Depression Mechanism of Strontium Ions in Bastnaesite Flotation with Salicylhydroxamic Acid as Collector
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Flotation Studies
2.3. Contact Angle Studies
2.4. Zeta-Potential Determination
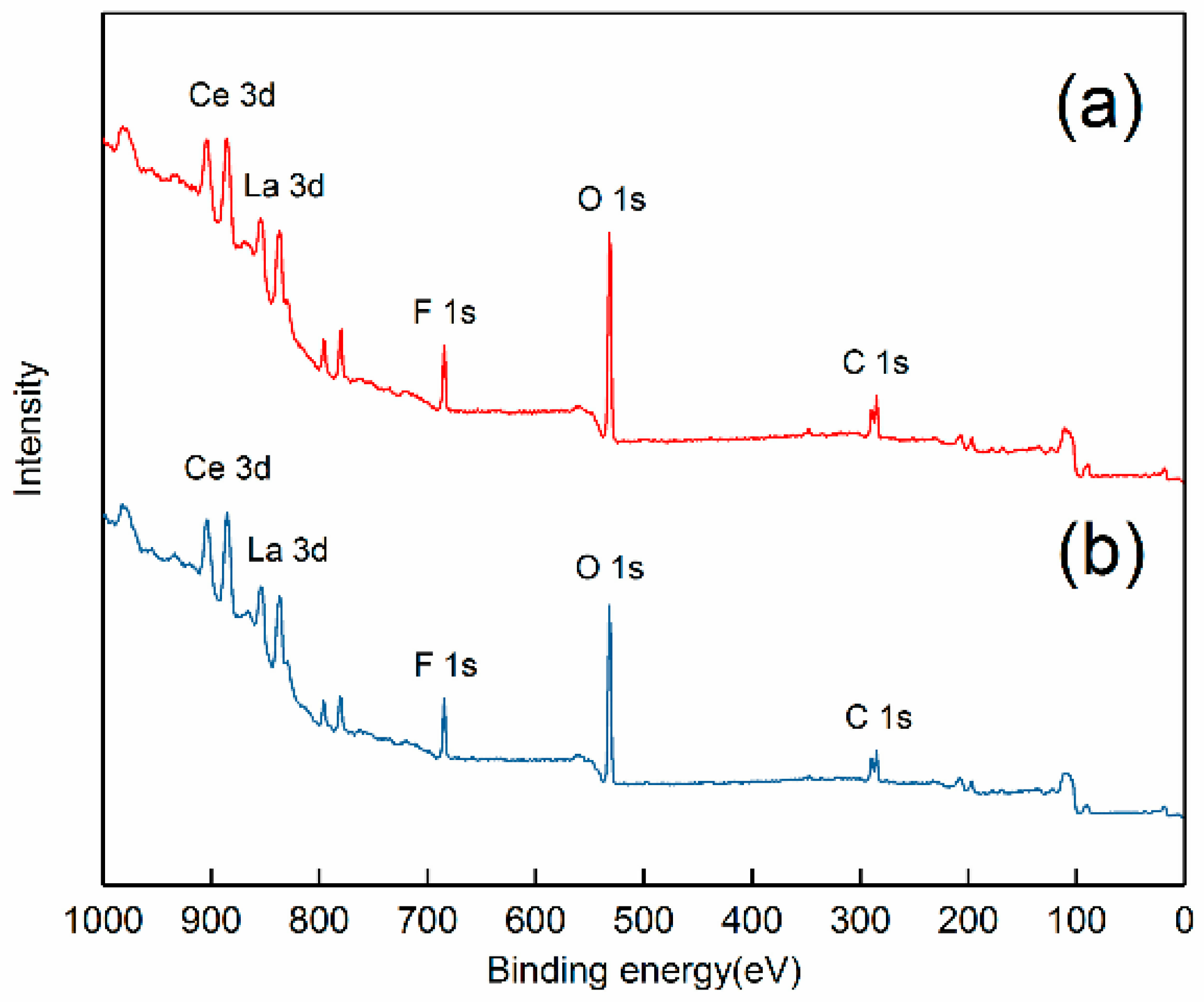
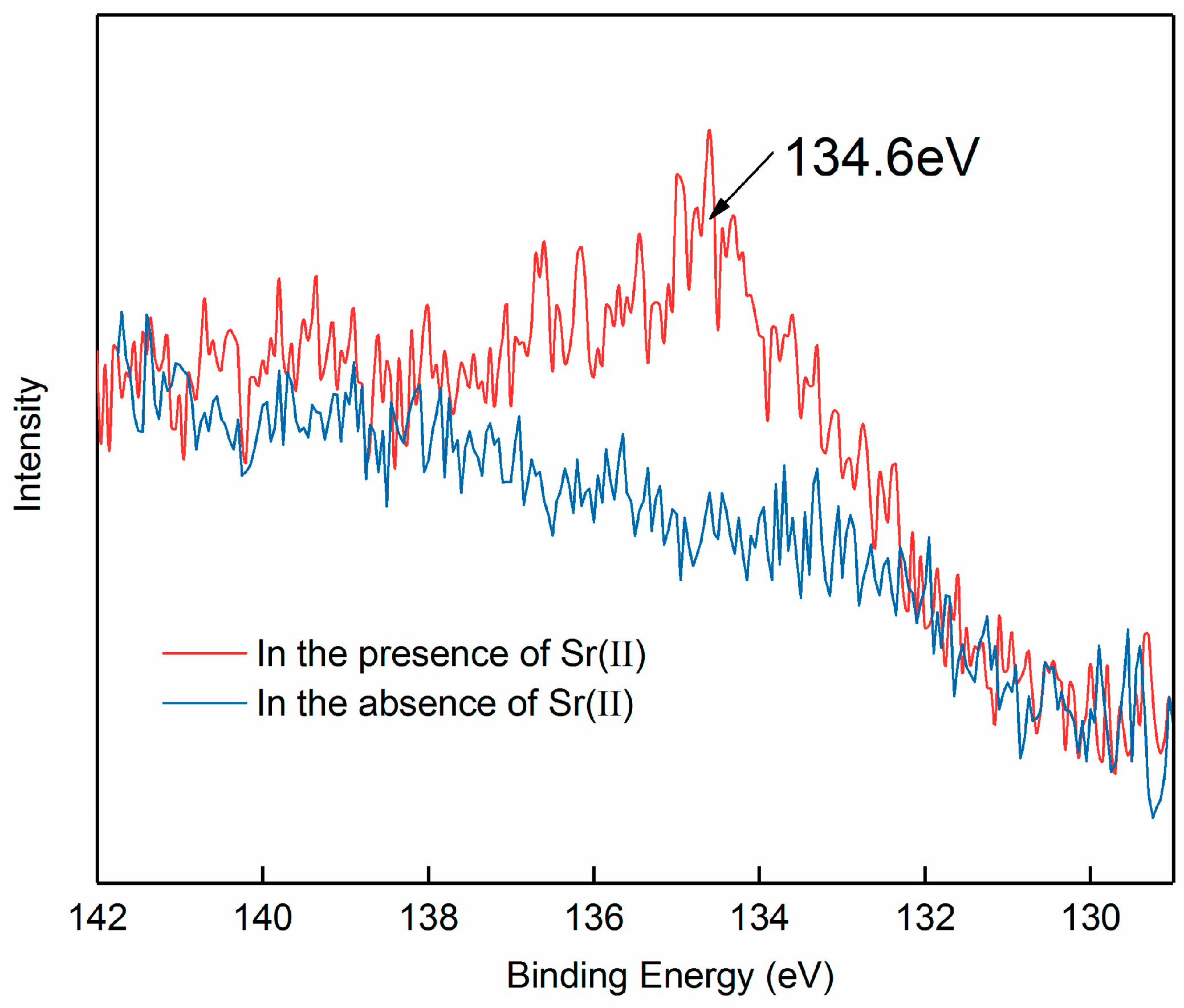
2.5. XPS Analysis
3. Results and Discussion
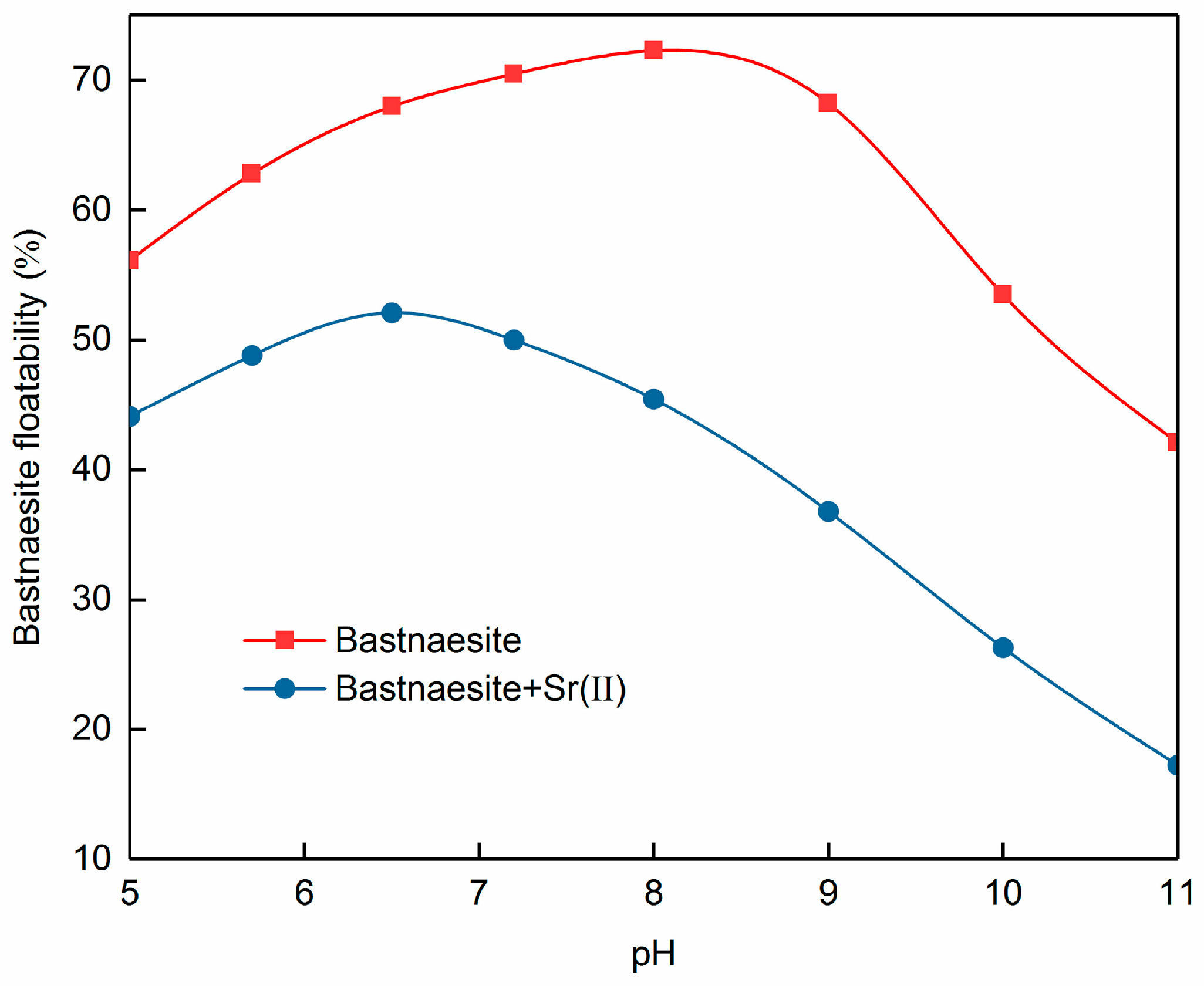
3.1. Effect of Strontium Ions on Bastnaesite Floatability
3.2. Effect of Strontium Ions on Zeta Potential of Bastnaesite
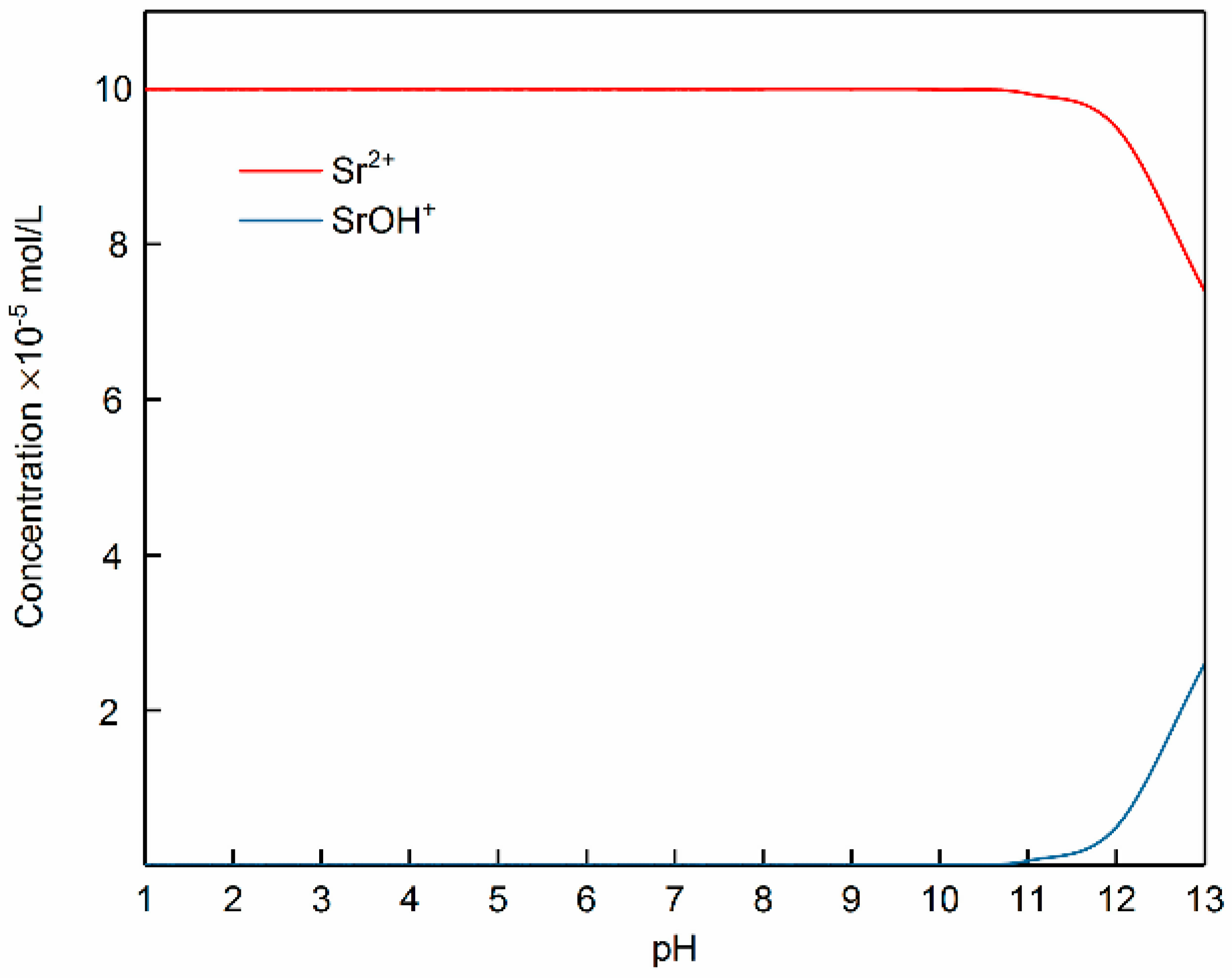
3.3. Adsorption Mechanism of Strontium Ions on Bastnaesite Surfaces
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chi, R.; Wang, D. Mineral Processing of Rare Earth Minerals; Science Press: Beijing, China, 2014; pp. 33–37. [Google Scholar]
- Dolg, M.; Stoll, H. Handbook on the Physics and Chemistry of Rare Earths; Elsevier: Amsterdam, The Netherlands, 1978. [Google Scholar]
- Zhang, X. Surface Chemistry Aspects of Fluorite and Bastnaesite Flotation Systems, Doctoral Dissertation; The University of Utah: Salt Lake City, UT, USA, 2014. [Google Scholar]
- Liu, W.; Wang, B.; Dai, S.; Ai-Xia, M.A.; Wei, D. Current application and development prospect of hydroximic acid in flotation. NonFerr. Min. Metall. 2006, 22, 25–27. [Google Scholar]
