Pyrophosphate-Inhibition of Apatite Formation Studied by In Situ X-Ray Diffraction
Abstract
:1. Introduction
2. Materials and Methods
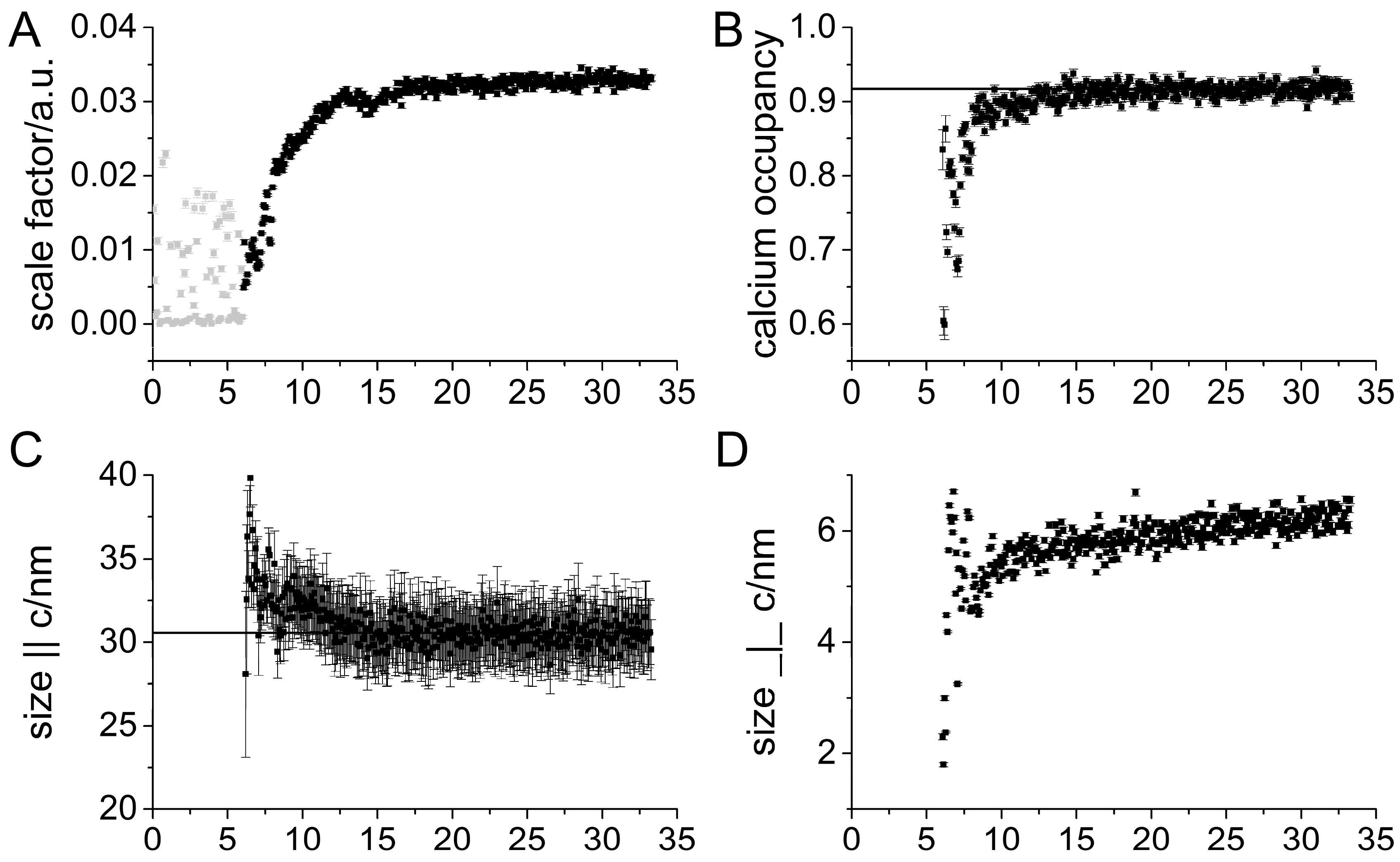
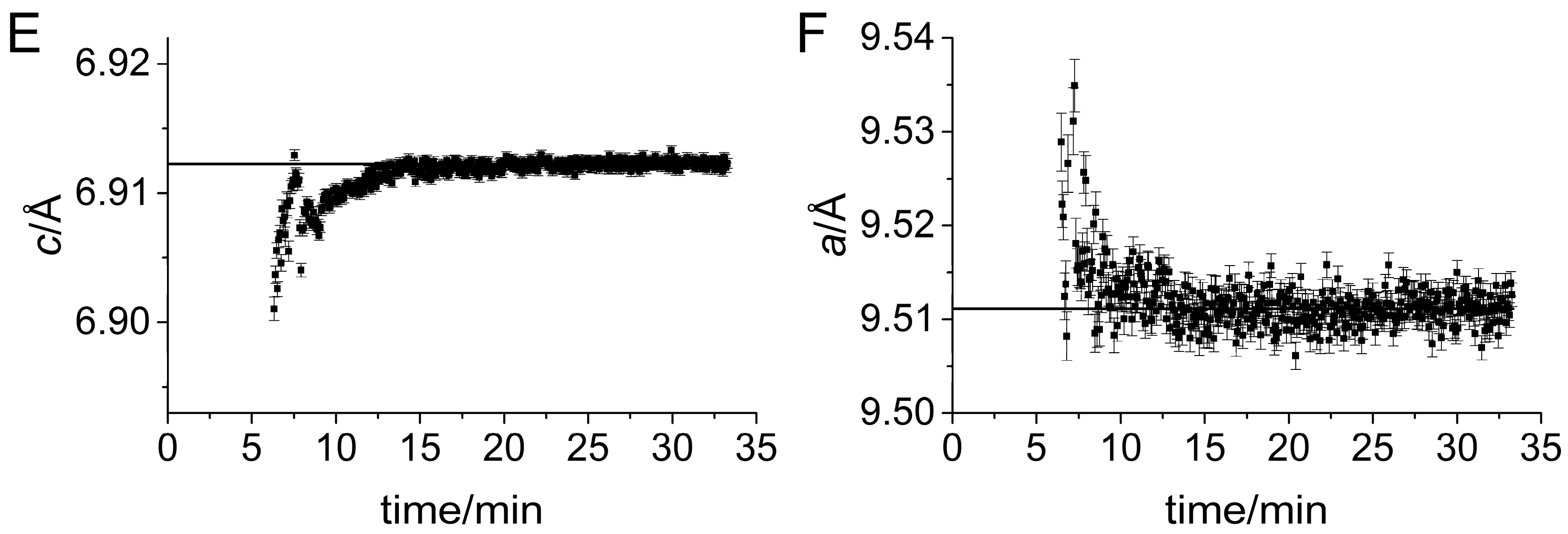
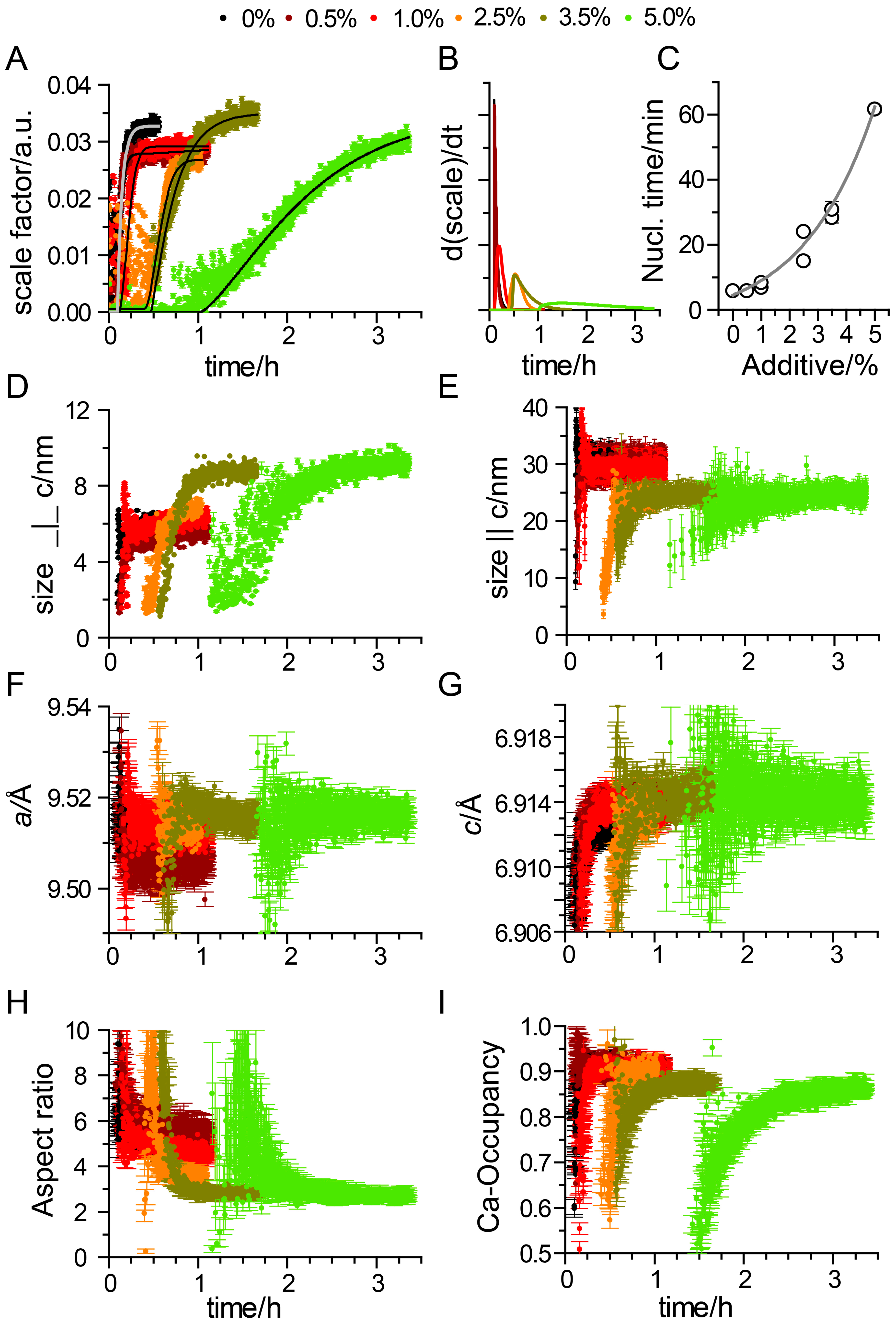
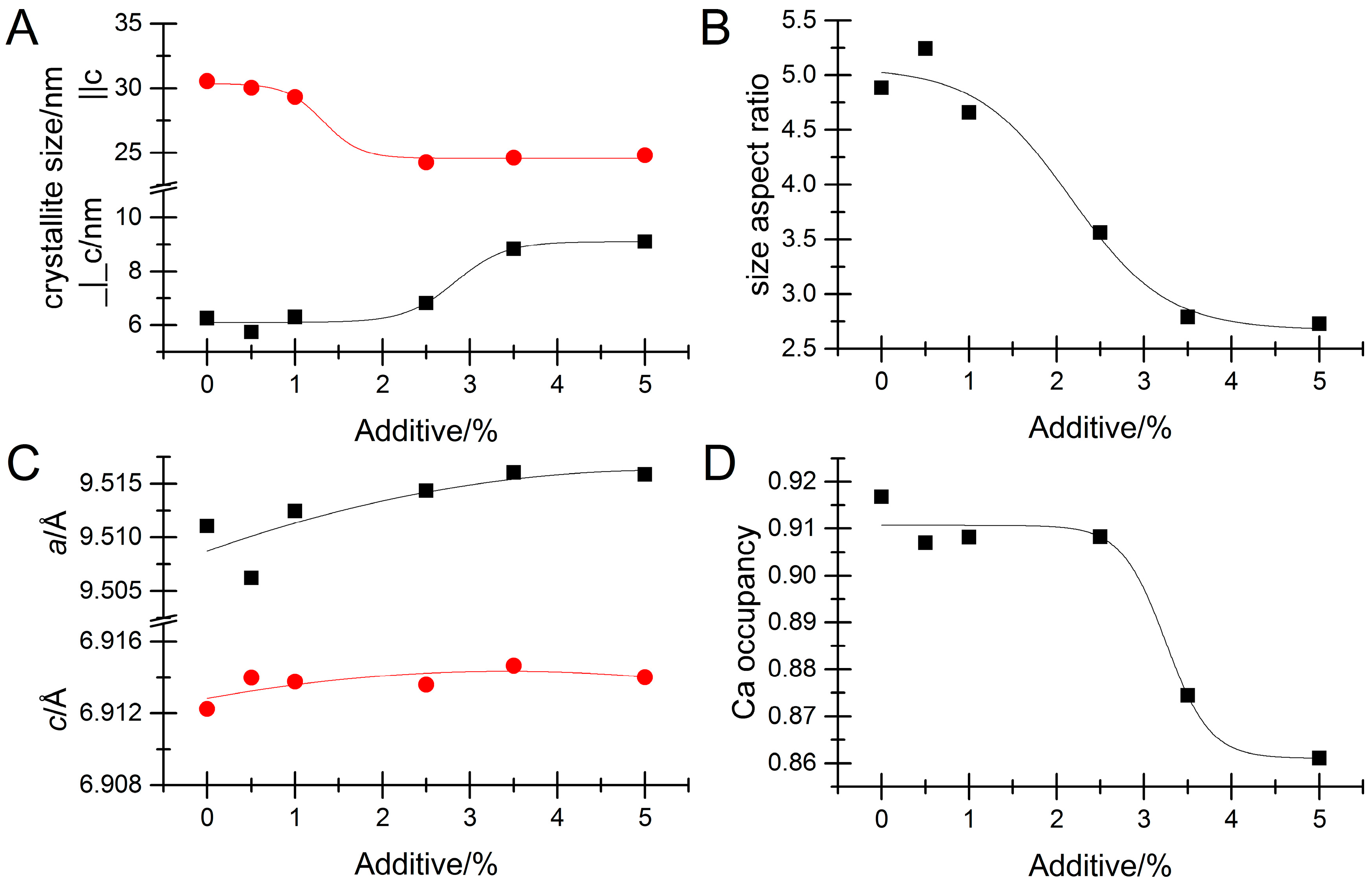
3. Results
Impact of Pyrophosphate
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Birkedal, H. Phase transformations in calcium phosphate crystallization. In New Perspectives on Mineral Nucleation and Growth: From Solution Precursors to Solid Materials; Van Driessche, A.E.S., Kellermeier, M., Benning, L.G., Gebauer, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 199–210. [Google Scholar]
- Davies, E.; Müller, K.H.; Wong, W.C.; Pickard, C.J.; Reid, D.G.; Skepper, J.N.; Duer, M.J. Citrate bridges between mineral platelets in bone. Proc. Natl. Acad. Sci. USA 2014, 111, E1354–E1363. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.-Y.; Rawal, A.; Schmidt-Rohr, K. Strongly bound citrate stabilizes the apatite nanocrystals in bone. Proc. Natl. Acad. Sci. USA 2010, 107, 22425–22429. [Google Scholar] [CrossRef] [PubMed]
- Reid, D.G.; Duer, M.J.; Jackson, G.E.; Murray, R.C.; Rodgers, A.L.; Shanahan, C.M. Citrate occurs widely in healthy and pathological apatitic biomineral: Mineralized articular cartilage, and intimal atherosclerotic plaque and apatitic kidney stones. Calcif. Tissue Int. 2013, 93, 253–260. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.C.S.; Ibsen, C.J.S.; Birkedal, H. Transparent aggregates of nanocrystalline hydroxyapatite. Cryst. Growth Des. 2014, 14, 6343–6349. [Google Scholar] [CrossRef]
