Pyrite Decay of Large Fossils: The Case Study of the Hall of Palms in Padova, Italy
Abstract
:1. Introduction
1.1. Pyrite and Pyrite Decay
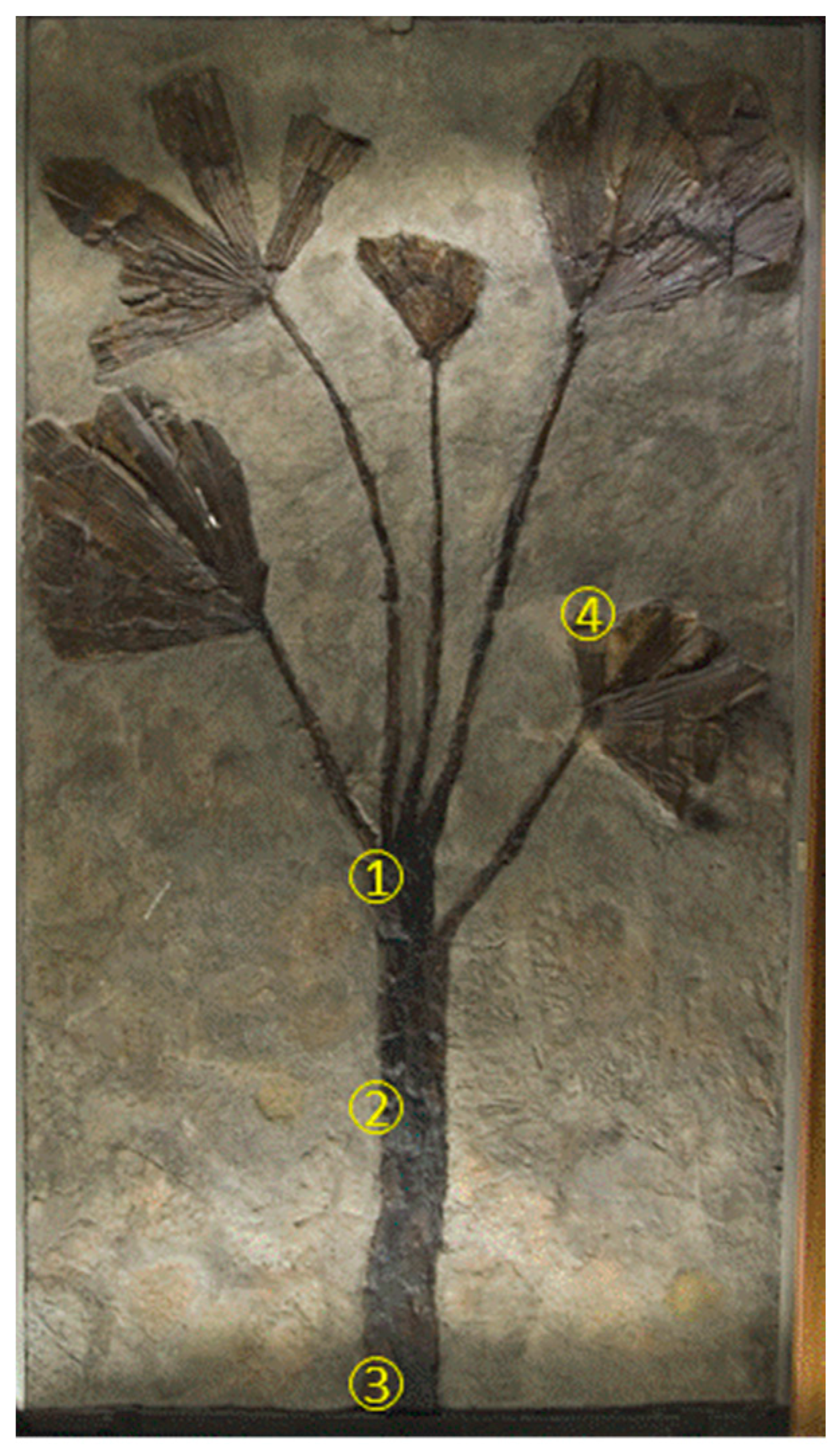
1.2. The Hall of Palms
2. Materials and Methods
2.1. Sampling and Analytical Techniques
2.2. On Field Investigations: Microclimatic Monitoring and Visual Inspections
- Climatic chamber VC3 4034 (Vötsch Industrietechnik, Balingen-Frommern, Germany);
- Selelogic sPRT—450 Smart Thermometer (Selelogic Instruments, Webresults advanced solutions, Bergamo, Italy), range T (−196 °C; 420 °C), accuracy 0.01 °C (−196 °C; 250 °C) and 0.02 °C (250 °C; 420 °C);
- Optidew High Performance Optical Dew Point transmitter (Michell Instruments, Michell Italia s.r.l, Milan, Italy), range RH (<0.5; 100%), accuracy ±0.2 °C dew point.