- Bulatovic, S.M. Handbook of Flotation Reagents; Elsevier: Amsterdam, The Netherlands, 2007. [Google Scholar]
- Ren, J.; Lu, S.; Song, S.; Niu, J. A new collector for rare earth mineral flotation. Miner. Eng. 1997, 10, 1395–1404. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. The adsorption of hydroxamate on semi-soluble minerals. Part I: Adsorption on barite, Calcite and Bastnaesite. Colloids Surf. 1983, 8, 103–119. [Google Scholar]
- Che, L.; Fu, Y.; Pang, J.; Zu, J.; Zhang, F. The application and development of hydroximic acid collector in the flotation of rare earth minerals. Rare Earth 2004, 3, 49–54. [Google Scholar]
- Fuerstenau, D.W. Adsorption of hydroxamate collectors on semisoluble minerals. Part II: Effect of temperature on adsorption. Colloids Surf. 1985, 15, 137–146. [Google Scholar]
- Zhu, Y.; Zhu, J. Chemical Principles of Flotation Reagents; Central South University of Technology Press: Changsha, China, 1996. [Google Scholar]
- Rao, J. The Flotation Behavior Study of Bastnaesite. Master’s Thesis, Central South University, Changsha, China, 2013. [Google Scholar]
- Wang, C.; Xian, Q.; Hu, Z.; Wang, T.; Li, H. Collecting mechanism of salicylhydroxamic acid on bastnaesite. J. Chin. Soc. Rare Earth 2014, 32, 727–735. [Google Scholar]
- Liu, J.; Wen, S.; Xian, Y.; Deng, J.; Huang, Y. Dissolubility and surface properties of a natural sphalerite in aqueous solution. Miner. Metall. Proc. 2012, 29, 113–120. [Google Scholar]
- Hu, Y.; Xu, J.; Qiu, G.; Wang, D. Effects of dissolved mineral species on the surface chemical characteristic, electrokinetic property and flotation behavior of fluorite and scheelite. J. Cent. South. Univ. Technol. 1994, 1, 63–67. [Google Scholar] [CrossRef]
- Hu, Y. Dissolution/surface property of salt-type minerals and design of schemes of flotation separation. J. Cent. South Inst. Min. Metall. 1992, 23, 273–279. [Google Scholar]
- Shin-Nosuke, S.; Tanaka, T.; Yamamoto, K. Crystal structure control of the dissolution of rare earth elements in water-mineral interactions. Geochem. J. 2007, 40, 437–446. [Google Scholar]
- Deng, J.; Wen, S.; Xian, Y.; Liu, J.; Bai, S. New discovery of unavoidable ions source in chalcopyrite flotation pulp: Fluid inclusions. Miner. Eng. 2013, 42, 22–28. [Google Scholar] [CrossRef]
- Deng, J.; Wen, S.; Liu, J.; Xian, Y.; Wu, D.; Bai, S. New source of unavoidable ions in bornite Flotation Aqueous Solution: Fluid Inclusions. Ind. Eng. Chem. Res. 2013, 52, 4895–4901. [Google Scholar] [CrossRef]
