X.X. Synthesis of pyridinium polysiloxane for antibacterial coating in supercritical carbon dioxide. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Zhao, J.k.; Ge, S.S.; Liu, L.R.; Shao, Q.; Mai, X.M.; Zhao, C.X.; Hao, L.H.; Wu, T.T.; Yu, Z.P.; Guo, Z.H. Microwave Solvothermal Fabrication of Zirconia Hollow Microspheres with Different Morphologies Using Pollen Templates and Their Dye Adsorption Removal. Ind. Eng. Chem. Res. 2018, 57, 231–241. [Google Scholar] [CrossRef]
- Wu, T.M.; Lin, Y.W. Doped polyaniline/multi-walled carbon nanotube composites: Preparation, characterization and properties. Polymer 2006, 47, 3576–3582. [Google Scholar] [CrossRef]
- Liu, Q.; Zhao, Q.; Li, Y.; Wang, X. CdCl2 center dot H2O nanorods oriented parallel on the Langmuir film of (phthalocyaninato) [tetrakis(4-pyridyl)porphyrinato] cerium complex. CrystEngComm 2012, 14, 1105–1110. [Google Scholar] [CrossRef]
- Ge, S.S.; Yang, X.K.; Shao, Q.; Liu, Q.Y.; Wang, T.J.; Wang, L.Y.; Wang, X.J. Self-assembled flower-like antimony trioxide microstructures with high infrared reflectance performance. J. Solid State Chem. 2013, 200, 136–142. [Google Scholar] [CrossRef]
- Liu, X.; Shao, X.Y.; Fang, G.B.; He, H.F.; Wan, Z.G. Preparation and properties of chemically reduced graphene oxide/copolymer-polyamide nanocomposites. E-Polymers 2017, 17, 3–14. [Google Scholar] [CrossRef]
- Tian, J.Y.; Shao, Q.; Dong, X.J.; Zheng, J.L.; Pan, D.; Zhang, X.Y.; Cao, H.L.; Hao, L.H.; Liu, J.R.; Mai, X.M.; et al. Bio-template synthesized NiO/C hollow microspheres with enhanced Li-ion battery electrochemical performance. Electrochim. Acta 2018, 261, 236–245. [Google Scholar] [CrossRef]
- He, M.S.; Jin, H.; Zhang, L.L.; Jiang, H.; Yang, T.; Cui, H.Z.; Fossard, F.; Wagner, J.B.; Karppinen, M.; Kauppinen, E.I.; et al. Environmental transmission electron microscopy investigations of Pt-Fe2O3 nanoparticles for nucleating carbon nanotubes. Carbon 2016, 110, 243–248. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Yang, Y.T.; Li, H.; Zhu, R.R.; Shao, Q.; Yang, S.G.; Xu, J.J. NiO nanoparticles modified with 5,10,15,20-tetrakis(4-carboxyl pheyl)-porphyrin: Promising peroxidase mimetics for H2O2 and glucose detection. Biosens. Bioelectron. 2015, 64, 147–153. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Zhu, J.Q.; Shao, Q.; Fan, J.F.; Wang, D.M.; Yina, Y.S. Highly ordered arrangement of -tetrakis (4-aminophenyl) porphyrin in self-assembled nanoaggregates hydrogen bonding. Chin. Chem. Lett. 2014, 25, 752–756. [Google Scholar] [CrossRef]
- Wu, T.H.; Shao, Q.; Ge, S.S. The facile preparation of novel magnetic zirconia composites with the aid of carboxymethyl chitosan and their efficient removal of dye. RSC Adv. 2016, 6, 58020–58027. [Google Scholar] [CrossRef]
- Ge, S.S.; Zhu, W.X.; Shao, Q. Fabrication and Characterization of Hollow Zirconia Microspheres Using Calcium Carbonate as Template. Z. Phys. Chem. 2016, 230, 1617–1628. [Google Scholar] [CrossRef]