3. Results and Discussion
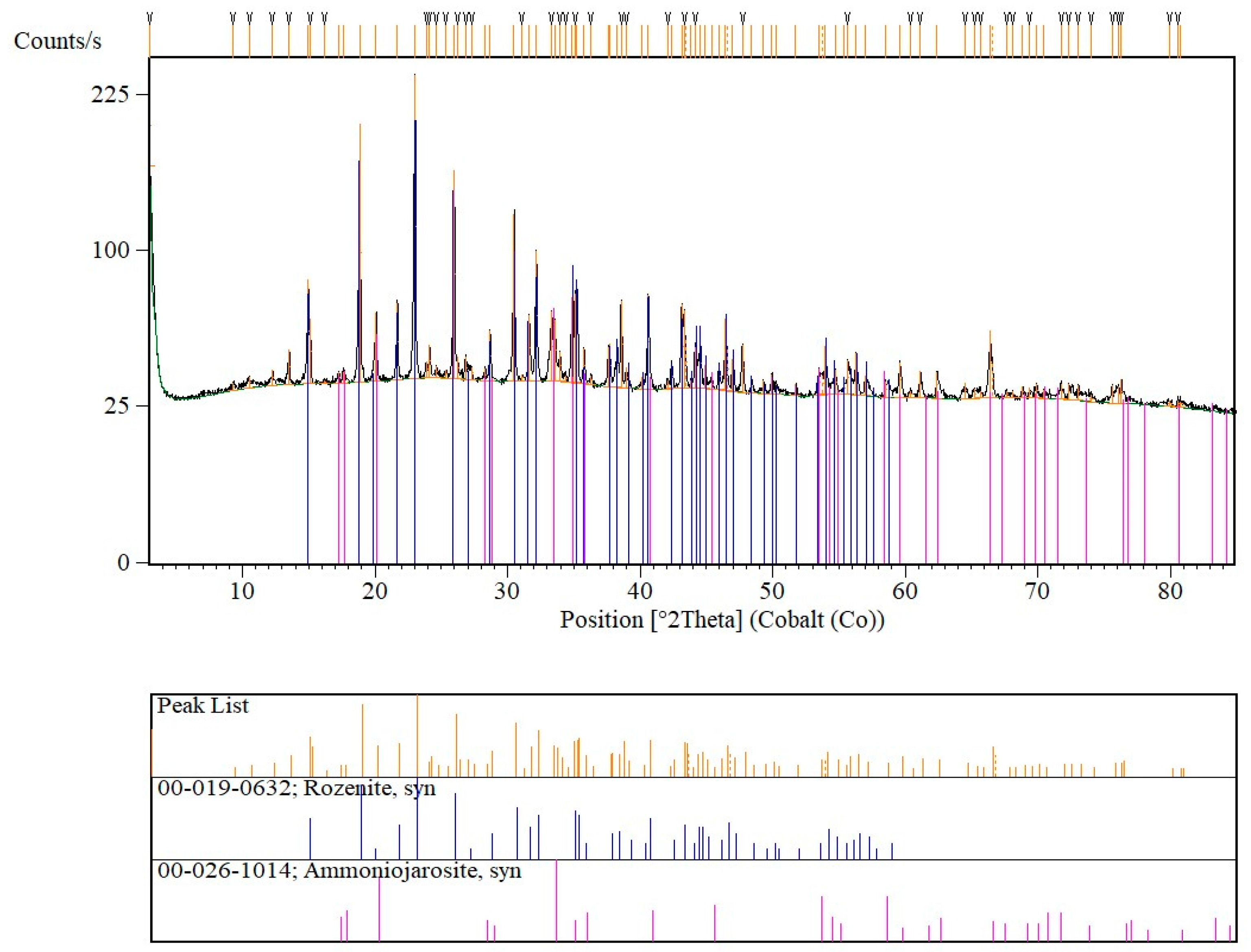
3.1. XRPD and Raman
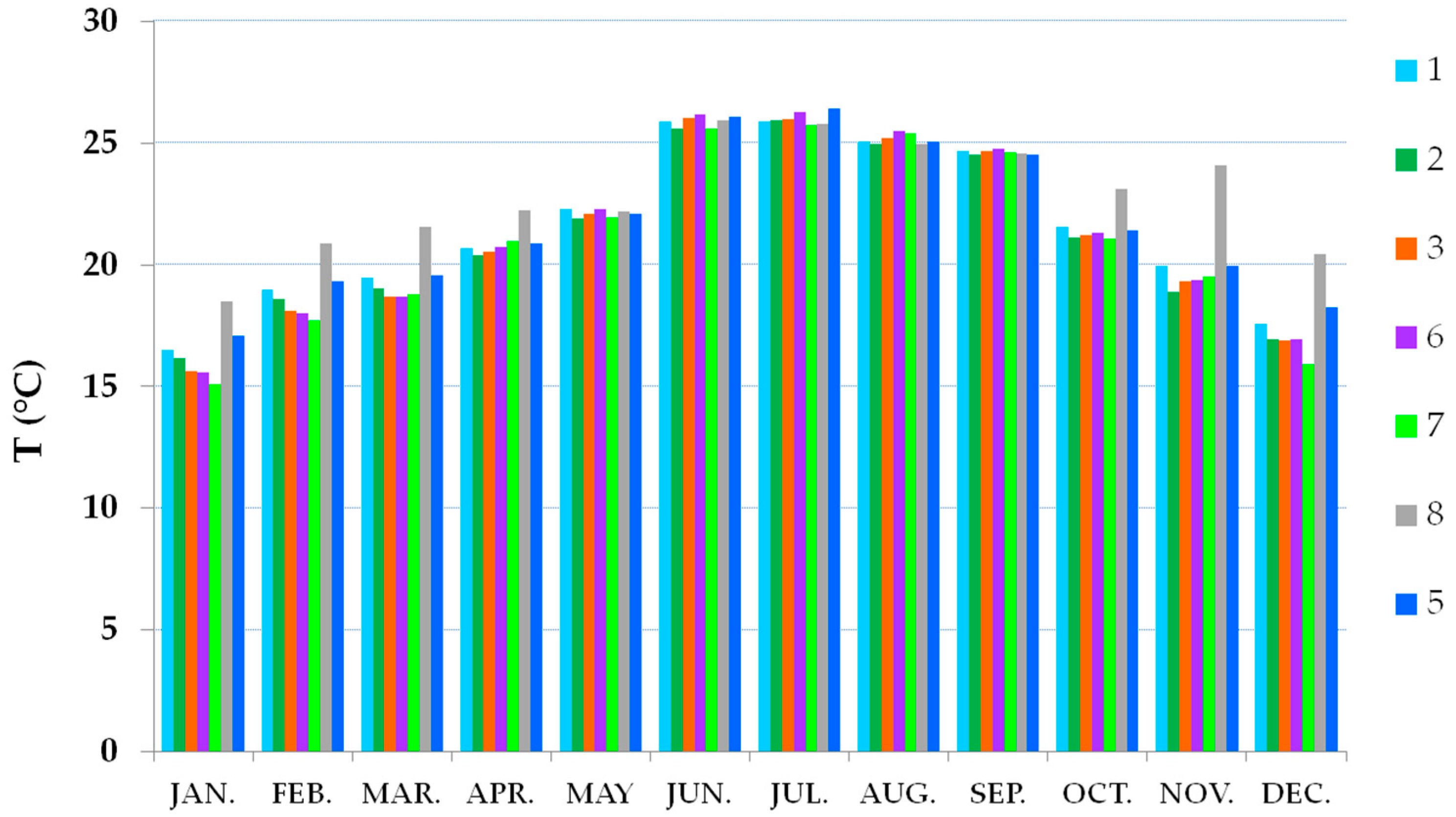
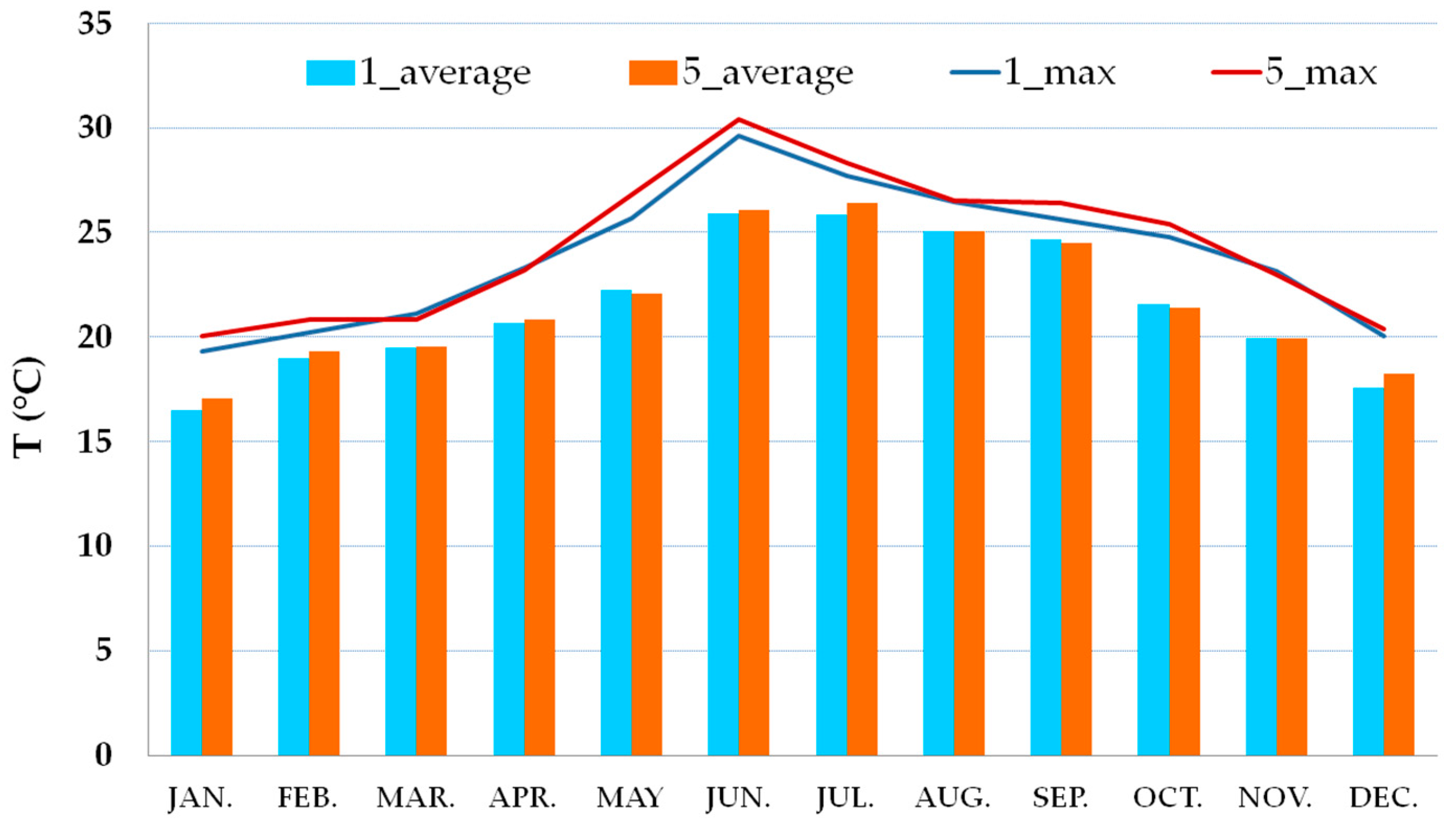
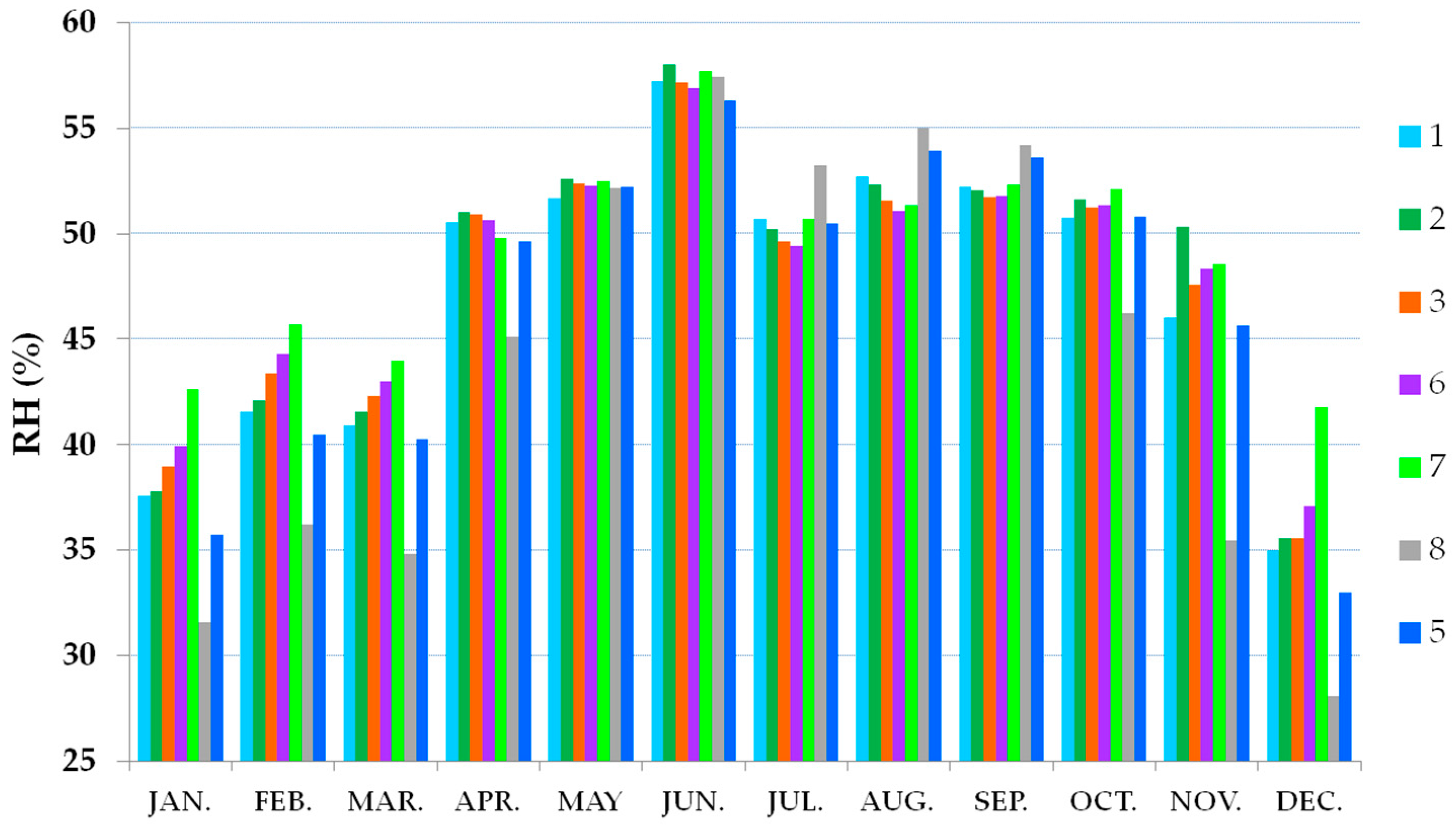
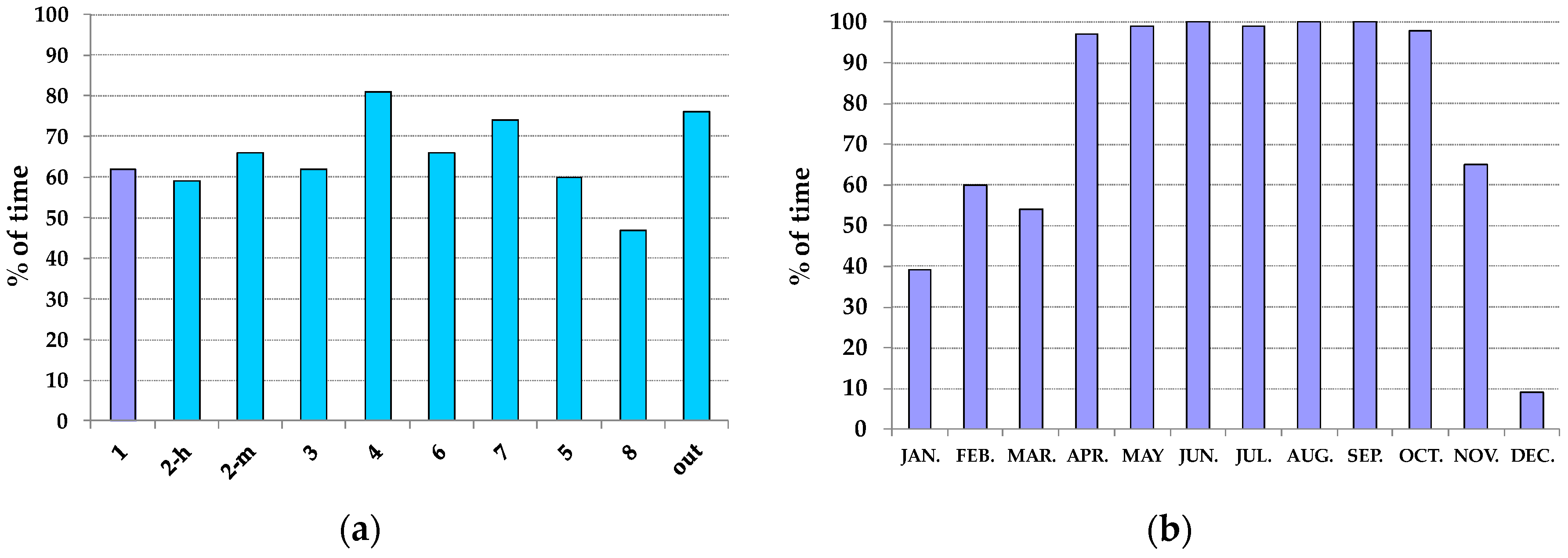
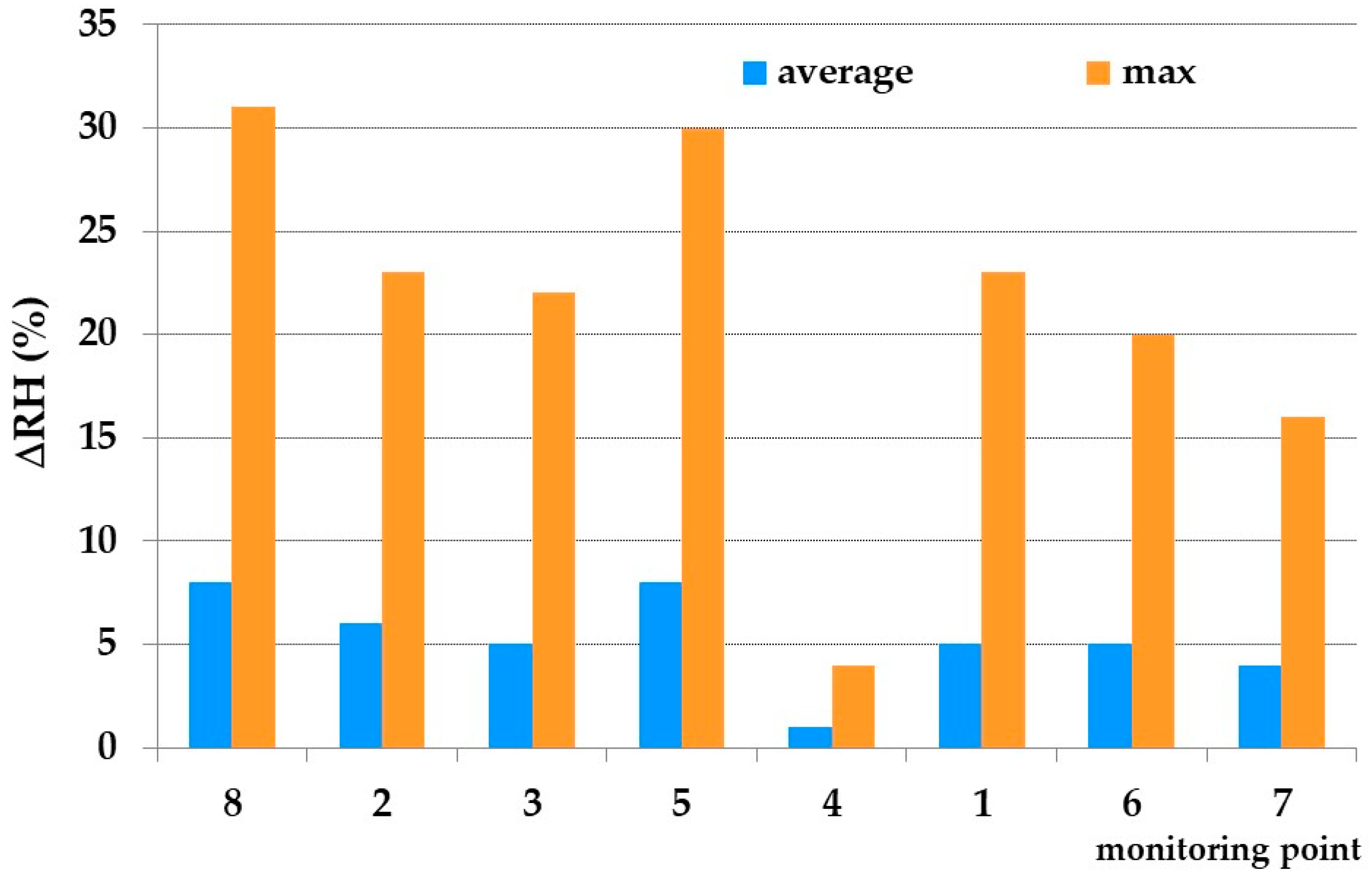
3.2. Thermo-Hygrometric Conditions
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Howie, F.M. Pyrite and conservation, part 2. Newslett. Geol. Curator. Group 1977, 1, 497–512. [Google Scholar]
- Larkin, N. Pyrite Decay: Cause and effect, prevention and cure. NatSCA News 2011, 21, 35–43. [Google Scholar]
- Howie, F.M. (Ed.) Pyrite and marcasite. In Care and Conservation of Geological Material; Butterworth & Heinmann: Oxford, UK, 1992; pp. 70–84. [Google Scholar]
- Canfield, D.E.; Raiswell, R. Pyrite formation and fossil preservation. In Taphonomy: Releasing the Data Locked in the Fossil Record; Allison, P.A., Briggs, D.E.G., Eds.; Plenum Press: New York, NY, USA, 1991; pp. 337–387. [Google Scholar]
- Andrew, K.J. Conservation of the Whitby Saurians—Large Scale, on Site Geological Conservation in North Yorkshire, United Kingdom. J. CAC 1999, 24, 3–10. [Google Scholar]
- Allison, P.A. Konservat-Lagerstaetten: Cause and classification. Paleobiology 1988, 14, 331–344. [Google Scholar] [CrossRef]