- Liu, J.; Wen, S.; Wu, D.; Bai, S.; Liu, D. Determination of the concentrations of calcium and magnesium released from fluid inclusions of sphalerite and quartz. Miner. Eng. 2013, 45, 41–43. [Google Scholar] [CrossRef]
- Zhang, X.; Du, H.; Wang, X.; Miller, J.D. Surface chemistry considerations in the flotation of rare-earth and other semisoluble salt minerals. Miner. Metall. Proc. 2013, 30, 24–37. [Google Scholar]
- Ren, J.; Song, S. Selective flotation of bastnaesite from monazite in rare earth concentrates using potassium alum as depressant. Int. J. Miner. Process. 2000, 59, 237–245. [Google Scholar] [CrossRef]
- Feng, Q.; Zhao, W.; Wen, S.; Cao, Q. Activation mechanism of lead ions in cassiterite flotation with salicylhydroxamic acid as collector. Sep. Purif. Technol. 2017, 178, 193–199. [Google Scholar] [CrossRef]
- Ren, J.; Lu, S. The depression mechanism of aluminum salt in the separation of bastnaesite and monazite by flotation. Nonferr. Metal. Eng. 1997, 2, 30–35. [Google Scholar]
- Deng, R.; Hu, Y.; Ku, J.; Zuo, W.; Yang, Z. Adsorption of Fe (III) on smithsonite surfaces and implications for flotation. Colloids Surf. A Physicochem. Eng. Asp. 2017, 533, 308–315. [Google Scholar] [CrossRef]