- Wang, D.M.; Cao, W.B.; Fan, J. Synthesis and luminescence properties of the europium quaternary complexes nanoparticles. Sci. China Chem. 2014, 57, 791–796. [Google Scholar] [CrossRef]
- Han, Z.Z.; Sun, B.; Zhao, H.; Yan, H.X.; Han, M.; Zhao, Y.Y.; Meng, R.R.; Zhuang, D.X.; Li, D.; Ma, Y.T. Isolation of Leclercia adcarboxglata Strain JLS1 from Dolostone Sample and Characterization of its Induced Struvite Minerals. Geomicrobiology 2016, 34, 500–510. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Synergistic role of bacterial urease and carbonic anhydrase in carbonate mineralization. Appl. Biochem. Biotechnol. 2014, 172, 2552–2561. [Google Scholar] [CrossRef] [PubMed]
- Margesin, R.; Schinner, F. Potential of halotolerant and halophilic microorganisms for biotechnology. Extremophiles 2001, 5, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Schultzelam, S.; Harauz, G.; Beveridge, T.J. Participation of a cyanobacterial S layer in fine-grain mineral formation. J. Bacteriol. 1992, 174, 7971–7981. [Google Scholar] [CrossRef]
- Badger, M.R.; Price, G.D. CO2 concentrating mechanisms in cyanobacteria: Molecular components, their diversity and evolution. J. Exp. Bot. 2003, 54, 609–622. [Google Scholar] [CrossRef]
- Smith, K.S.; Ferry, J.G. Prokaryotic carbonic carbonic anhydrases. FEMS Microbiol. Rev. 2000, 24, 335–366. [Google Scholar] [CrossRef]
- Faridi, S.; Satyanarayana, T. Characteristics of recombinant α-carbonic anhydrase of polyextremophilic bacterium Bacillus halodurans TSLV1. Int. J. Biol. Macromol. 2016, 89, 659–668. [Google Scholar] [CrossRef]
- Dreybrodt, W.; Eisenlohr, L.; Madry, B.; Ringer, S. Precipitation kinetics of calcite in the system CaCO3–H2O–CO2: The conversion to CO2 by the slow process H++HCO3− → CO2+H2O as a rate limiting step. Geochim. Cosmochim. Acta 1997, 61, 3897–3904. [Google Scholar] [CrossRef]
- Liu, N.; Bond, G.M.; Abel, A.; Mcpherson, B.J.; Stringer, J. Biomimetic sequestration of CO2 in carbonate form: Role of produced waters and other brines. Fuel Process. Technol. 2005, 86, 1615–1625. [Google Scholar] [CrossRef]
- Li, W.; Chen, W.S.; Zhou, P.P.; Cao, L.; Yu, L.J. Influence of initial pH on the precipitation and crystal morphology of calcium carbonate induced by microbial carbonic anhydrase. Colloids Surf. B. 2013, 102, 281–287. [Google Scholar] [CrossRef]
- Kim, I.G.; Jo, B.H.; Kang, D.G.; Kim, C.S.; Choi, Y.S.; Cha, H.J. Biomineralization-based conversion of carbon dioxide to calcium carbonate using recombinant carbonic anhydrase. Chemosphere 2012, 87, 1091–1096. [Google Scholar] [CrossRef]
- Bond, G.M.; Liu, N. Biomimetic Sequestration of CO2 in Carbonate Form:Role of Produced Waters and Other Brines. Prepr. Symp. Am. Chem. Soc. Div. Fuel Chem. 2004, 49, 420–421. [Google Scholar]
- Sundaram, S.; Thakur, I.S. Induction of calcite precipitation through heightened production of extracellular carbonic anhydrase by CO2 sequestering bacteria. Bioresour. Technol. 2018, 253, 368–371. [Google Scholar] [CrossRef] [PubMed]
- Mirjafari, P.; Asghari, K.; Mahinpey, N. Investigating the Application of Enzyme Carbonic Anhydrase for CO2 Sequestration Purposes. Ind. Eng. Chem. Res. 2007, 46, 921–926. [Google Scholar] [CrossRef]