- Omelon, S.; Georgiou, J.; Henneman, Z.J.; Wise, L.M.; Sukhu, B.; Hunt, T.; Wynnyckyj, C.; Holmyard, D.; Bielecki, R.; Grynpas, M.D. Control of vertebrate skeletal mineralization by polyphosphates. PLoS ONE 2009, 4, e5634. [Google Scholar] [CrossRef] [PubMed]
- Omelon, S.; Grynpas, M.D. Relationships between polyphosphate chemistry, biochemistry and apatite biomineralization. Chem. Rev. 2008, 108, 4694–4715. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Garimella, R.; Tague, S.E. The role of matrix vesicles in growth plate development and biomineralization. Front. Biosci. 2005, 10, 822–837. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Shapiro, I.M. The epiphyseal growth plate. In Bone and Development; Bronner, F., Farach-Carson, M.C., Roach, H.I., Eds.; Springer: London, UK, 2010; pp. 39–64. [Google Scholar]
- Anderson, H.C.; Sipe, J.B.; Hessle, L.; Dhamyamraju, R.; Atti, E.; Camacho, N.P.; Millán, J.L. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am. J. Pathol. 2004, 164, 841–847. [Google Scholar] [CrossRef]
- Orriss, I.R.; Arnett, T.R.; Russell, R.G.G. Pyrophosphate: A key inhibitor of mineralisation. Curr. Opin. Pharmacol. 2016, 28, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Hessle, L.; Johnson, K.A.; Anderson, H.C.; Narisawa, S.; Sali, A.; Goding, J.W.; Terkeltaub, R.; Millán, J.L. Tissue-nonspecific alkaline phosphatase and plasma cell membrane glycoprotein-1 are central antagonistic regulators of bone mineralization. Proc. Natl. Acad. Sci. USA 2002, 99, 9445–9449. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Russell, R.G.G.; Straumann, F. Effect of pyrophosphate on hydroxyapatite and its implications in calcium homeostasis. Nature 1966, 212, 901–903. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Russell, R.G.G.; Bisaz, S.; Termine, J.D.; Posner, A.S. Influence of pyrophosphate on the transformation of amorphous to crystalline calcium phosphate. Calcif. Tissue Res. 1968, 2, 49–59. [Google Scholar] [CrossRef]
- Fleisch, H.; Bisaz, S. Isolation from urine of pyrophosphate, a calcification inhibitor. Am. J. Physiol. Leg. Content 1962, 203, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Straumann, F.; Schenk, R.; Bisaz, S.; Allgöwer, M. Effect of condensed phosphates on calcification of chick embryo femurs in tissue culture. Am. J. Physiol. 1966, 211, 821–825. [Google Scholar] [CrossRef] [PubMed]
- Francis, M.D. The inhibition of calcium hydroxyapatite crystal growth by polyphosphonates and polyphosphates. Calcif. Tissue Res. 1969, 3, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Fleisch, H.; Neuman, W.F. Mechanisms of calcification: Role of collagen, polyphosphates, and phosphatase. Am. J. Physiol. 1961, 200, 1296–1300. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.L.; McCall, J.T.; Smith, L.H. Inhibition of calcium phosphate crystallization by nucleoside phosphates. Calcif. Tissue Res. 1974, 15, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Meyer, J.L. Can biological calcification occur in the presence of pyrophosphate? Arch. Biochem. Biophys. 1984, 231, 1–8. [Google Scholar] [CrossRef]