- Sonderegger, J.L.; Brower, K.R.; Lefebre, V.G. A preliminary investigation of strontianite dissolution kinetics. Am. J. Sci. 1976, 8, 997–1022. [Google Scholar] [CrossRef]
- Mavromatis, V.; Harrison, A.L.; Eisenhauer, A.; Dietzel, M. Strontium isotope fractionation during strontianite (SrCO3) dissolution, precipitation and at equilibrium. Geochim. Cosmochim. Acta 2017, 218, 201–214. [Google Scholar] [CrossRef]
- Martińez, A.L.; Uribe, A.S. Interfacial properties of celestite and strontianite in aqueous solutions. Miner. Eng. 1995, 8, 1009–1022. [Google Scholar] [CrossRef]
- Bose, S.; Hu, X.; Higgins, S.R. Dissolution kinetics and topographic relaxation on celestite (001) surfaces: The effect of solution saturation state studied using Atomic Force Microscopy. Geochim. Cosmochim. Acta 2008, 72, 759–770. [Google Scholar] [CrossRef]
- Dove, P.M.; Czank, C.A. Crystal chemical controls on the dissolution kinetics of the isostructural sulfates: Celestite, anglesite, and barite. Geochim. Cosmochim. Acta 1995, 59, 1907–1915. [Google Scholar] [CrossRef]
- Donnay, G.; Donnay, J.D. The crystallography of bastnaesite, parisite, roentgenite, and synchisite. Am. Miner. 1953, 38, 932–963. [Google Scholar]