- Borkow, L.S.; Babcock, L.E. Turning pyrite concretions outside-in: Role of biofilms in pyritization fossils. In The Sedimentary Record; Society for Sedimentary Geology: Tulsa, OK, USA, 2003; Volume 1, pp. 4–7. [Google Scholar]
- Brock, F.; Parkes, R.J.; Briggs, D.E.G. Experimental pyrite formation associated with decay of plant material. Palaios 2006, 21, 499–506. [Google Scholar] [CrossRef]
- Clark, G.R.; Lutz, R.A. Pyritization in the shells of living bivalves. Geology 1980, 8, 268–271. [Google Scholar] [CrossRef]
- Oddy, W.A. The Conservation of Pyritic Stone Antiquities. Stud. Conserv. 1977, 22, 68–72. [Google Scholar] [CrossRef]
- Fors, Y.; Sandström, M. Sulfur and iron in shipwrecks cause conservation concerns. Chem. Soc. Rev. 2006, 35, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Guinea, J.; Martinez-Frias, J.; Gonzales-Martin, R.; Zamora, L. Framboidal pyrite in antique books. Nature 1997, 388, 631. [Google Scholar] [CrossRef]
- Shinya, A.; Bergwall, L. Pyrite oxidation: Review and prevention practices. In Proceedings of the Poster to the Society of Vertebrate Paleontology 67th Annual Meeting, Austin, TX, USA, 17–20 October 2007. [Google Scholar]
