Mobility and Attenuation Dynamics of Potentially Toxic Chemical Species at an Abandoned Copper Mine Tailings Dump
Abstract
:1. Introduction
2. Materials and Methods
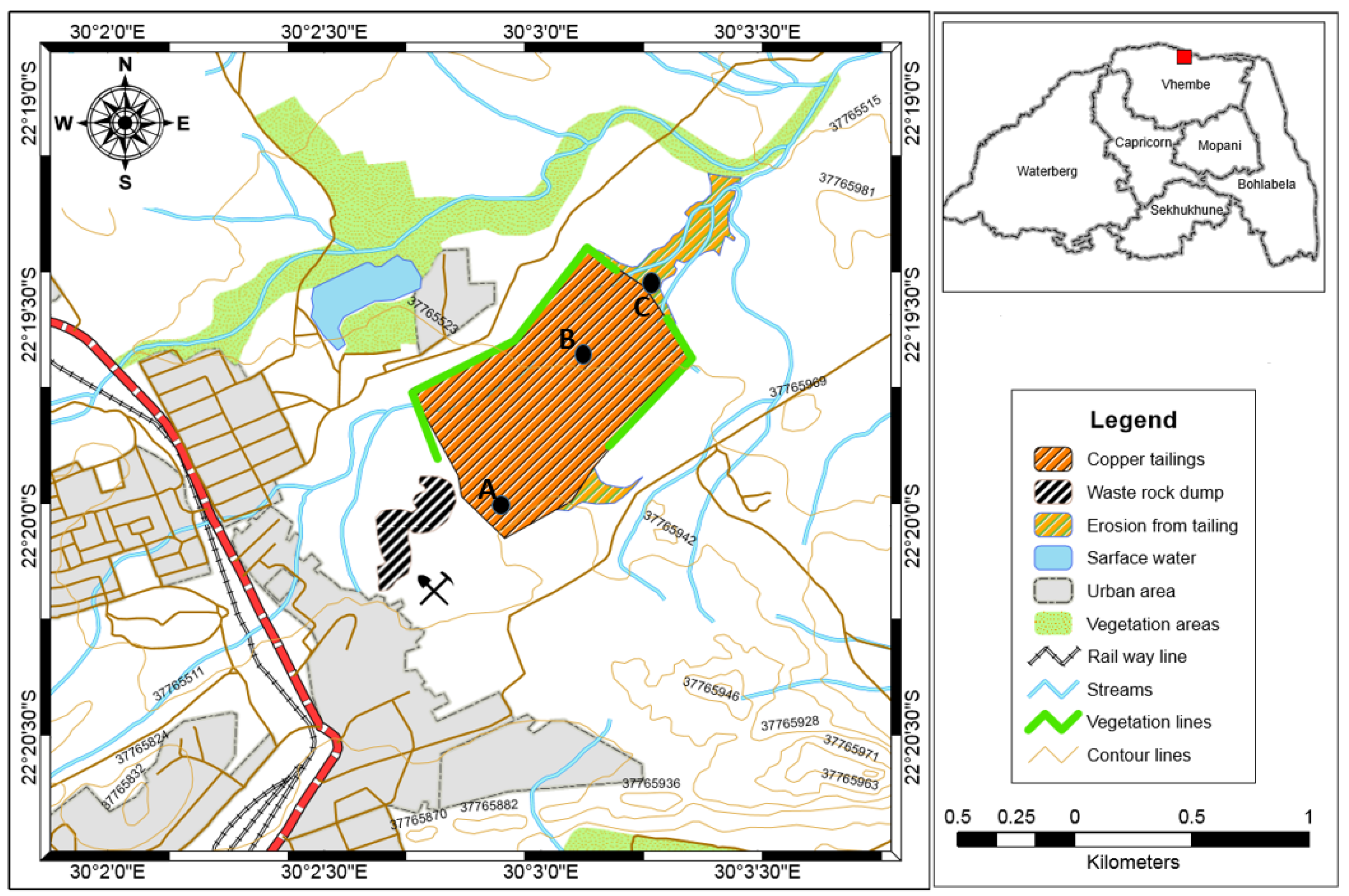
2.1. Study Area and Sampling Techniques
2.2. Determination of Tailings Physical Properties
2.3. Geochemical Analysis
2.3.1. Paste pH and Eh
2.3.2. Bulk Chemical Element Composition
2.4. Mineralogical Analysis
2.5. Batch Leaching Tests or Pore Water Chemistry
3. Results and Discussion
3.1. Macroscopic Description and Physical Properties of Copper Tailings Samples