- Wuthier, R.E.; Bisaz, S.; Russell, R.G.G.; Fleisch, H. Relationship between pyrophosphate, amorphous calcium phosphate and other factors in the sequence of calcificationin vivo. Calcif. Tissue Res. 1972, 10, 198–206. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, C.J.S.; Chernyshov, D.; Birkedal, H. Apatite formation from amorphous calcium phosphate and mixed amorphous calcium phosphate/amorphous calcium carbonate. Chem. Eur. J. 2016, 22, 12347–12357. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.C.S.; Hinge, M.; Birkedal, H. Calcite nucleation on the surface of PNIPAM-PAAc micelles studied by time resolved in situ PXRD. Cryst. Eng. Commun. 2015, 17, 6940–6946. [Google Scholar] [CrossRef]
- Ibsen, C.J.S.; Birkedal, H. Influence of poly(acrylic acid) on apatite formation studied by in situ X-ray diffraction using an X-ray scattering reaction cell with high-precision temperature control. J. Appl. Crystallogr. 2012, 45, 976–981. [Google Scholar] [CrossRef]
- Ibsen, C.J.S.; Birkedal, H. Modification of bone-like apatite nanoparticle size and growth kinetics by alizarin red S. Nanoscale 2010, 2, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Ibsen, C.J.S.; Leemreize, H.; Mikladal, B.F.; Skovgaard, J.; Eltzholtz, J.R.; Bremholm, M.; Iversen, B.B.; Birkedal, H. Crystallization kinetics of bone-like apatite nanocrystals formed from amorphous calcium phosphate in water by in situ synchrotron powder diffraction: Counter ions matter. 2018; in preparation. [Google Scholar]