- Fellowes, D.; Hagan, P. Pyrite oxidation: The conservation of historic shipwrecks and geological and paleontological specimens. Stud. Conserv. 2003, 48, 26–38. [Google Scholar] [CrossRef]
- Rouchon, V.; Badet, H.; Belhadj, O.; Bonnerot, O.; Lavédrine, B.; Michard, J.G.; Miska, S. Raman and FTIR spectroscopy applied to the conservation report of paleontological collections: Identification of Raman and FTIR signatures of several iron sulfate species such as ferrinatrite and sideronatrite. J. Raman Spectrosc. 2012, 43, 1265–1274. [Google Scholar] [CrossRef]
- Newman, A. Pyrite oxidation and museum collections: A review of theory and conservation treatments. Geol. Curator 1998, 6, 363–371. [Google Scholar]
- Buttler, C.J. Environmental effects on geological material: Pyrite decay. In Conservation of Geological Collections; Child, R.E., Ed.; Archetype Publications: London, UK, 1994; pp. 4–8. [Google Scholar]
- McPhail, D.; Lam, E.; Doyle, A. The heat sealing of Escal® barrier films. Conservator 2003, 27, 107–116. [Google Scholar] [CrossRef]
- Mathias, C.; Ramsdale, K.; Nixon, D. Saving the Archaeological iron using the Revolutionary Preservation System. In Proceedings of the Metal 2004, National Museum of Australia, Canberra ACT, Canberra, Australia, 4–8 October 2004; pp. 28–42. [Google Scholar]