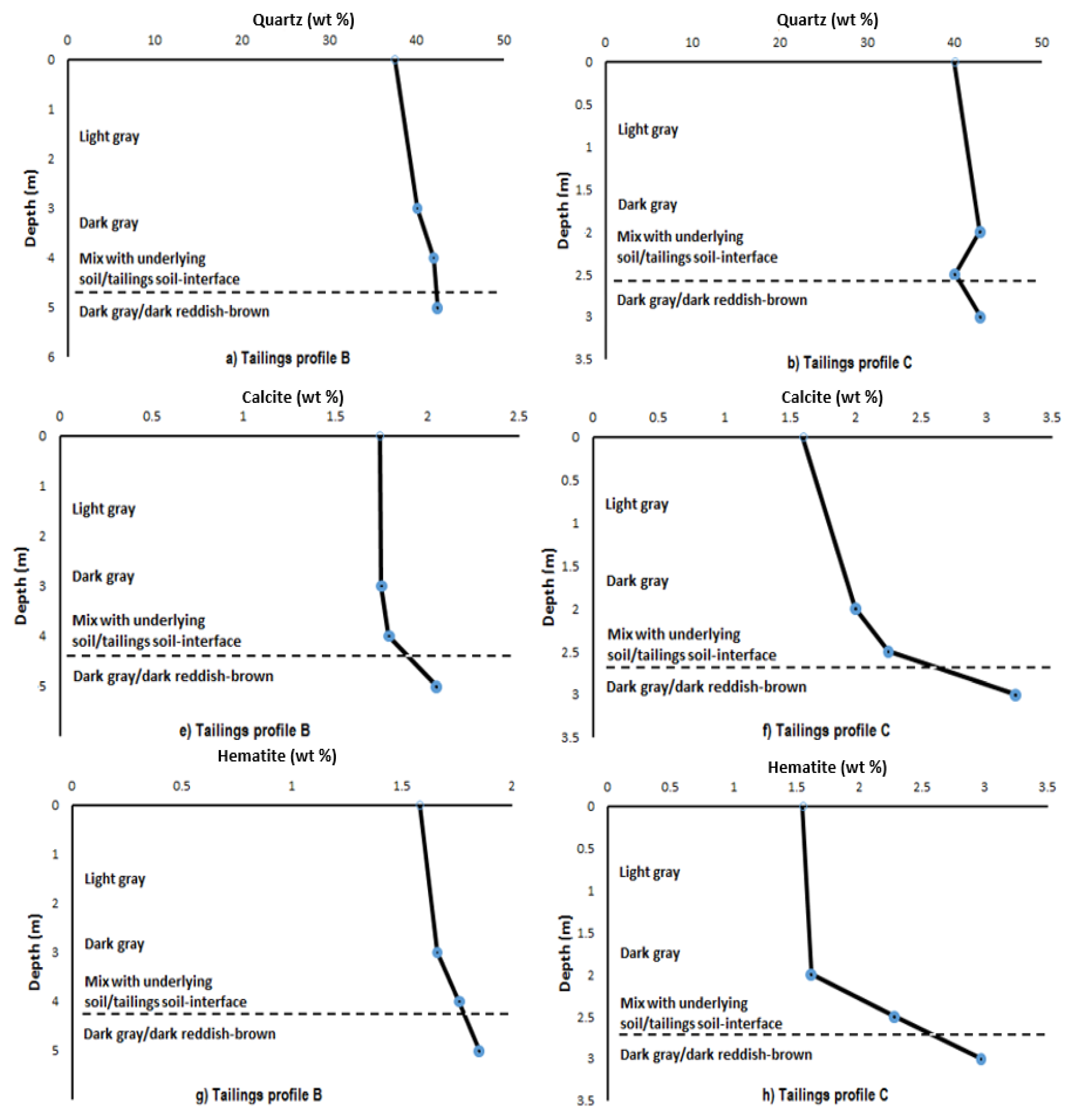
3.2. Mineralogical Composition within the Respective Copper Tailings Samples
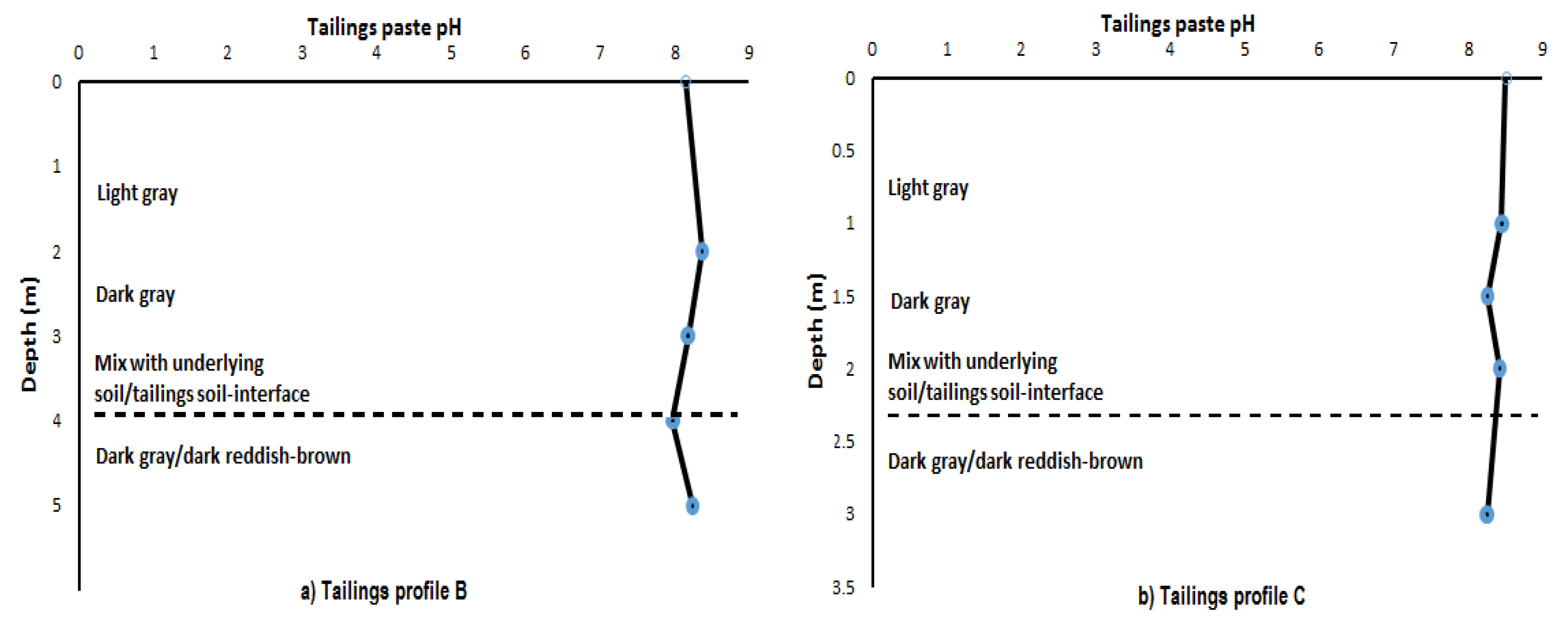
3.3. Tailings Paste pH and Eh
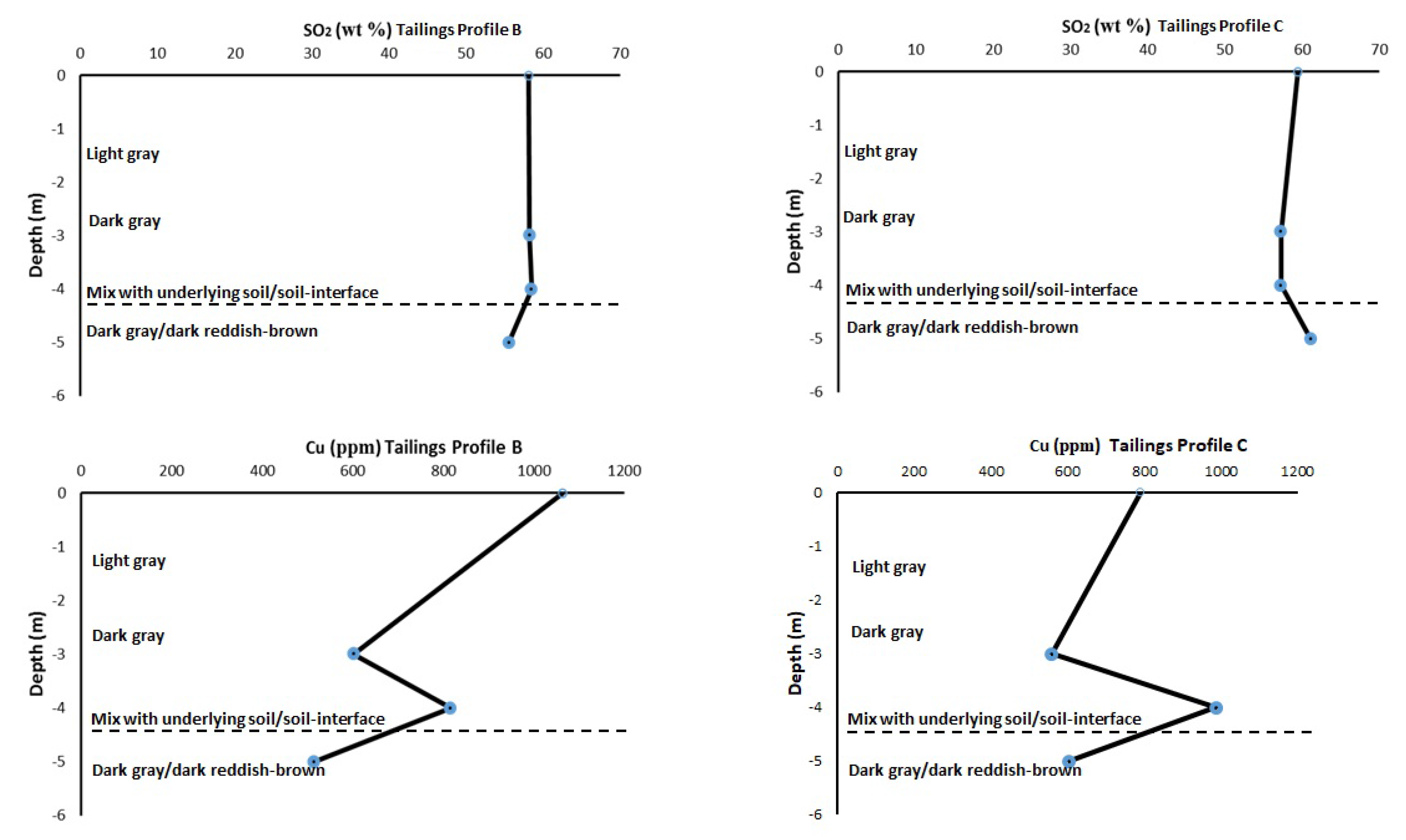
3.4. Bulk Chemical Elemental Composition within the Tailings Profiles
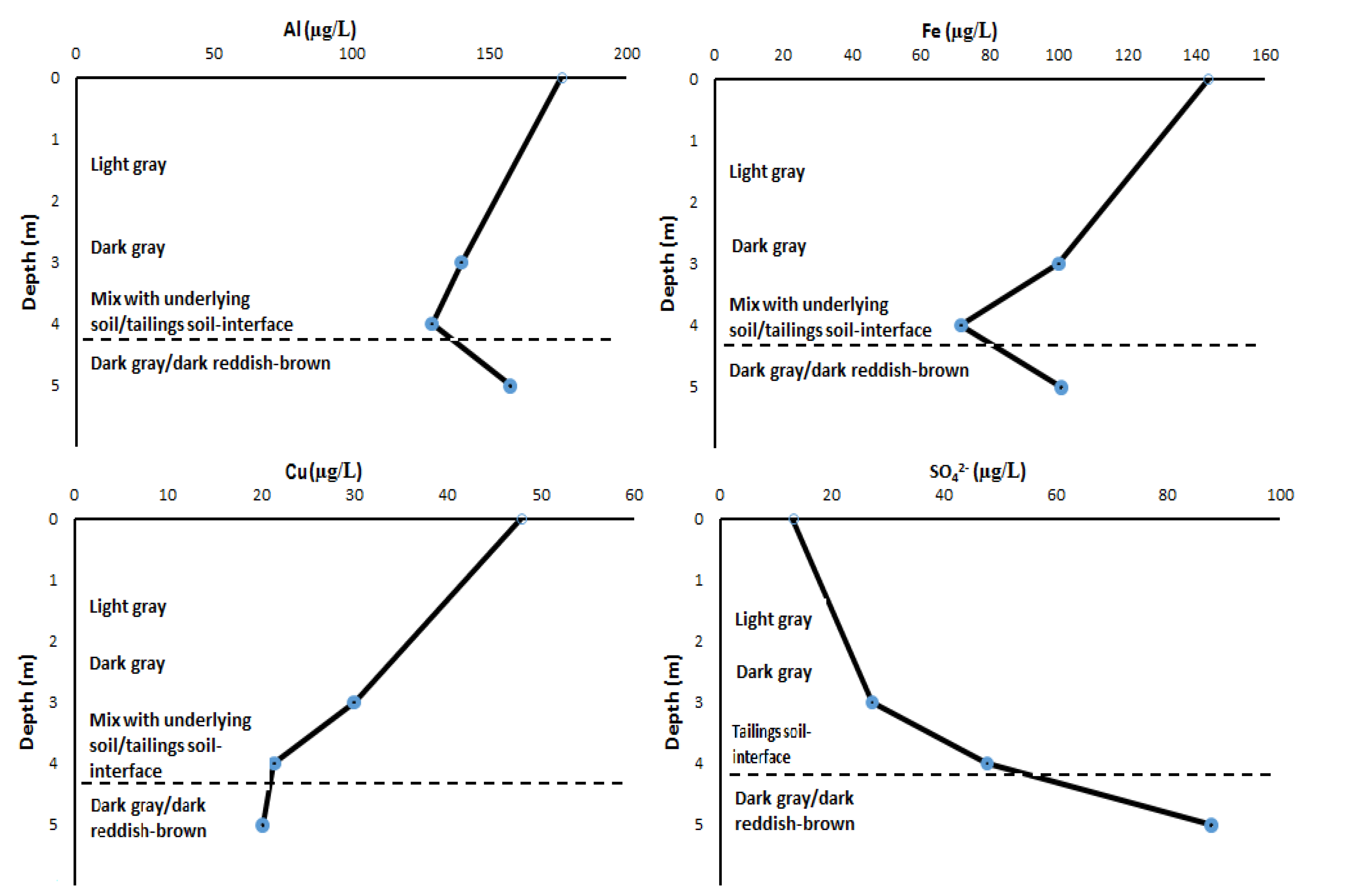
3.5. Pore-Water Chemistry, Solubility, and Mobility Patterns for Potentially Toxic Chemical Species with Tailings Depth
4. Summary and Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Department of Mineral Resources (DMR). The National