Effect of Dissolved Silica on Immobilization of Boron by Magnesium Oxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sorption Experiments for Boron Using Magnesium Oxide
2.2. Sorption Experiment for Boron Using Magnesium Oxide with Soluble Silica
2.3. Sorption Experiment for Boron Using Precipitates Reacting with Magnesium Oxide and Amorphous Silica
2.4. Analysis of the Solutions
2.5. Analysis of the Solid Phases
3. Results
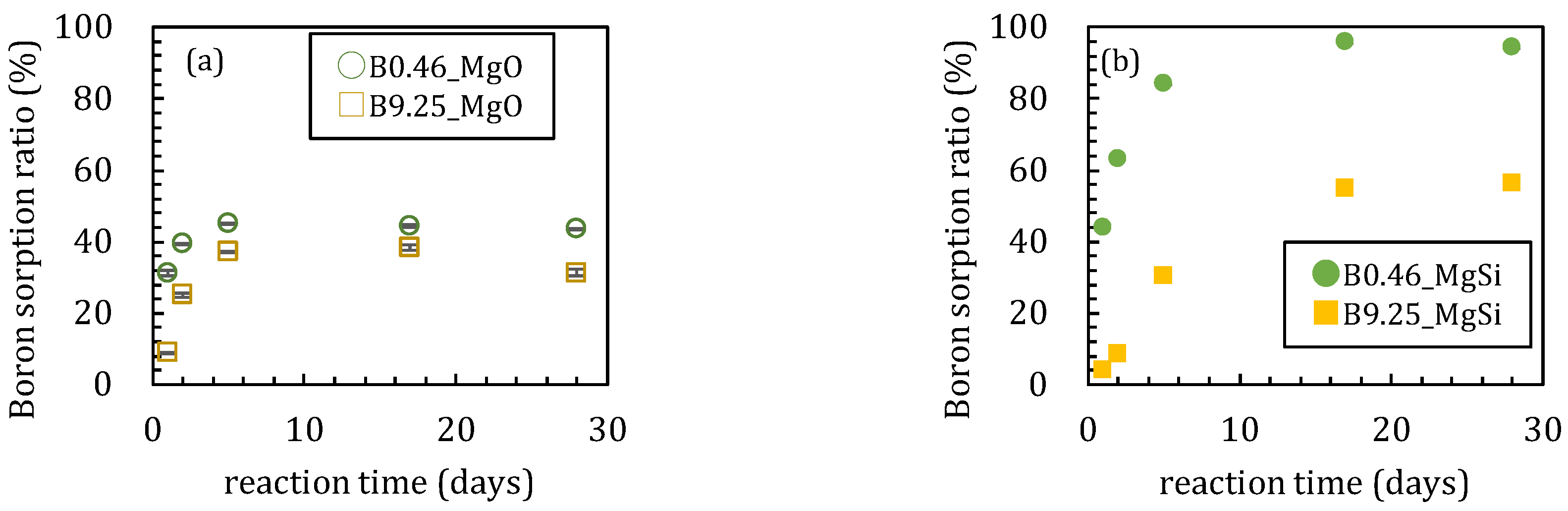
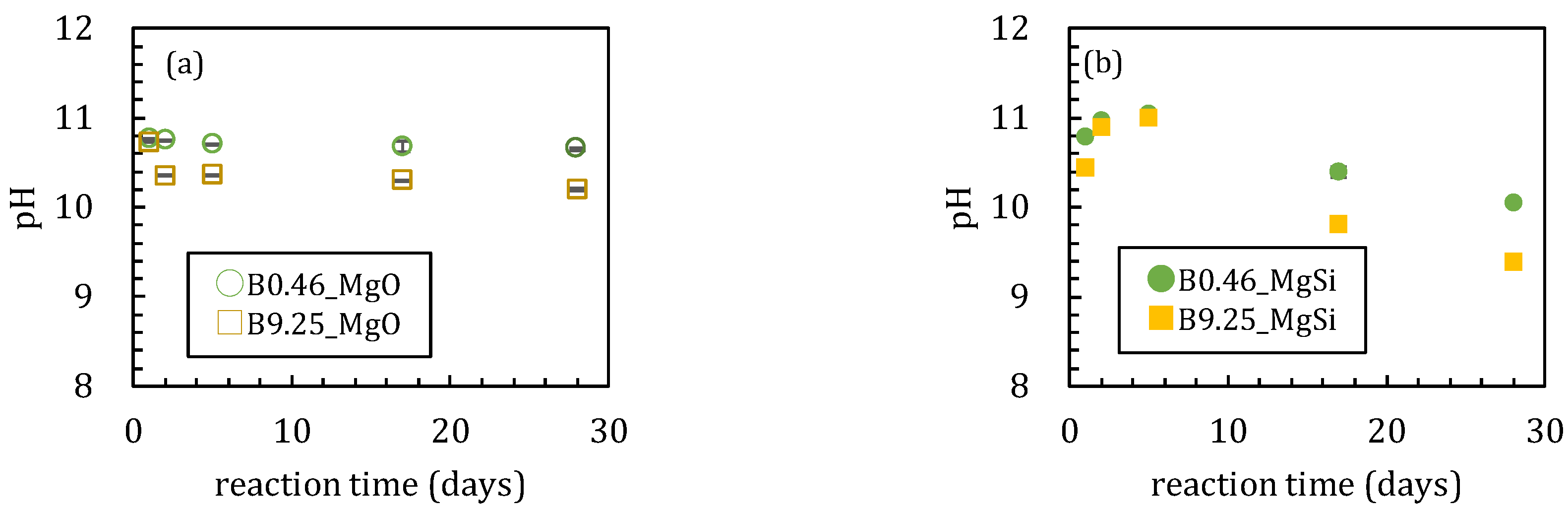
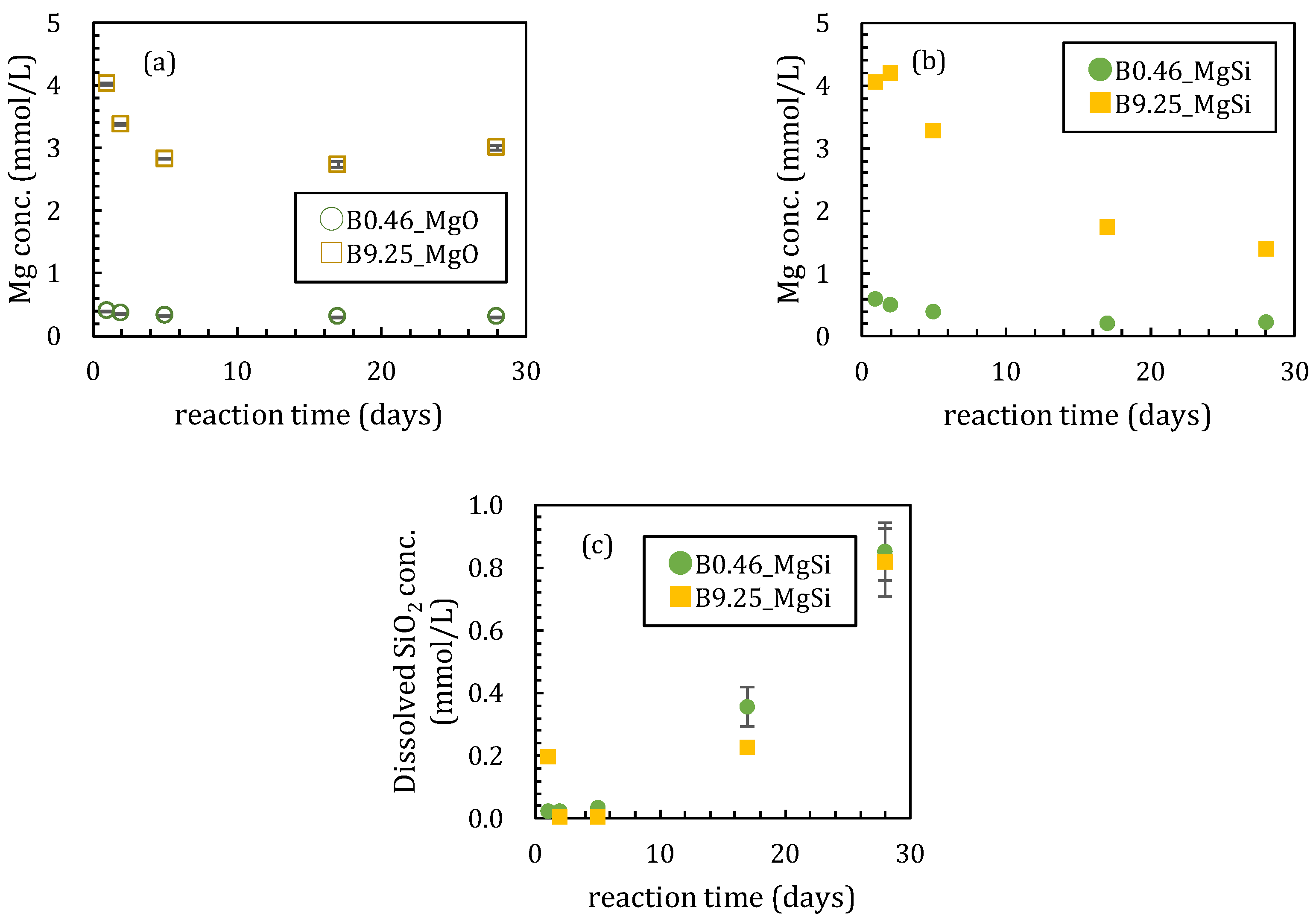
3.1. Sorption Experiments Using Magnesium Oxide
3.1.1. Sorption Experiments with Boron and Magnesium Oxide Both Added Together
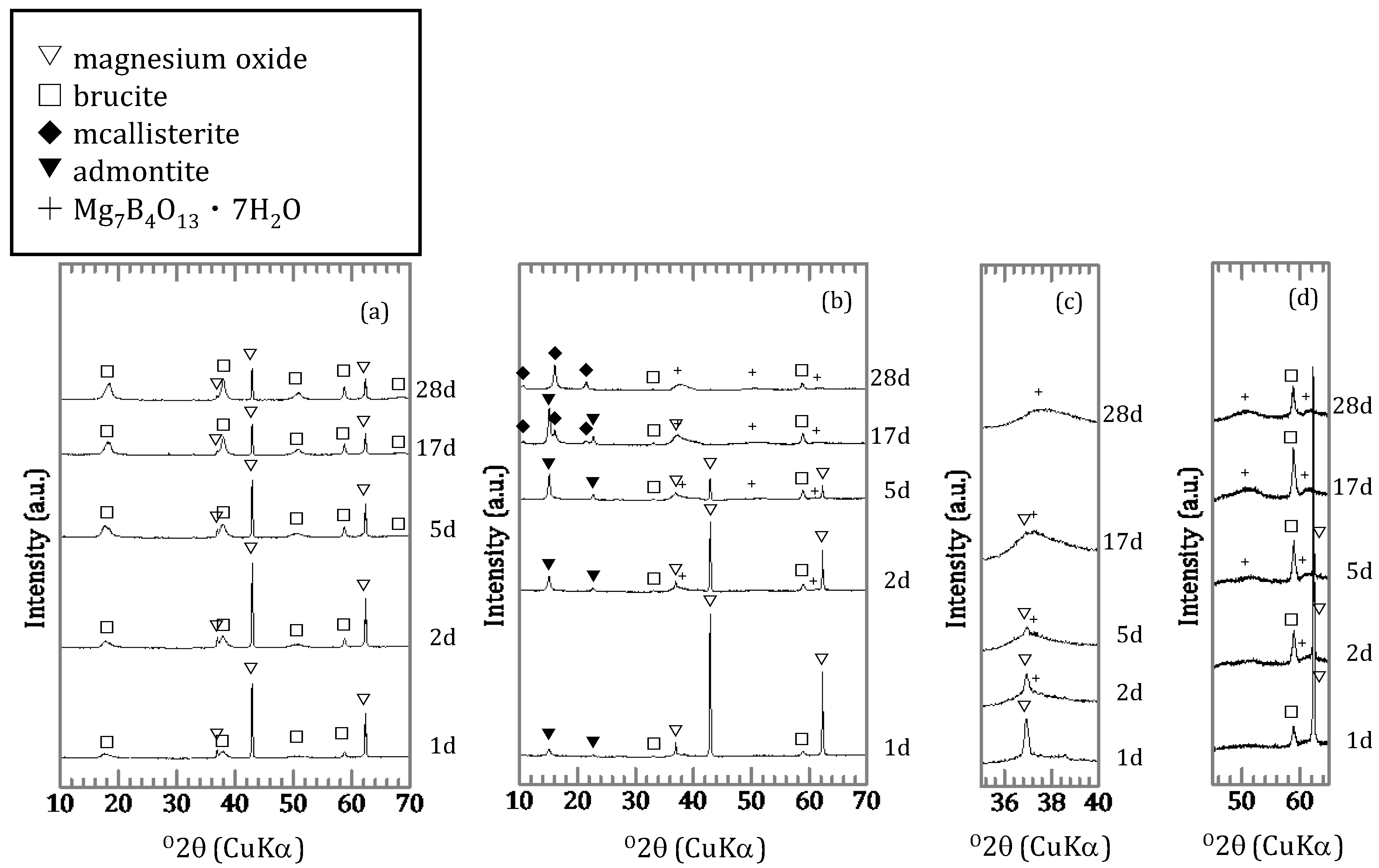
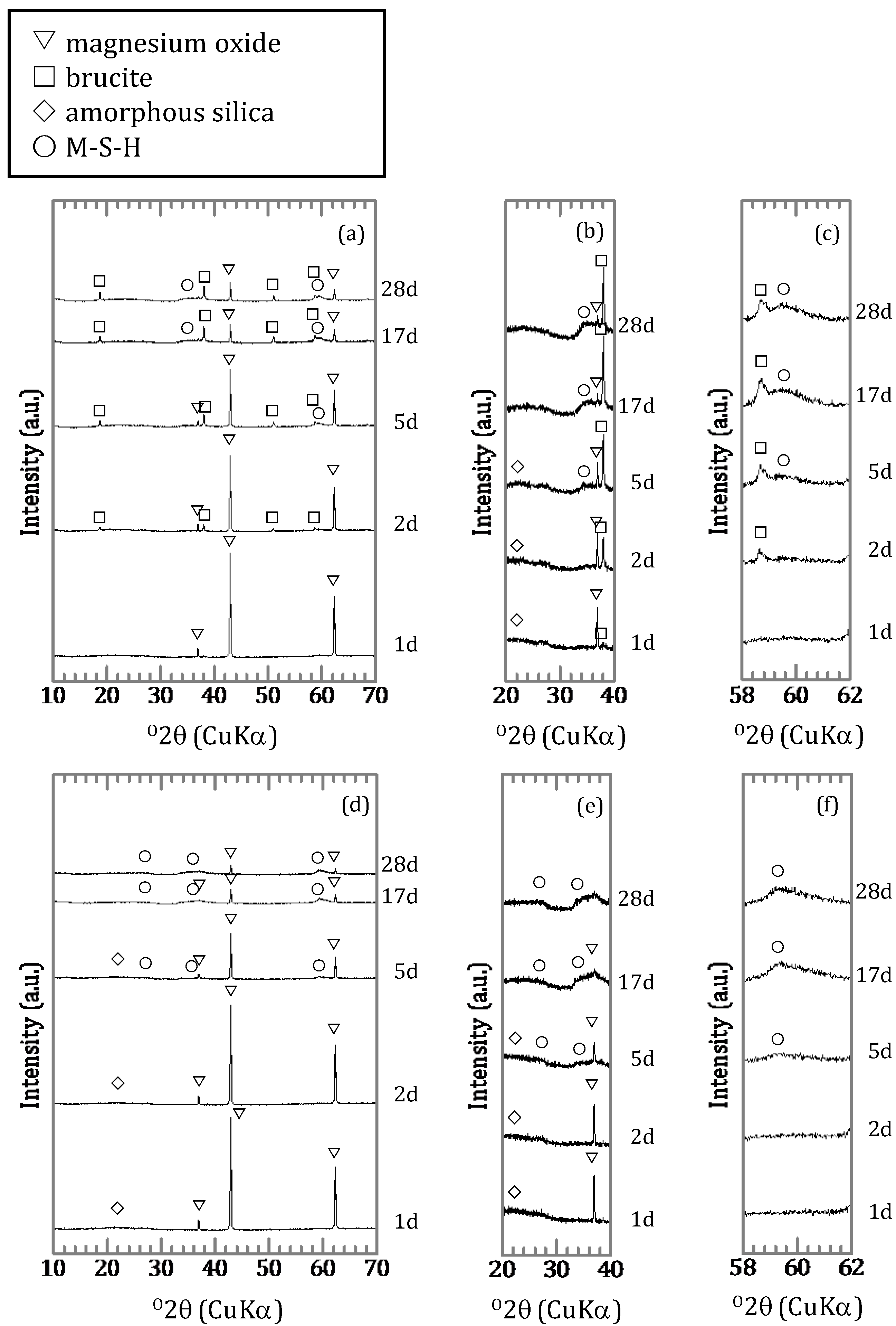
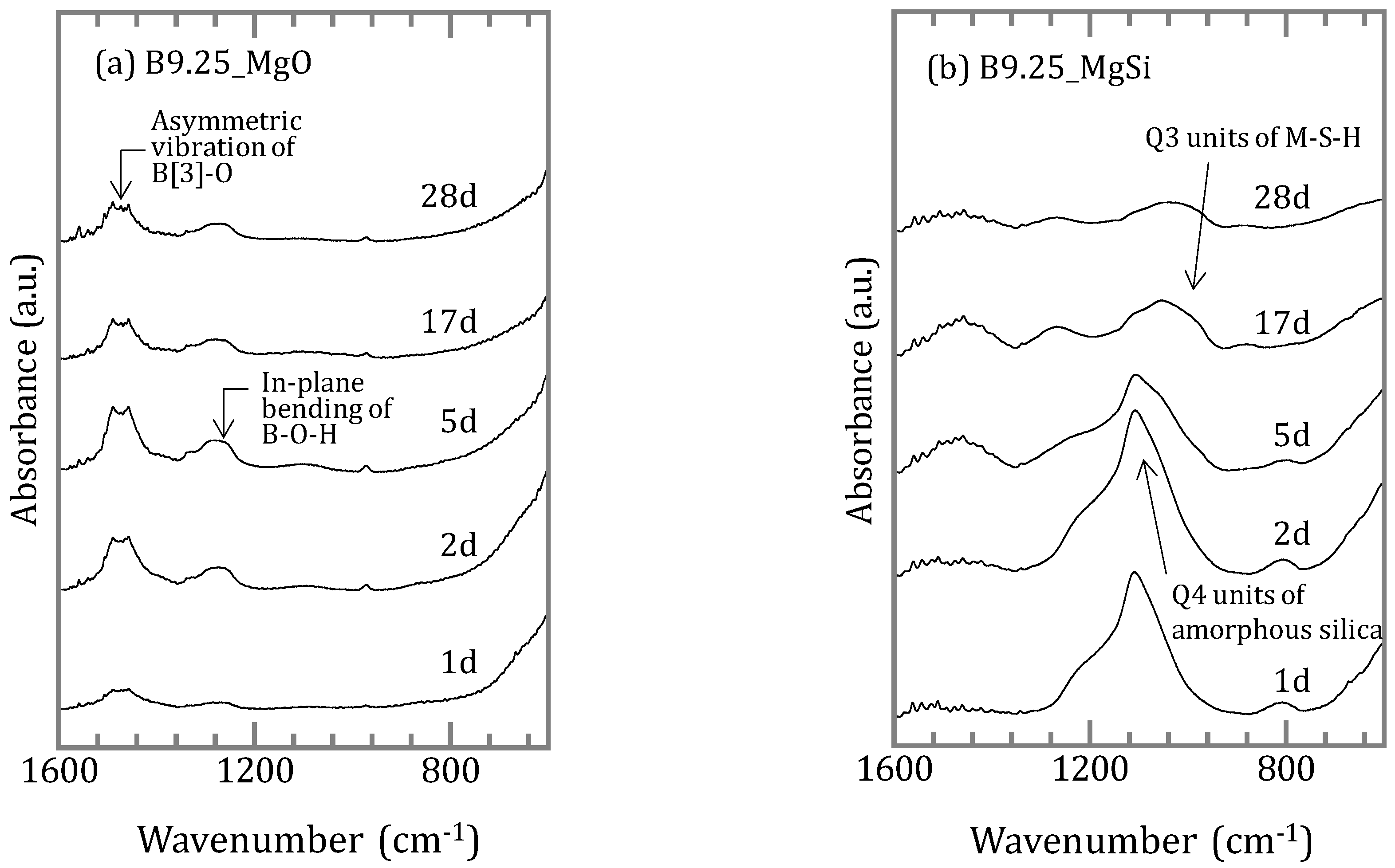
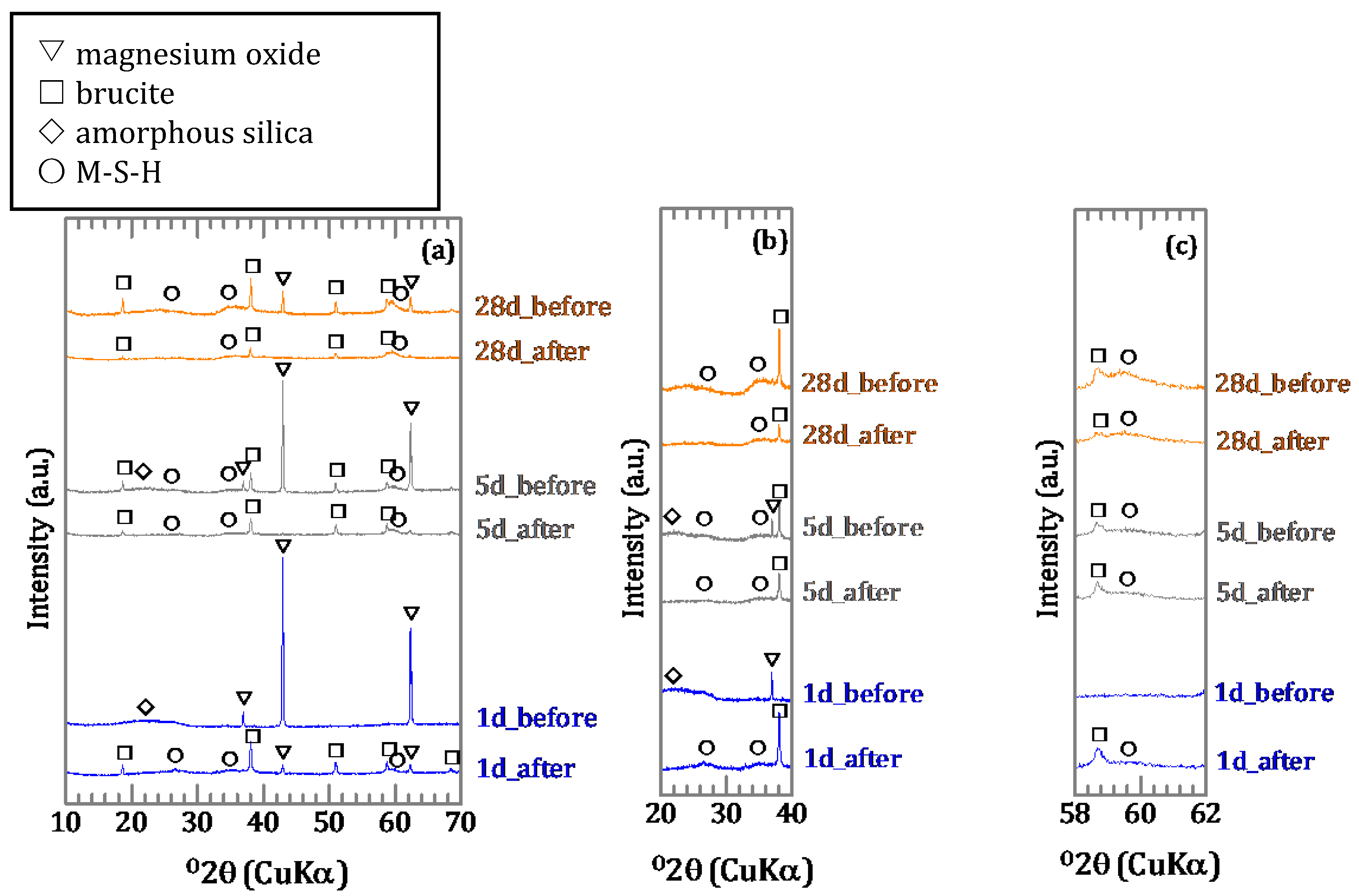
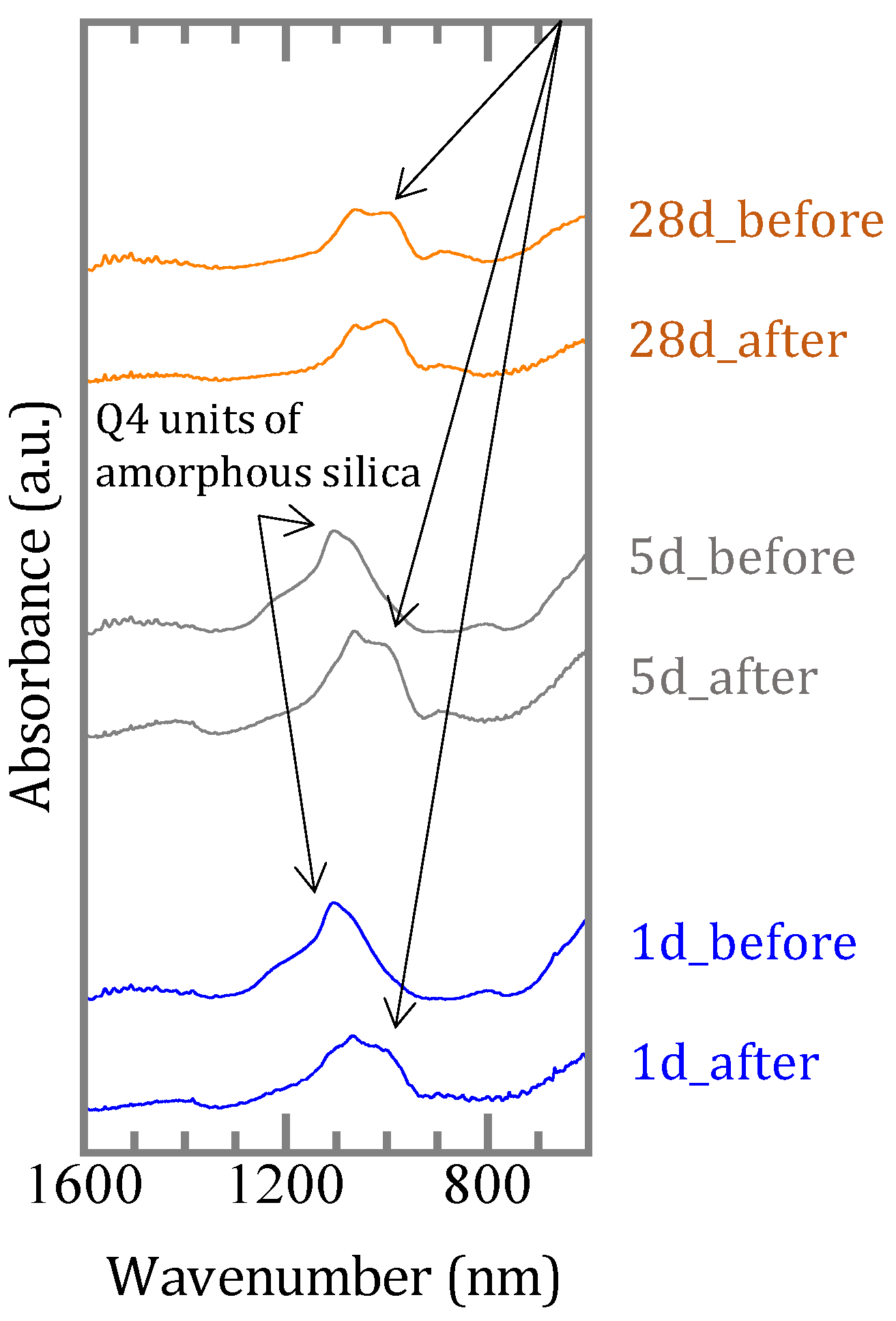
3.1.2. Characterization of the Precipitates