- Waller, R. An experimental ammonia gas treatment method for oxidized pyrite mineral specimens. In Proceedings of the ICOM Committee for Conservation: 8th Triennial Meeting, Sydney, Australia, 6–11 September 1987; Grimstad, K., Ed.; Getty Conservation Institute: Los Angeles, CA, USA, 1987; pp. 623–630. [Google Scholar]
- Cornish, L.; Doyle, A.M. Use of ethanolamine thioglycollate in the conservation of pyritized fossils. Palaeontology 1984, 2, 421–424. [Google Scholar]
- Cornish, L. The treatment of decaying pyritiferous fossil material using ethanolamine thioglycollate. Geol. Curator 1986, 4, 451–454. [Google Scholar]
- Cornish, L.; Doyle, A.M.; Swannel, J. The Gallery 30 Project: Conservation of a collection of fossil marine reptiles. Conservator 1995, 19, 20–28. [Google Scholar] [CrossRef]
- Camuffo, D. Microclimate for Cultural Heritage, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 1998. [Google Scholar]
- Bernardi, A. Microclimate inside Cultural Heritage Buildings; Il Prato: Padova, Italy, 2008. [Google Scholar]
- Becherini, F.; Bernardi, A.; Vivarelli, A.; Pockelé, L.; De Grandi, S.; Gandini, A.; García, O.; Zubiaga, M.; Espada Suárez, J.C.; Sperandio, B. Environmental Risk Assessment and Preventive Conservation Strategy for the Porch of the Glory, Santiago of Compostela Cathedral. J. Environ. Sci. Eng. B 2013, 2, 299–303. [Google Scholar] [CrossRef]