for the Management of Derelict and Ownerless Mines in South Africa; Department of Mineral Resources: Pretoria, South Africa, 2009.
- Fosso-Kankeu, E. Ni2+ Extraction from Low Grade Leachate of Tailings Dumps Material Using Cloned Indigenous Bacterial Species; University of Johannesburg: Johannesburg, South Africa, 2011. [Google Scholar]
- Godfrey, L.; Oelofse, S.; Phiri, A.; Nahman, A.; Hall, J. Mineral Waste: The Required Governance Environment to Enable Reuse; Final Report, CSIR/NRE/ PW/IR/2007/0080/C; Council of Scientific and Industrial Research: New Delhi, India, 2007. [Google Scholar]
- Thobakgale, R. Evaluation of the Geochemical and Mineralogical Transformation at an Old Copper Mine Tailings Dump, Limpopo Province, South Africa. Master’s Thesis, University of Venda, Thohoyandou, South Africa, 2017. [Google Scholar]
- AngloGold Ashanti. Stakeholder Involvement in the Closure Planning Process at Ergo. In Report to Society for 2004; AngloGold Ashanti: Johannesburg, South Africa, 2004; pp. E45–E48. Available online: https://thevault.exchange/?get_group_doc=143/1502780448-Reporttosociety2004.pdf (accessed on 7 February 2018).