- Hsu, L.C. Synthesis and stability of bastnaesites in a part of the system (Ce,La)-F-H-C-O. Mineral. Petrol. 1992, 47, 87–101. [Google Scholar] [CrossRef]
- Ni, Y.; Hughes, J.M.; Mariano, A.N. The atomic arrangement of bastnaesite-(Ce), Ce(CO3)F, and structural elements of synchysite-(Ce), roentgenite-(Ce), and parisite-(Ce). Am. Mineral. 1993, 78, 415–418. [Google Scholar]
- Parks, G.A. Surface energy and adsorption at mineral/water interfaces: An introduction. Rev. Mineral. Geochem. 1990, 9, 1–2. [Google Scholar]
- Davis, J.A.; Leckie, J.O. Surface ionization and complexation at the oxide/water interface II. Surface properties of amorphous iron oxyhydroxide and adsorption of metal ions. J. Colloid Interf. Sci. 1978, 67, 90–107. [Google Scholar] [CrossRef]
- Fuerstenau, D.W. The role of inorganic and organic reagents in the flotation separation of rare-earth ores. Int. J. Miner. Process. 1991, 32, 1–22. [Google Scholar]
- Houot, R.; Cuif, J.P.; Mottot, Y.; Samama, J.C. Recovery of rare earth minerals, with emphasis on flotation process. Mater. Sci. Forum 1991, 2, 301–324. [Google Scholar] [CrossRef]
- Wang, D.; Hu, Y. Flotation Solution Chemistry; Hunan Science and Technology Press: Changsha, China, 1988. [Google Scholar]