- Becherini, F.; Bernardi, A.; Di Tuccio, M.C.; Vivarelli, A.; Pockelé, L.; De Grandi, S.; Fortuna, S.; Quendolo, A. Microclimatic monitoring for the investigation of the different state of conservation of the stucco statues of the Longobard Temple in Cividale del Friuli (Udine, Italy). J. Cult. Herit. 2016, 18, 375–379. [Google Scholar] [CrossRef]
- Becherini, F.; Bernardi, A.; Frassoldati, E. Microclimate inside a semi-confined environment: Valutation of suitability for the conservation of heritage materials. J. Cult. Herit. 2010, 11, 471–476. [Google Scholar] [CrossRef]
- Barbieri, G.; Medizza, F. Contributo alla conoscenza della regione di Bolca (Monti Lessini). Mem. Ist. Geol. Min. Univ. Padova 1969, 27, 1–36. [Google Scholar]
- Giusberti, L.; Roghi, G.; Martinetto, E.; Fornasiero, M.; Simonetto, L. Le flore del Paleogene dell’Italia Settentrionale. The Paleogene flora of northern Italy. In La Storia Delle Piante Fossili in Italia/Palaeobotany of Italy, 2nd ed.; Kustatscher, E., Roghi, G., Bertini, A., Miola, A., Eds.; Museum of Nature South Tyrol: Bolzano, Italy, 2016; Volume 9, pp. 206–231. [Google Scholar]