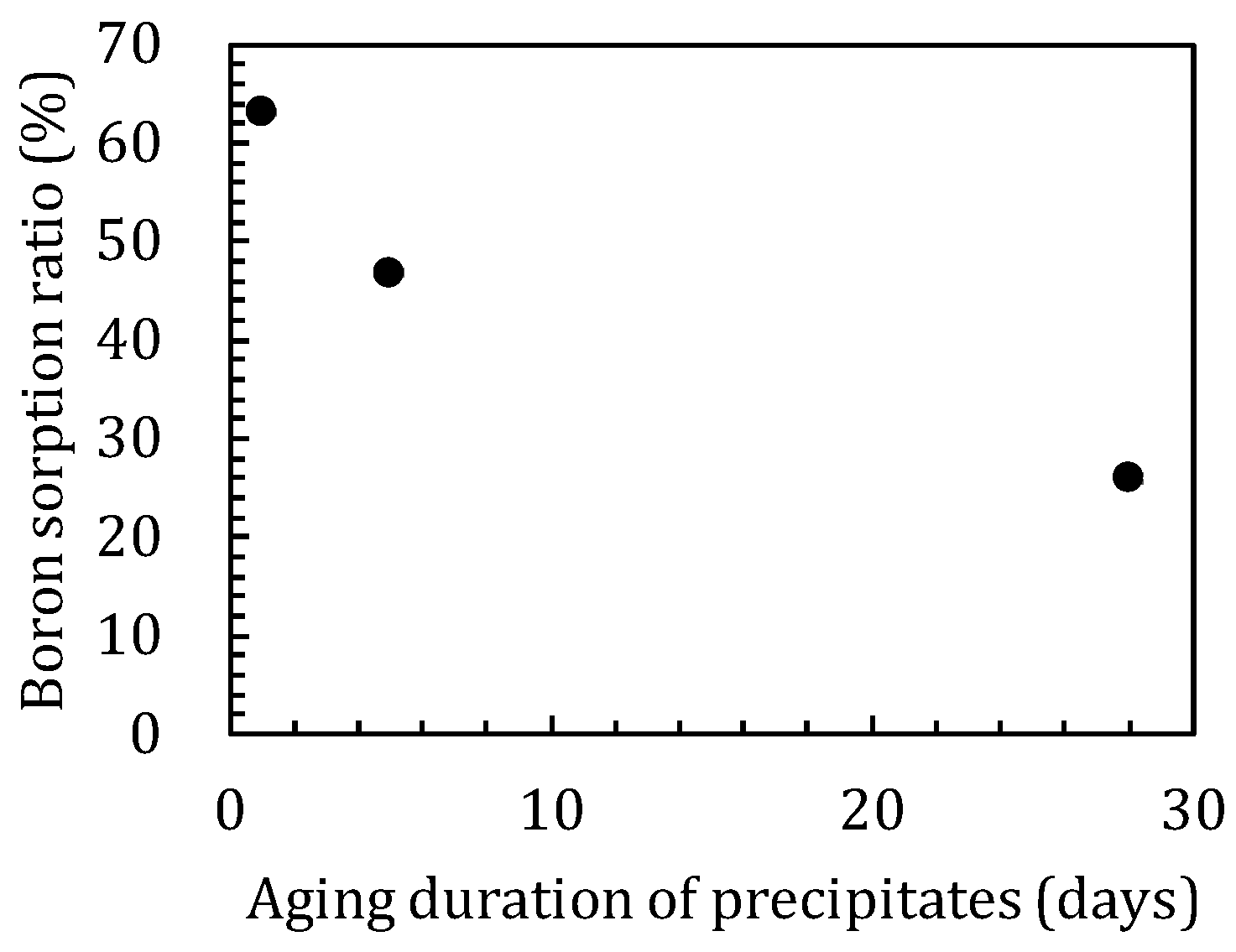
3.2. Sorption Experiments with Boron Added after Reaction of Magnesium Oxide and Amorphous Silica
4. Discussion
4.1. Influence of Silica on Boron Sorption Reactions
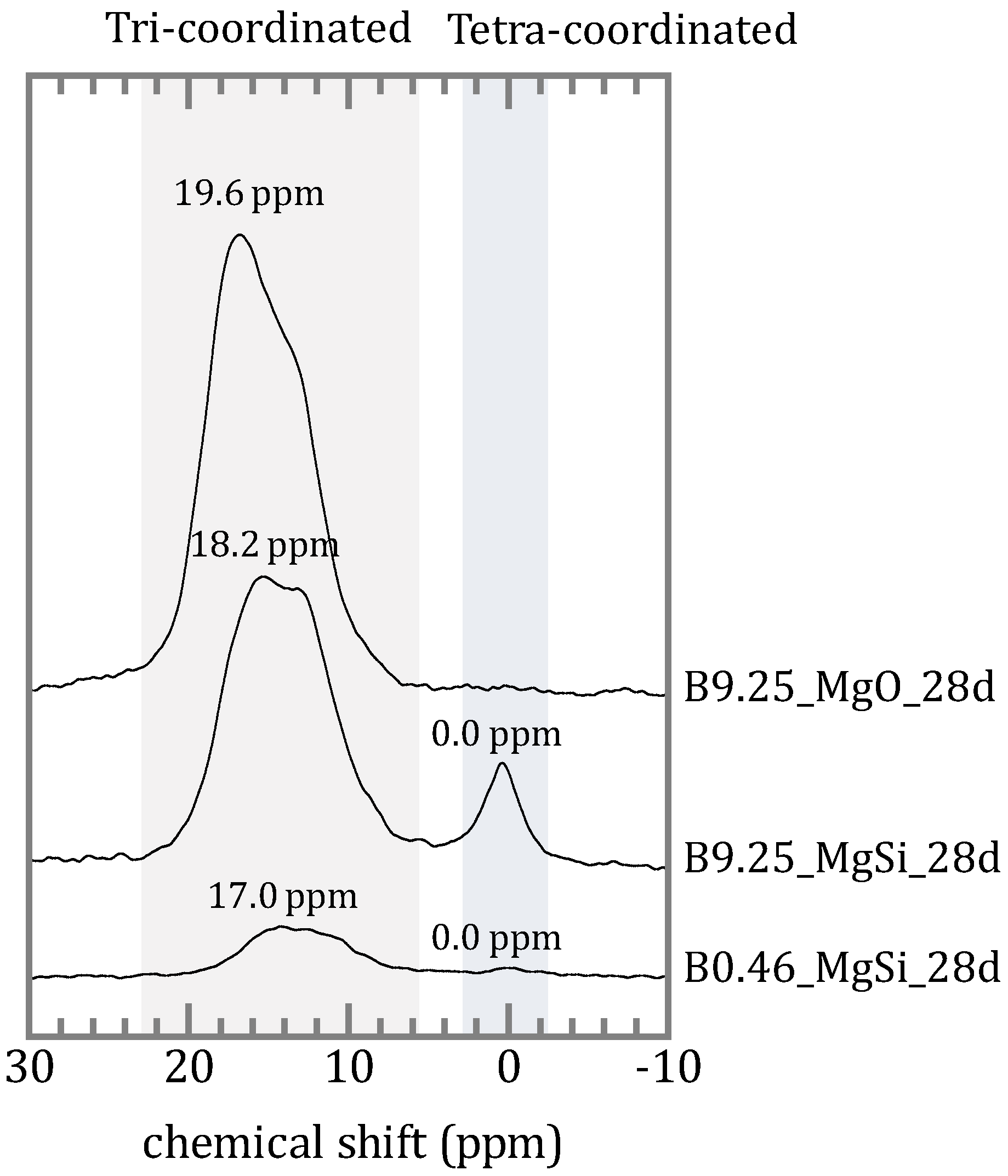
4.2. Chemical Form of Boron in the Precipitates
4.3. The Mechanism of Boron Incorporation in Precipitates
4.4. Implications for Immobilization of Boron in Contaminated Soils
5. Conclusions
Acknowledgments
Author contributions
Conflicts of interest
References
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. Isrn Ecol. 2011, 2011, 1–20. [Google Scholar] [CrossRef]
- Wada, S.I.; Morishita, T. Stabilization of Heavy Metals Contaminated Soils by Magnesium Oxide and Related Chemical and Mineralogical Reactions. Nendo Kagaku 2013, 51, 107–117. [Google Scholar]
- Morimoto, K.; Sato, T.; Yoneda, T. Complexation Reactions of Oxyanions on Brucite Surfaces. Nendo Kagaku 2009, 48, 9. [Google Scholar]