- Lottermoser, B.G. Mine Wastes: Characterization, Treatment and Environmental Impacts, 3rd ed.; Springer: Berlin, Germany, 2010; pp. 204–240. [Google Scholar]
- Romero, F.M.; Armienta, M.A. Gonzales-Hernandez, G. Solid-phase control on the mobility of potentially toxic elements in an abandoned lead/zinc tailings impoundment, Taxco, Mexico. Appl. Geochem. 2007, 22, 109–127. [Google Scholar] [CrossRef]
- Arenas-Lago, D.; Andrade, M.L.; Lago-Vila, M.; Rodríguez-Seijo, A.; Vega, F.A. Sequential extraction of heavy metals in soils from a copper mine: Distribution in geochemical fractions. Geoderma 2014, 10, 108–118. [Google Scholar] [CrossRef]
- Hiller, E.; Petrák, M.; Tóth, R.; Voleková, B.L.; Jurkovic, L.; Kučerová, G.; Radková, A.; Šottník, P.; Vozár, J. Geochemical and mineralogical characterization of neutral, low-sulfide/high-carbonate tailings impoundments, Markušovce, eastern Slovakia. Environ. Sci. Pollut. Res. 2013, 20, 7627–7642. [Google Scholar] [CrossRef] [PubMed]
- Rice, K.C.; Herman, J.S. Acidification of Earth: An assessment across mechanisms and scales. Appl. Geochem. 2012, 27, 1–14. [Google Scholar] [CrossRef]
- Sracek, O.; Mihaljevic, M.B.; Krˇibek, B.; Majer, V.; Veselovsky, F. Geochemistry and mineralogy of Cu and Co in mine tailings at the Copper belt, Zambia. J. Afr. Earth Sci. 2010, 57, 14–30. [Google Scholar] [CrossRef]
- McGregor, R.G.; Blowes, D.W. The physical, chemical and mineralogical Properties of three cemented layers within sulfide-bearing mine tailings. J. Geochem. Explor. Can. 2002, 76, 195–207. [Google Scholar] [CrossRef]
- Ashton, P.; Love, D.; Mahachi, H.; Dirks, P. An Overview of the Impact of Mining and Mineral Processing Operations on Water Resources and Water Quality in the Zambezi, Limpopo and Olifants Catchments in Southern Africa; Project by CSIR Environmentek and Geology Department, University of Zimbabwe, Harare, Zimbabwe; Report No. ENV-P-C 2001-042; Mining, Minerals and Sustainable Development: Pretoria, South Africa, 2001. [Google Scholar]
- Naicker, K.; Cukrowska, E.; McCarthy, T.S. Acid mine drainage arising from gold mining activity in Johannesburg, South Africa. Environ. Pollut. 2003, 122, 29–40. [Google Scholar] [CrossRef]
- Bortnikova, S.; Bessonova, E.; Gaskova, O. Geochemistry of arsenic and metals in stored tailings of a Co-Ni arsenide-ore. Khovu-Aksy area, Russia. Appl. Geochem. 2012, 27, 2238–2250. [Google Scholar] [CrossRef]
- Conesa, H.M.; Robinson, B.H.; Schulin, R.; Nowack, B. Metal extractability in acidic and neutral mine tailings from the Cartagena-La Unión mining district (SE Spain). Appl. Geochem. 2008, 23, 1232–1240. [Google Scholar] [CrossRef]
- Gitari, M.W.; Akinyemi, S.A.; Thobakgale, R.; Ngoejana, P.C.; Ramugondo, L.; Matidza, M.; Mhlongo, S.E.; Dacosta, F.A.; Nemapate, N. Physicochemical and Mineralogical Characterization of Musina Mine Copper and New Union Gold Mine Tailings: Implications for Fabrication of Beneficial Geopolymeric Construction Materials. J. Afr. Earth Sci. 2018, 137, 218–228. [Google Scholar] [CrossRef]
- Matshusa, K.; Makgae, M. Overview of Abandoned Mines in the Limpopo Province, South Africa: Rehabilitation Challenges. J. Environ. Sci. Eng. 2014, 15, 156–161. [Google Scholar]
- Singo, N.K. An Assessment of Heavy Metal Pollution near an Old Copper Mine Tailings Dump in Musina, South Africa. Master’s Thesis, University of South Africa, Johannesburg, South Africa, 2013. [Google Scholar]
- Beale, C.O. Copper in South Africa-Part II. J. S. Afr. Inst. Min. Metall. 1985, 85, 109–124. [Google Scholar]
- Cairncross, B.; Dixon, R. Minerals of South Africa; Geological Society of South Africa: Johannesburg, South Africa, 1995; ISBN 0-620-19324-7. [Google Scholar]
- Brandl, G. The Geology of the Musina Area; Government Printer: Pretoria, South Africa, 1981.
- ASTM D2216. Standard Test Methods for Laboratory Determination of Water (Moisture) Content of Soil and Rock by Mass; ASTM D2216-98; ASTM International: West Conshohocken, PA, USA, 1998. [Google Scholar]
- The European Committee for Standardization. Characterization of Waste. Leaching. Compliance Test for Leaching of Granular Waste Materials and Sludge. One Stage Batch Test at a Liquid to Solid Ratio of 10 L/kg for Materials with Particle Size below 4 mm (without or with Size Reduction); BS EN 12457-2:2002; The European Committee for Standardization (CEN): Brussels, Belgium, 2002. [Google Scholar]
- World Health Organization (WHO). Guidelines for Drinking Water Quality, 4th ed.; World Health Organization (WHO): Geneva, Switzerland, 2011. [Google Scholar]
- Gilbert, S.E.; Cooke, D.R.; Hollings, P. The effect of hardpan layers on the water chemistry from the leaching pyrrhotite-rich tailings material. Environ. Geol. 2003, 44, 687–697. [Google Scholar] [CrossRef]
- Lottermoser, B.G.; Ashley, P.M. Mobility and retention of trace elements in hardpan-cemented cassiterite tailings, North Queensland, Australia. Environ. Geol. 2006, 50, 835–846. [Google Scholar] [CrossRef]
- Government Gazette of South Africa. South African Soil Quality Guidelines for All Land Uses; Report No. 35160 (561); Government Gazette: Pretoria, South Africa, 2012.