- Srivastava, S.; Bharti, R.K.; Verma, P.K.; Thakur, I.S. Cloning and expression of gamma carbonic anhydrase from Serratia sp. ISTD04 for sequestration of carbon dioxide and formation of calcite. Bioresour. Technol. 2015, 188, 209–213. [Google Scholar] [CrossRef]
- Ramanan, R.; Kannan, K.; Vinayagamoorthy, N.; Ramkumar, K.M.; Sivanesan, S.D.; Chakrabarti, T. Purification and characterization of a novel plant-type carbonic anhydrase from Bacillus subtilis. Biotechnol. Bioprocess Eng. 2009, 14, 32–37. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Diego, J.; Shaw, S.; Bots, P.; Roncal-Herrero, T.; Benning, L.G. The role of Mg in the crystallization of monohydrocalcite. Geochim. Cosmochim. Acta 2014, 127, 204–220. [Google Scholar] [CrossRef] [Green Version]
- Ito, T. The occurrence of monohydrocalcite from calcareous sinter of cold spring of Shiowakka, Asyoro, Hokkaido. J. Mineral. Petrol. Econ. Geol. 1993, 88, 485–491. [Google Scholar] [CrossRef]
- Onac, B. Mineralogical studies and Uranium-series dating of speleothems from Scåri¿oara Glacier Cave (Bihor Mountains, Romania). Theor. Appliedkarstol. 2001, 13–14, 33–38. [Google Scholar]
- Solotchina, E.P.; Prokopenko, A.A.; Kuzmin, M.I.; Solotchin, P.A.; Zhdanova, A.N. Climate signals in sediment mineralogy of Lake Baikal and Lake Hovsgol during the LGM-Holocene transition and the 1-Ma carbonate record from the HDP-04 drill core. Quat. Int. 2009, 205, 38–52. [Google Scholar] [CrossRef]
- Lowenstam, H.A. Minerals formed by organisms. Science 1981, 211, 1126–1131. [Google Scholar] [CrossRef] [PubMed]
- Señorale-Pose, M.; Chalar, C.; Dauphin, Y.; Massard, P.; Pradel, P.; Marín, M. Monohydrocalcite in calcareous corpuscles of Mesocestoides corti. Exp. Parasitol. 2008, 118, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Carlström, D. A crystallographic study of vertebrate otoliths. Biol. Bull. 1963, 125, 441–463. [Google Scholar] [CrossRef]
- Catherine, H.; Skinner, W.; Osbaldiston, G.W.; Wilner, A.N. Monohydrocalcite in a guinea pig bladder stone, a novel occurrence. Am. Mineral. 1977, 62, 273–277. [Google Scholar]
- Garvie, L.A.J. Decay of cacti and carbon cycling. Naturwissenschaften 2006, 93, 114–118. [Google Scholar] [CrossRef]
- Garvie, L.A.J. Decay-induced biomineralization of the saguaro cactus (Carnegiea gigantea). Am. Mineral. 2003, 88, 1879–1888. [Google Scholar] [CrossRef]
- Taylor, G.F. The occurrence of monohydrocalcite in two small lakes in the South-East of South Australia. Am. Mineral. 1975, 60, 690–697. [Google Scholar]
- Rivadeneyra, M.A.; Párraga, J.; Delgado, R.; Ramos-Cormenzana1, A.; Delgado, G. Biomineralization of carbonates by Halobacillus trueperi in solid and liquid media with different salinities. FEMS Microbiol. Ecol. 2004, 48, 39–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishiyama, R.; Munemoto, T.; Fukushi, K. Formation condition of monohydrocalcite from CaCl2 –MgCl2 –Na2CO3 solutions. Geochim. Cosmochim. Acta 2013, 100, 217–231. [Google Scholar] [CrossRef]