- Del Favero, L.; Fornasiero, M.; Molin, G.; Reggiani, P.; Zorzi, F. Il restauro dei vegetali fossili esposti nella ‘Sala delle Palme’ del Museo di Geologia e Paleontologia dell’Università di Padova. Museol. Sci. Nuova Ser. 2012, 6, 49–57. [Google Scholar]
- Chapter 4: Museum Collections Environment. Available online: https://www.nps.gov/museum/publications/MHI/chap4.pdf (accessed on 5 September 2017).
- Stolow, N. Conservation policy and the exhibition of museum collections. J. Am. Inst. Conserv. 1977, 16, 12–20. [Google Scholar] [CrossRef]
- Brunton, C.H.C.; Besterman, T.P.; Cooper, J.A. Guidelines Curation of Geological Materials; Geological Society: London, UK, 1985. [Google Scholar]
- Environmental Guidelines ICOM-CC and IIC Declaration. Available online: http://www.icom-cc.org/332/-icom-cc-documents/declaration-on-environmental-guidelines/ (accessed on 1 September 2017).





| Season | Hall | Hall | Showcases | Showcases |
|---|---|---|---|---|
| T (°C) | RH (%) | T (°C) | RH (%) | |
| Summer | 24–25 | 50–55 | 20–22 | <40 |
| Winter | 18–20 | 50–55 | 18–20 | <40 |
| Identification Number/Name | Location | Showcase Dimensions | Height from the Floor |
|---|---|---|---|
| 1 | Inside showcase | 5.87 × 3.75 × 0.64 1 | 1.5 m |