- García, M.A.; Chimenos, J.M.; Fernández, A.I.; Miralles, L.; Segarra, M.; Espiell, F. Low-grade MgO used to stabilize heavy metals in highly contaminated soils. Chemosphere 2004, 56, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Gorchev, H.G.; Ozolins, G. Guidelines for Drinking-water Quality. WHO Guidel. Drink. Qual. 2011, 38, 104–108. [Google Scholar]
- Shimada, N. The Essence of Problems on Groundwater and Soil Pollutions Caused by Naturally Occurring Heavy Metals and Harmful Elements: Boron. Oyo Tech. Rep. 2013, 32, 29–55. [Google Scholar]
- Remy, P.; Muhr, H.; Plasari, E.; Ouerdiane, I. Removal of boron from wastewater by precipitation of a sparingly soluble salt. Environ. Prog. 2005, 24, 105–110. [Google Scholar] [CrossRef]
- Kabay, N.; Sarp, S.; Yuksel, M.; Arar, Ö.; Bryjak, M. Removal of boron from seawater by selective ion exchange resins. React. Funct. Polym. 2007, 67, 1643–1650. [Google Scholar] [CrossRef]
- Bicak, N.; Gazi, M.; Bulutcu, N. N,N-bis(2,3-dihydroxypropyl) octadecylamine for liquid-liquid extraction of boric acid. Sep. Sci. Technol. 2003, 38, 165–177. [Google Scholar] [CrossRef]
- Bengü, L.; Taştan, B.E.; Dönmez, G. Detection of boron removal capacities of different microorganisms in wastewater and effective removal process. Water Sci. Technol. 2015, 72, 1832–1839. [Google Scholar] [CrossRef]
- Sasaki, K.; Qiu, X.; Moriyama, S.; Tokoro, C.; Ideta, K.; Miyawaki, J. Characteristic Sorption of H3BO3/B(OH)4− on Magnesium Oxide. Mater. Trans. 2013, 54, 1809–1817. [Google Scholar] [CrossRef]
- de la Fuente García-Soto, M.M.; Muñoz Camacho, E. Boron removal by means of adsorption processes with magnesium oxide—Modelization and mechanism. Desalination 2009, 249, 626–634. [Google Scholar] [CrossRef]
- Jin, J.; Martens, D.C.; Zelazny, L.W. Distribution and Plant Availability of Soil Boron Fractions1. Soil Sci. Soc. Am. J. 1987, 51, 1228. [Google Scholar] [CrossRef]