- Last, F.M.; Last, W.M.; Halden, N.M. Carbonate microbialites and hardgrounds from Manito Lake, an alkaline, hypersaline lake in the northern Great Plains of Canada. Sediment. Geol. 2010, 225, 34–49. [Google Scholar] [CrossRef]
- Stoffers, P.; Fischbeck, R. Monohydrocalcite in the sediments of Lake Kivu (East Africa). Sedimentology 1974, 21, 163–170. [Google Scholar] [CrossRef]
- Zhang, Y.; Dawe, R.A. Influence of Mg2+ on the kinetics of calcite precipitation and calcite crystal morphology. Chem. Geol. 2000, 163, 129–138. [Google Scholar] [CrossRef]
- Davis, K.; Dove, P.; De Yoreo, J. The role of Mg2+ as an impurity in calcite growth. Science 2000, 290, 1134–1137. [Google Scholar] [CrossRef]
- Moomaw, A.S. The unique nature of Mg2+ channels. Physiology 2008, 23, 275–285. [Google Scholar] [CrossRef]
- Mucci, A.; Morse, J.W. The incorporation of Mg2+ and Sr2+ into calcite overgrowths: Influences of growth rate and solution composition. Geochim. Cosmochim. Acta 1983, 47, 217–233. [Google Scholar] [CrossRef]
- Fukushi, K.; Suzuki, Y.; Kawano, J.; Ohno, T.; Ogawa, M.; Yaji, T.; Takahashi, Y. Speciation of magnesium in monohydrocalcite: XANES, ab initio and geochemical modeling. Geochim. Cosmochim. Acta 2017, 213, 457–474. [Google Scholar] [CrossRef]
- Ludi, B.; Niederberger, M. Zinc oxide nanoparticles: Chemical mechanisms and classical and non-classical crystallization. Dalton Trans. 2013, 42, 12554–12568. [Google Scholar] [CrossRef] [PubMed]
- Morse, J.W.; Given, R.K.; Wilkinson, B.H. Kinetic control of morphology, composition, and mineralogy of abiotic sedimentary carbonates; discussion and reply. Science 1985, 55, 109–119. [Google Scholar] [CrossRef]
- Fernández-Díaz, L.; Putnis, A.; Prieto, M.; Putnis, C.V. The role of magnesium in the crystallization of calcite and aragonite in a porous medium. J. Intercul. Stud. 1996, 15, 73–84. [Google Scholar]













| Time (h) | CO32− (mol/L) | Na2CO3 (g/100 mL) | pH |
|---|---|---|---|
| 72 | 0.0035 | 0.0371 | 10.80 |
| 87 | 0.008 | 0.0848 | 11.01 |
| 92 | 0.012 | 0.1272 | 11.08 |
| 111 | 0.022 | 0.2332 | 11.20 |
| 120 | 0.0255 | 0.2703 | 11.22 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, Z.; Yu, W.; Zhao, H.; Zhao, Y.; Tucker, M.E.; Yan, H. The Significant Roles of Mg/Ca Ratio, Cl− and SO42− in Carbonate Mineral Precipitation by the Halophile Staphylococcus epidermis Y2. Minerals 2018, 8, 594. https://doi.org/10.3390/min8120594
Han Z, Yu W, Zhao H, Zhao Y, Tucker ME, Yan H. The Significant Roles of Mg/Ca Ratio, Cl− and SO42− in Carbonate Mineral Precipitation by the Halophile Staphylococcus epidermis Y2. Minerals. 2018; 8(12):594. https://doi.org/10.3390/min8120594
Chicago/Turabian StyleHan, Zuozhen, Wenwen Yu, Hui Zhao, Yanhong Zhao, Maurice E. Tucker, and Huaxiao Yan. 2018. "The Significant Roles of Mg/Ca Ratio, Cl− and SO42− in Carbonate Mineral Precipitation by the Halophile Staphylococcus epidermis Y2" Minerals 8, no. 12: 594. https://doi.org/10.3390/min8120594