| 2 | Inside showcase | 5.87 × 3.75 × 0.64 1 | 1.5 and 2.6 m |
| 3 | Inside showcase | 22.85 × 3.75 × 0.64 2 | 1.5 m |
| 4 | Inside showcase | 3.5 × 0.5 × 0.11 | 1.5 m |
| 5 | Inside room | 2.5 m | |
| 6 | Inside showcase | 22.85 × 3.75 × 0.64 2 | 1.5 m |
| 7 | Inside showcase | 3.23 × 2.67 × 0.20 | 1.5 m |
| 8 | Inside room | 2.5 m | |
| 9 | Inside showcase | 1.67 × 3.75 × 0.64 | 1.5 m |
| 10 | Inside showcase | 4.00 × 3.76 × 0.63 | 1.5 m |
| out | Outside | 7 m |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becherini, F.; Del Favero, L.; Fornasiero, M.; Guastoni, A.; Bernardi, A. Pyrite Decay of Large Fossils: The Case Study of the Hall of Palms in Padova, Italy. Minerals 2018, 8, 40. https://doi.org/10.3390/min8020040
Becherini F, Del Favero L, Fornasiero M, Guastoni A, Bernardi A. Pyrite Decay of Large Fossils: The Case Study of the Hall of Palms in Padova, Italy. Minerals. 2018; 8(2):40. https://doi.org/10.3390/min8020040
Chicago/Turabian StyleBecherini, Francesca, Letizia Del Favero, Mariagabriella Fornasiero, Alessandro Guastoni, and Adriana Bernardi. 2018. "Pyrite Decay of Large Fossils: The Case Study of the Hall of Palms in Padova, Italy" Minerals 8, no. 2: 40. https://doi.org/10.3390/min8020040