- Kipcak, A.S.; Gurses, P.; Kunt, K.; Derun, E.M.; Piskin, S. Magnesium borate synthesis by microwave energy: A new method. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2013, 7, 290–295. [Google Scholar] [CrossRef]
- Liu, J.; Li, Y.; Huang, X.; Li, Z.; Li, G.; Zeng, H. Hydrothermal Synthesis of Single-Crystal Szaibelyite MgBO2(OH) Nanobelt as a New Host Material for Red-Emitting Rare-Earth Ions. Chem. Mater. 2008, 20, 250–257. [Google Scholar] [CrossRef]
- Rao, T.G.V.M.; Rupesh Kumar, A.; Neeraja, K.; Veeraiah, N.; Rami Reddy, M. Optical and structural investigation of Dy3+-Nd3+ co-doped in magnesium lead borosilicate glasses. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 118, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, M.E.; Sonderby, C.; Li, Z.; Sogaard, E.G. XPS and FT-IR investigation of silicate polymers. J. Mater. Sci. 2009, 44, 2079–2088. [Google Scholar] [CrossRef]
- Xiong, Y. Thermodynamic properties of brucite determined by solubility studies and their significance to nuclear waste isolation. Aquat. Geochem. 2008, 14, 223–238. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Rentsch, D.; Pochard, I.; Dauzères, A. Formation of magnesium silicate hydrates (M-S-H). Phys. Chem. Earth 2017, 99, 142–157. [Google Scholar] [CrossRef]
- Nied, D.; Enemark-Rasmussen, K.; L’Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Concr. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Roosz, C.; Grangeon, S.; Blanc, P.; Montouillout, V.; Lothenbach, B.; Henocq, P.; Giffaut, E.; Vieillard, P.; Gaboreau, S. Crystal structure of magnesium silicate hydrates (M-S-H): The relation with 2:1 Mg-Si phyllosilicates. Cem. Concr. Res. 2015, 73, 228–237. [Google Scholar] [CrossRef]