- Kobayashi, H.; Satoh, K.; Sawada, K. Adsorption of divalent heavy metal ions on calcium carbonate (calcite). Bunseki Kagaku 2004, 53, 101–107. [Google Scholar] [CrossRef]
- Christie, A.B.; Lee, J.; Sutherland, I.; Walls, J.M. An XPS study of ion-induced compositional changes with group II and group IV compounds. Appl. Surf. Sci. 1983, 15, 224–237. [Google Scholar] [CrossRef]
- Moulder, J.F.; Chastain, J.; King, R.C.J. Handbook of X-ray photoelectron spectroscopy: A reference book of standard spectra for identification and interpretation of XPS data. Chem. Phys. Lett. 1979, 220, 7–10. [Google Scholar]
- Cui, J.; Hope, G.A.; Buckley, A.N. Spectroscopic investigation of the interaction of hydroxamate with bastnaesite (cerium) and rare earth oxides. Miner. Eng. 2012, 5, 91–99. [Google Scholar] [CrossRef]
- Nowak, P.; Laajalehto, K.; Kartio, I. A flotation related X-ray photoelectron spectroscopy study of the oxidation of galena surface. Colloids Surface. A Physicochem Eng. Asp. 2000, 161, 447–460. [Google Scholar] [CrossRef]
- Kotani, A.; Ogasawara, H. Theory of core-level spectroscopy of rare-earth oxides. J. Electron Spectrosc. Relat. Phenom. 1992, 60, 257–299. [Google Scholar] [CrossRef]