- Dold, B. Basic concepts of environmental geochemistry of sulfide mine-waste. In Mineralogia, Geoquimica y Geomicrobiologia para el Manejo Ambiental de Desechos Mineros, Proceedings of the XXIV Curso Latinoamericano de Metalogenia UNESCO-SEG, Lima, Peru, 22 August–2 September 2005; Society of Economic Geologists (SEG): Littleton, CO, USA, 2005. [Google Scholar]
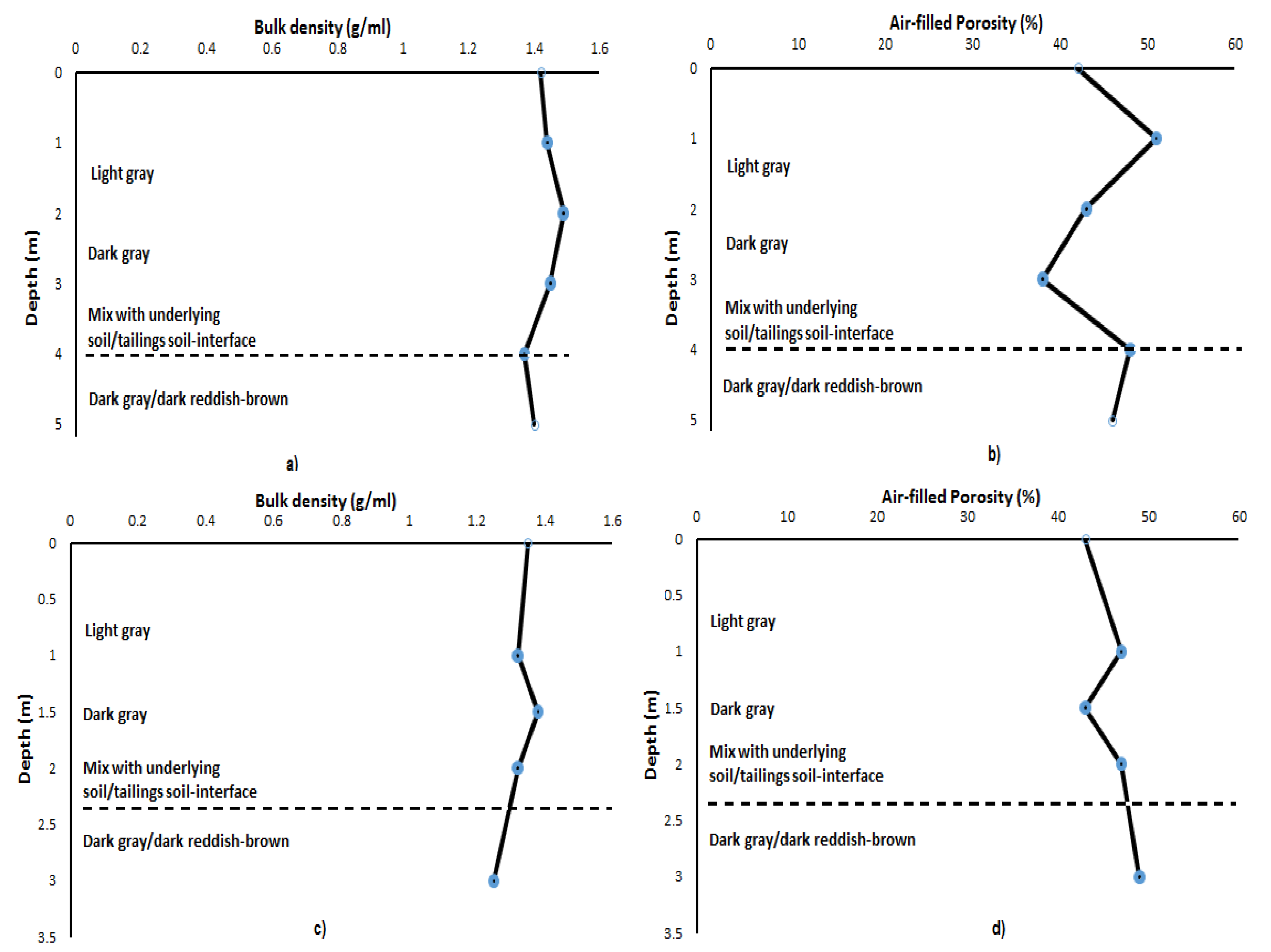

| Depth (m) | Bulk Density (g/mL) | Porosity (%) | Moisture (%) |
|---|---|---|---|
| 1 | 1.42 | 42 | 0.09 |
| 2 | 1.44 | 51 | 0.93 |
| 3 | 1.49 | 43 | 0.43 |
| 4 | 1.45 | 38 | 1.19 |
| 5 | 1.37 | 50 | 1.39 |
| 6 | 1.40 | 46 | 0.23 |
| Profile B (0–2 m) | Weight (%) | 3σ Error | Profile B (2–4 m) | Weight (%) | 3σ Error | Profile B (5 m) | Weight (%) | 3σ Error |
| Actinolite | 4.12 | 0.87 | Actinolite | 3.08 | 0.93 | Actinolite | 2.97 | 0.9 |
| Calcite | 1.74 | 0.33 | Calcite | 1.79 | 0.36 | Calcite | 2.05 | 0.36 |
| Chlorite | 17.34 | 0.84 | Chlorite | 18.87 | 0.9 | Chlorite | 17.38 | 0.93 |
| Epidote | 21.64 | 0.99 | Epidote | 20.41 | 1.02 | Epidote | 21.61 | 1.05 |
| Hematite | 1.58 | 0.33 | Hematite | 1.76 | 0.36 | Hematite | 1.85 | 0.33 |
| Muscovite | 8.41 | 0.72 | Muscovite | 8.18 | 0.81 | Muscovite | 8.2 | 0.84 |
| Plagioclase | 7.65 | 0.9 | Plagioclase | 3.98 | 0.84 | Plagioclase | 3.54 | 0.72 |
| Quartz | 37.5 | 0.99 | Quartz | 41.92 | 1.08 | Quartz | 42.4 | 1.08 |
| Profile C (0–2 m) | Weight (%) | 3σ Error | Profile C (2 m Top–2 m Bottom) | Weight (%) | 3σ Error | Profile C (3 m) | Weight (%) | 3σ Error |
| Actinolite | 4.1 | 0.9 | Calcite | 2.24 | 0.39 | Actinolite | 2.47 | 0.75 |
| Calcite | 2 | 0.39 | Chlorite | 16.7 | 0.84 | Calcite | 3.22 | 0.36 |
| Chlorite | 16.82 | 0.87 | Epidote | 23.56 | 0.93 | Chlorite | 13.31 | 0.78 |
| Epidote | 22.23 | 0.93 | Hematite | 2.28 | 0.3 | Epidote | 19.94 | 0.87 |
| Hematite | 1.62 | 0.3 | Muscovite | 8.55 | 0.75 | Hematite | 2.79 | 0.3 |
| Muscovite | 7.13 | 0.78 | Plagioclase | 6.59 | 0.87 | Muscovite | 8.68 | 0.69 |
| Plagioclase | 3.16 | 0.75 | Quartz | 40.07 | 0.96 | Plagioclase | 6.64 | 0.75 |
| Quartz | 42.94 | 1.05 | - | - | - | Quartz | 42.96 | 1.05 |
| Paste pH | 8.14 | 8.37 | 8.18 | 7.97 | 8.24 | |
| Eh (mV) | −71.3 | −83.9 | −73.3 | −61.9 | −77.2 | |
| Depth (m) | 0–1 | 1–2 | 2–3 | 3–4 | 4–5 | |
| Colour | Dark gray | Light/dark gray | Light/dark gray | Light/dark gray | Dark-reddish brown | |
| wt % | ||||||
| SiO2 | 58.1176 | 58.1431 | 58.2518 | 58.4938 | 58.5819 | |