- Frølich, S.; Birkedal, H. Multiref: Software platform for automated and intelligent rietveld refinement of multiple powder diffractograms from in situ, scanning or diffraction tomography experiments. J. Appl. Cryst. 2015, 48, 2019–2025. [Google Scholar] [CrossRef]
- Ibsen, C.J.S.; Gebauer, D.; Birkedal, H. Osteopontin strongly stabilizes metastable states prior to nucleation during apatite formation. Chem. Mater. 2016, 28, 8550–8555. [Google Scholar] [CrossRef]
- Olliges-Stadler, I.; Rossell, M.D.; Suess, M.J.; Ludi, B.; Bunk, O.; Pedersen, J.S.; Birkedal, H.; Niederberger, M. A comprehensive study of the crystallization mechanism involved in the nonaqueous formation of tungstite. Nanoscale 2013, 5, 8517–8525. [Google Scholar] [CrossRef] [PubMed]
- Jensen, G.V.; Bremholm, M.; Lock, N.; Deen, G.R.; Jensen, T.R.; Iversen, B.B.; Niederberger, M.; Pedersen, J.S.; Birkedal, H. Anisotropic crystal growth kinetics of anatase TiO2 nanoparticles synthesized in a nonaqueous medium. Chem. Mater. 2010, 22, 6044–6055. [Google Scholar] [CrossRef]
- Bisaz, S.; Russell, R.G.G.; Fleisch, H. Isolation of inorganic pyrophosphate from bovine and human teeth. Arch. Oral Biol. 1968, 13, 683–696. [Google Scholar] [CrossRef]
- Larson, A.C.; Von Dreele, R.B. Los Alamos National Laboratory Report Laur 86-748; Los Alamos National Laboratory: Los Alamos, NM, USA, 2000.