- Mullins, D.R.; Overbury, S.H.; Huntley, D.R. Electron spectroscopy of single crystal and polycrystalline cerium oxide surfaces. Surf. Sci. 1998, 409, 307–319. [Google Scholar] [CrossRef]


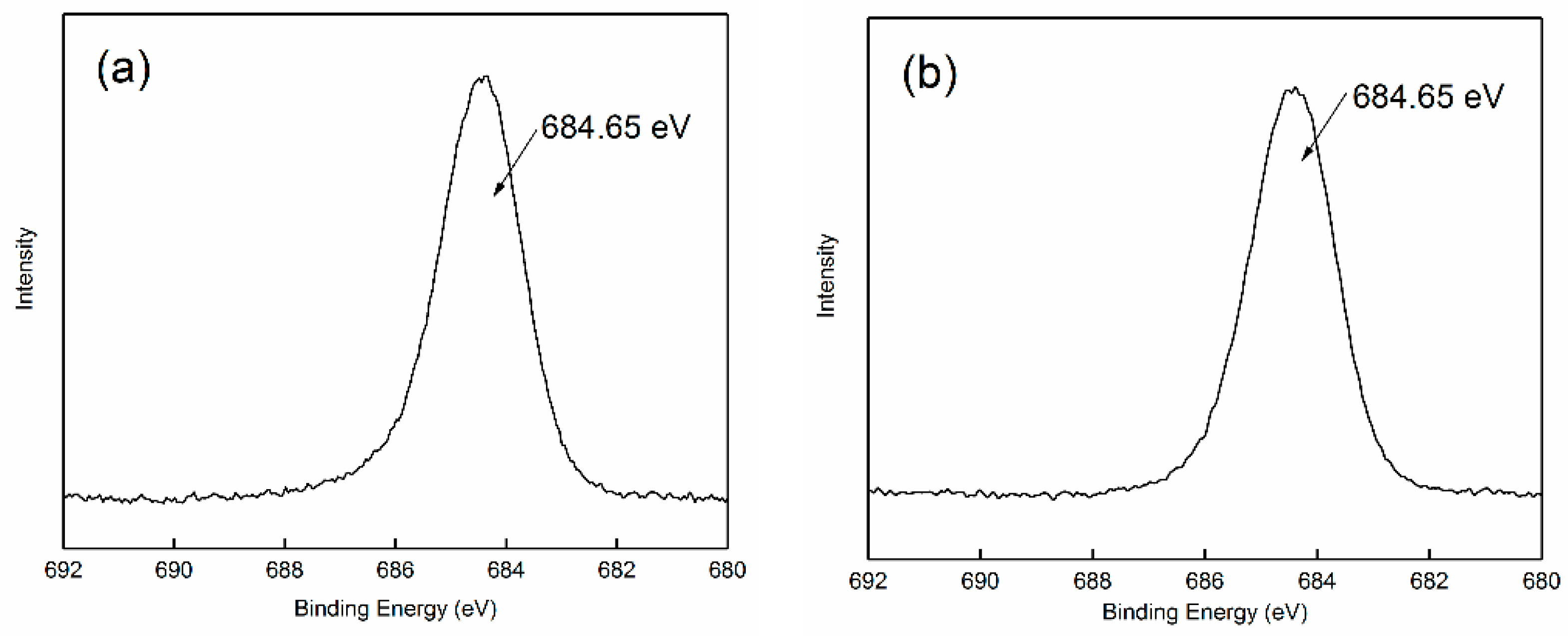

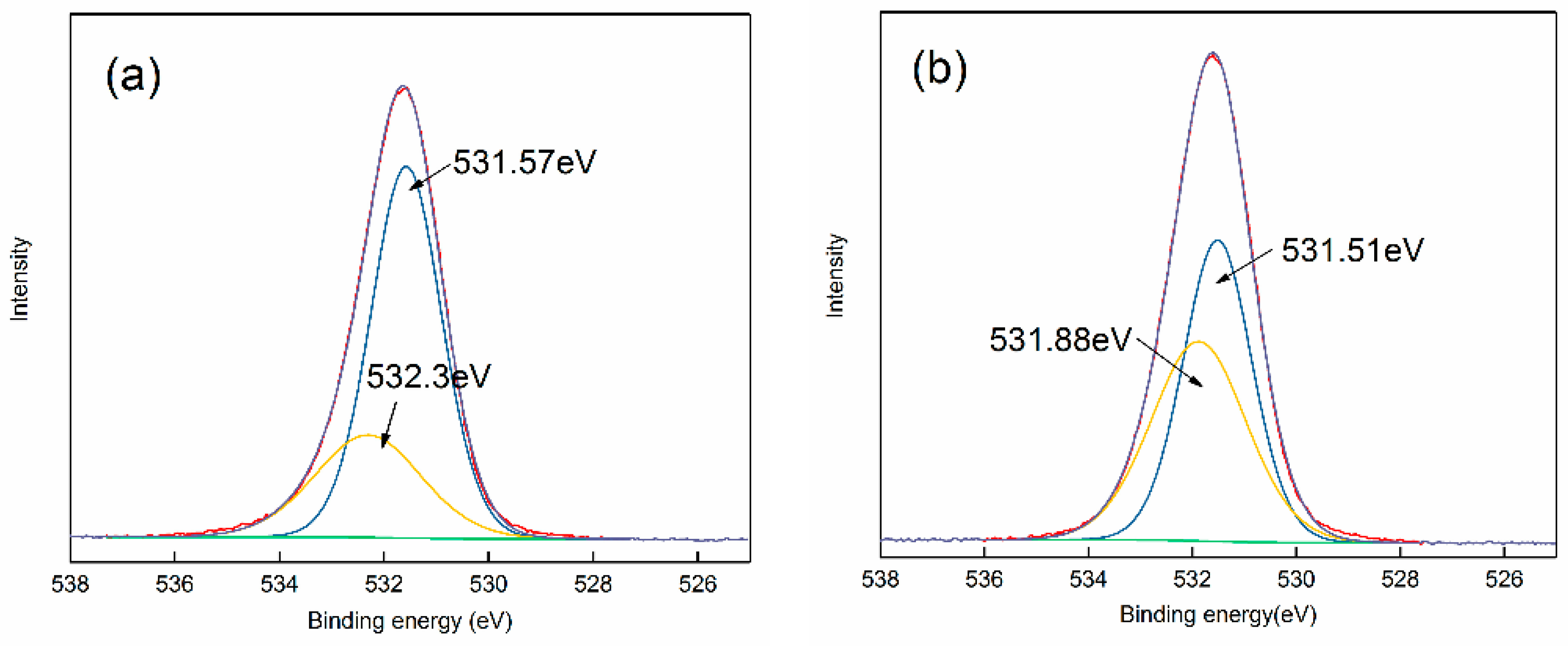
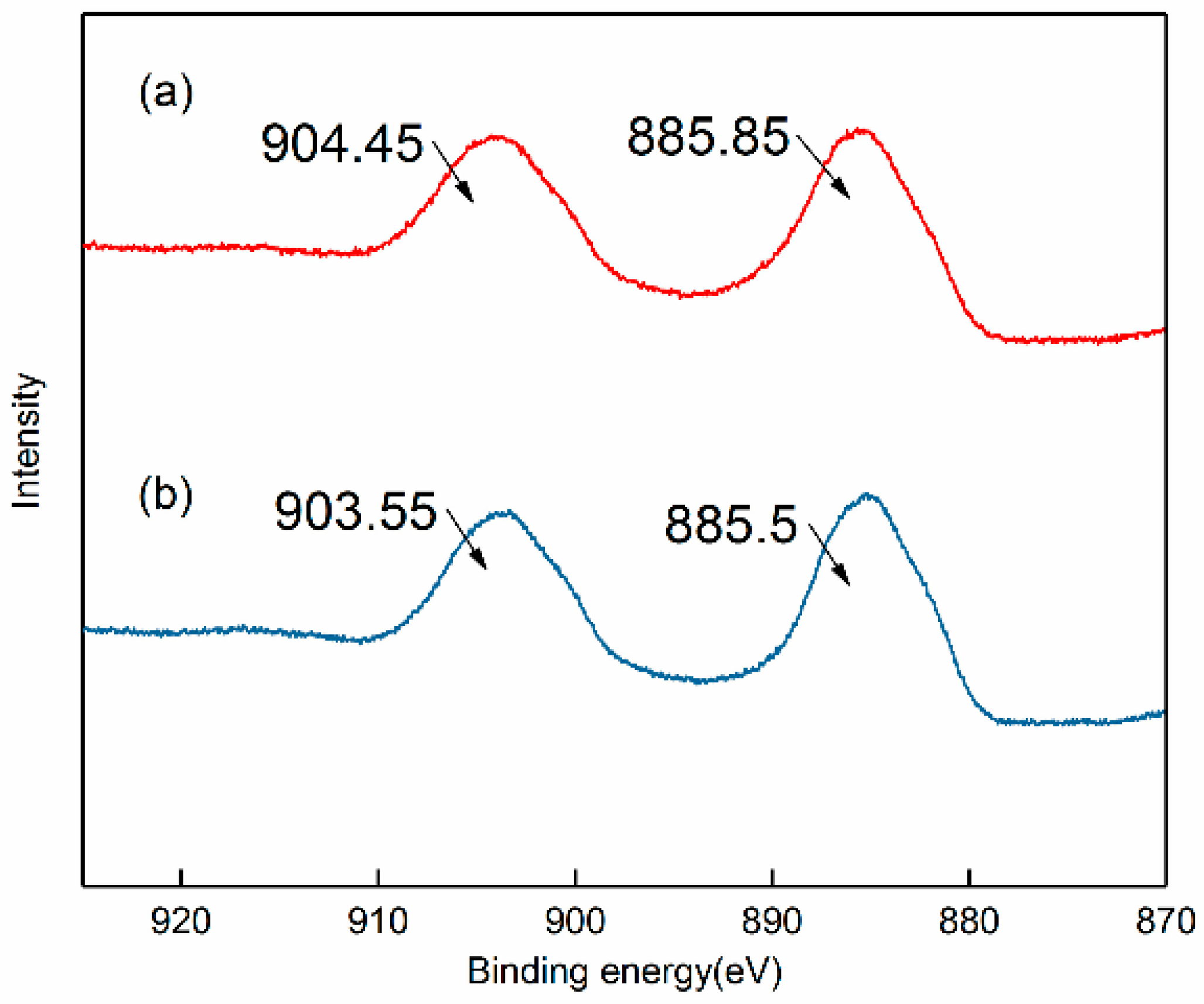

| Atom | Atomic Concentration (%) | |
|---|---|---|
| Untreated Bastnaesite | Bastnaesite Treated with Strontium Ions | |
| Ce | 6.05 | 7.98 |
| C (in ) | 19.73 | 7.97 |
| O | 59.66 | 66.25 |
| F | 14.55 | 17.25 |
| Sr | 0 | 0.55 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, S.; Cao, Y.; Liao, Y.; Ma, Z. Depression Mechanism of Strontium Ions in Bastnaesite Flotation with Salicylhydroxamic Acid as Collector. Minerals 2018, 8, 66. https://doi.org/10.3390/min8020066
Cao S, Cao Y, Liao Y, Ma Z. Depression Mechanism of Strontium Ions in Bastnaesite Flotation with Salicylhydroxamic Acid as Collector. Minerals. 2018; 8(2):66. https://doi.org/10.3390/min8020066
Chicago/Turabian StyleCao, Shiming, Yijun Cao, Yinfei Liao, and Zilong Ma. 2018. "Depression Mechanism of Strontium Ions in Bastnaesite Flotation with Salicylhydroxamic Acid as Collector" Minerals 8, no. 2: 66. https://doi.org/10.3390/min8020066