| TiO2 | 0.798 | 0.9266 | 0.9151 | 0.9105 | 0.9079 | |
| Al2O3 | 14.1711 | 13.9807 | 13.9559 | 13.9314 | 13.564 | |
| Fe2O3 | 10.5766 | 11.3313 | 11.2364 | 11.0953 | 11.7616 | |
| MnO | 0.0502 | 0.0524 | 0.0544 | 0.0537 | 0.0556 | |
| MgO | 2.6372 | 2.4388 | 2.5485 | 2.6219 | 2.66 | |
| CaO | 7.3781 | 7.6648 | 7.5943 | 7.3809 | 7.2605 | |
| Na2O | 0.3797 | 0.324 | 0.3671 | 0.361 | 0.4474 | |
| K2O | 1.0167 | 0.8587 | 0.8272 | 0.8588 | 0.8066 | |
| LOI | 3.93 | 3.23 | 2.96 | 3.08 | 2.8908 | |
| TOTAL | 99.5507 | 99.509 | 99.1803 | 99.2741 | 99.4133 | |
| ppm | 0–1 m | 1–2 m | 2–3 m | 3–4 m | 4–5 m | South African Soil Quality Guidelines [28] |
| Cu | 1063 | 862 | 602 | 816 | 515 | 16 |
| Ni | 36 | 16 | 20 | 35 | 14 | 91 |
| Pb | - | - | - | - | - | 20 |
| Zn | 25 | 13 | 15 | 24 | 10 | 240 |
| Zr | 151 | 223 | 187 | 256 | 123 | - |
| Sr | 236 | 192 | 197 | 252 | 133 | - |
| Sample Name | Relative Mobility or % Leach of Chemical Species | |||||
|---|---|---|---|---|---|---|
| Cu | Co | Ni | Cd | Zn | Pb | |
| PB (0–2 m) | 0.006 | n.m | 0.081 | n.m | 0.005 | n.m |
| PB (2–4 m) | 0.003 | n.m | 0.003 | n.m | 0.015 | n.m |
| PB (4–5 m) | 0.005 | n.m | 0.006 | n.m | 0.027 | n.m |
| Bulk Chemical Composition | Units | PB (0–2 m) | PB (2–4 m) | PB (4–5 m) | WHO [25] |
|---|---|---|---|---|---|
| pHH20 | - | 8.46 ± 0.098 | 8.36 ± 0.021 | 8.41 ± 0.04 | - |
| EC | µS/cm | 156.5 ± 16.82 | 312 ± 7.07 | 364 ± 1.44 | - |
| TDS | mg/L | 18.8 ± 0.07 | 18.75 ± 0.06 | 18.85 ± 0.06 | - |
| Al | µg/L | 176.23 ± 59.67 | 129.37 ± 25 | 157.86 ± 0.26 | ≤100 |
| Fe | µg/L | 143.51 ± 95.58 | 71.65 ± 2.99 | 100.83 ± 1.47 | ≤1000–3000 |
| Ti | µg/L | 1.18 ± 0.15 | 0.78 ± 0.14 | 0.87 ± 0.12 | - |
| V | µg/L | 1.18 ± 0.04 | 0.63 ± 0.08 | 1.02 ± 0.03 | - |
| Cr | µg/L | 0.90 ± 0.26 | 0.60 ± 0.04 | 1.13 ± 0.04 | ≤50 |
| Mn | µg/L | 17.68 ± 2.40 | 37.10 ± 8.07 | 16.63 ± 1.80 | ≤500 |
| Co | µg/L | 0.09 ± 0.02 | 0.08 ± 0.01 | 0.09 ± 0.01 | ≤500 |
| Ni | µg/L | 0.54 ± 0.01 | 0.72 ± 0.08 | 0.62 ± 0.02 | ≤70 |
| Cu | µg/L | 47.9 ± 4.27 | 21.43 ± 1.74 | 20.21 ± 0.01 | ≤2000 |
| Zn | µg/L | 0.88 ± 0.21 | 1.54 ± 1.02 | 1.8 ± 0.52 | ≤5000 |
| As | µg/L | 0.31 ± 0.22 | 0.22 ± 0.01 | 0.29 ± 0.12 | ≤10 |
| Se | µg/L | 17.41 ± 1.23 | 20.93 ± 1.51 | 10.81 ± 2.88 | - |
| Sr | µg/L | 57.54 ± 1.96 | 123.88 ± 10.1 | 106.33 ± 4.22 | - |
| Mo | µg/L | 41.4 ± 2.9 | 59.44 ± 2.98 | 66.11 ± 1.22 | - |
| Cd | µg/L | 0.02 ± 0.01 | 0.01 | 0.02 ± 0.01 | ≤10 |