- Leemreize, H.; Eltzholtz, J.R.; Birkedal, H. Lattice macro and microstrain fluctuations in the calcified byssus of Anomia simplex. Eur. J. Miner. 2014, 26, 517–522. [Google Scholar]
- Frølich, S.; Sørensen, H.O.; Hakim, S.S.; Marin, F.; Stipp, S.L.S.; Birkedal, H. Smaller calcite lattice deformation caused by occluded organic material in coccoliths than in mollusk shell. Cryst. Growth Des. 2015, 15, 2761–2767. [Google Scholar] [CrossRef]
- Pokroy, B.; Fitch, A.N.; Lee, P.L.; Quintana, J.P.; Caspi, E.N.; Zolotoyabko, E. Anisotropic lattice distortions in mollusk-made aragonite: A widespread phenomenon. J. Struct. Biol. 2006, 153, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Pokroy, B.; Fitch, A.N.; Marin, F.; Kapon, M.; Adir, N.; Zolotoyabko, E. Anisotropic lattice distortions in biogenic calcite induced by intra-crystalline organic moleucles. J. Struct. Biol. 2006, 155, 96–103. [Google Scholar] [CrossRef] [PubMed]
- Pokroy, B.; Quintana, J.P.; Caspi, E.N.; Berner, A.; Zolotoyabko, E. Anisotropic lattice distortions in biogenic aragonite. Nat. Mater. 2004, 3, 900–902. [Google Scholar] [CrossRef] [PubMed]
- Brif, A.; Ankonina, G.; Drathen, C.; Pokroy, B. Bio-inspired band gap engineering of zinc oxide by intracrystalline incorporation of amino acids. Adv. Mater. 2014, 26, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Brif, A.; Bloch, L.; Pokroy, B. Bio-inspired engineering of a zinc oxide/amino acid composite: Synchrotron microstructure study. Cryst. Eng. Comm. 2014, 16, 3268–3273. [Google Scholar] [CrossRef]
- Habraken, W.J.E.M.; Tao, J.; Brylka, L.J.; Friedrich, H.; Bertinetti, L.; Schenk, A.S.; Verch, A.; Dmitrovic, V.; Bomans, P.H.H.; Frederik, P.M.; et al. Ion-association complexes unite classical and non-classical theories for the biomimetic nucleation of calcium phosphate. Nat. Comm. 2013, 4, 1507. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibsen, C.J.S.; Birkedal, H. Pyrophosphate-Inhibition of Apatite Formation Studied by In Situ X-Ray Diffraction. Minerals 2018, 8, 65. https://doi.org/10.3390/min8020065
Ibsen CJS, Birkedal H. Pyrophosphate-Inhibition of Apatite Formation Studied by In Situ X-Ray Diffraction. Minerals. 2018; 8(2):65. https://doi.org/10.3390/min8020065
Chicago/Turabian StyleIbsen, Casper Jon Steenberg, and Henrik Birkedal. 2018. "Pyrophosphate-Inhibition of Apatite Formation Studied by In Situ X-Ray Diffraction" Minerals 8, no. 2: 65. https://doi.org/10.3390/min8020065