| Ba | µg/L | 13.1 ± 1.86 | 19.02 ± 1.98 | 16.17 ± 0.88 | - |
| Hg | µg/L | 0.28 ± 0.08 | 0.15 ± 0.07 | 0.10 ± 0.02 | ≤6 |
| Pb | µg/L | 0.27 ± 0.08 | 0.28 ± 0.09 | 0.34 ± 0.01 | ≤5-10 |
| Ca | mg/L | 26.71 ± 0.7 | 55.515 ± 3.92 | 30.75 ± 2.07 | ≤150 |
| K | mg/L | 9.09 ± 1.35 | 9.11 ± 1.9 | 8.45 ± 1.92 | - |
| Mg | mg/L | 4.88 ± 0.04 | 12.67 ± 1.77 | 8.01 ± 0.41 | ≤70 |
| Na | mg/L | 2.08 ± 0.13 | 4.4 ± 0.2 | 4.88 ± 0.11 | ≤200 |
| S | mg/L | 15.71 ± 0.03 | 55.94 ± 2.24 | 25.56 ± 1.98 | - |
| SO42− | ppm | 13.02 ± 3.16 | 47.74 ± 0.93 | 87.76 ± 4.463 | ≤500 |
| Cl− | ppm | 2.106 ± 0.35 | 16.93 ± 0.83 | 24.38 ± 0.44 | ≤300 |
| Saturation Index | Profile B | Profile C |
| Cd (OH)2 (s) | −7.074 | −3.06 |
| CoO (s) | −6.092 | −5.605 |
| Cr (OH)2 (s) | −5.33 | −5.022 |
| Cuprite | 5.488 | 1.403 |
| Fe(OH)2 (am) | −2.78 | −2.453 |
| Ni (OH)2 (s) | −4.611 | −4.06 |
| Quartz | −2.925 | −2.909 |
| Zn(OH)2 (am) | −4.171 | −3.535 |
| Main Species (%) | Profile B | Profile C |
| Cd | - | - |
| Cd2+ | 98.845 | 97.869 |
| CdOH+ | 1.145 | 2.013 |
| Co | - | - |
| Co2+ | 96.854 | 94.082 |
| CoOH+ | 2.817 | 4.861 |
| Co (OH)2 (aq) | 0.328 | 1.007 |
| Cr | - | - |
| Cr2+ | 0.084 | 0.047 |
| CrOH+ | 99.916 | 99.953 |
| Cu | - | - |
| Cu+ | 100 | 1.21 |
| CuSO3− | N/A | 98.79 |
| Fe | - | - |
| Fe2+ | 94.508 | 90.637 |
| FeOH+ | 5.485 | 9.343 |
| Ni | - | - |
| Ni2+ | 97.992 | 96.181 |
| NiOH+ | 1.798 | 3.135 |
| Ni (OH)2 (aq) | 0.21 | 0.65 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gitari, W.M.; Thobakgale, R.; Akinyemi, S.A. Mobility and Attenuation Dynamics of Potentially Toxic Chemical Species at an Abandoned Copper Mine Tailings Dump. Minerals 2018, 8, 64. https://doi.org/10.3390/min8020064
Gitari WM, Thobakgale R, Akinyemi SA. Mobility and Attenuation Dynamics of Potentially Toxic Chemical Species at an Abandoned Copper Mine Tailings Dump. Minerals. 2018; 8(2):64. https://doi.org/10.3390/min8020064
Chicago/Turabian StyleGitari, Wilson Mugera, Rendani Thobakgale, and Segun Ajayi Akinyemi. 2018. "Mobility and Attenuation Dynamics of Potentially Toxic Chemical Species at an Abandoned Copper Mine Tailings Dump" Minerals 8, no. 2: 64. https://doi.org/10.3390/min8020064
APA StyleGitari, W. M., Thobakgale, R., & Akinyemi, S. A. (2018). Mobility and Attenuation Dynamics of Potentially Toxic Chemical Species at an Abandoned Copper Mine Tailings Dump. Minerals, 8(2), 64. https://doi.org/10.3390/min8020064