- Dauzeres, A.; Achiedo, G.; Nied, D.; Bernard, E.; Alahrache, S.; Lothenbach, B. Magnesium perturbation in low-pH concretes placed in clayey environment—Solid characterizations and modeling. Cem. Concr. Res. 2016, 79, 137–150. [Google Scholar] [CrossRef]
- García Calvo, J.L.; Hidalgo, A.; Alonso, C.; Fernández Luco, L. Development of low-pH cementitious materials for HLRW repositories. Resistance against ground waters aggression. Cem. Concr. Res. 2010, 40, 1290–1297. [Google Scholar] [CrossRef] [Green Version]
- Jenni, A.; Mäder, U.; Lerouge, C.; Gaboreau, S.; Schwyn, B. In situ interaction between different concretes and Opalinus Clay. Phys. Chem. Earth 2014, 70–71, 71–83. [Google Scholar] [CrossRef]
- Brew, D.R.M.; Glasser, F.P. Synthesis and characterisation of magnesium silicate hydrate gels. Cem. Concr. Res. 2005, 35, 85–98. [Google Scholar] [CrossRef]
- Lothenbach, B.; Nied, D.; L’Hôpital, E.; Achiedo, G.; Dauzères, A. Magnesium and calcium silicate hydrates. Cem. Concr. Res. 2015, 77, 60–68. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Xu, Y.; Sang, S.; Jin, S. Enhanced formation of magnesium silica hydrates (M-S-H) using sodium metasilicate and caustic magnesia in magnesia castables. Ceram. Int. 2017, 43, 9110–9116. [Google Scholar] [CrossRef]
- Yu, P.; Kirkpatrick, R.J.; Poe, B.; McMillan, P.F.; Cong, X. Structure of Calcium Silicate Hydrate (C-S-H): Near-, Mid-, and Far-Infrared Spectroscopy. J. Am. Ceram. Soc. 2004, 82, 742–748. [Google Scholar] [CrossRef]
- Zhou, B.; Michaelis, V.K.; Pan, Y.; Yao, Y.; Tait, K.T.; Hyde, B.C.; Wren, J.E.C.; Sherriff, B.L.; Kroeker, S. Crystal structure refinements of borate dimorphs inderite and kurnakovite using 11B and 25Mg nuclear magnetic resonance and DFT calculations. Am. Mineral. 2012, 97, 1858–1865. [Google Scholar] [CrossRef]
- Angeli, F.; Charpentier, T.; De Ligny, D.; Cailleteau, C. Boron speciation in soda-lime borosilicate glasses containing zirconium. J. Am. Ceram. Soc. 2010, 93, 2693–2704. [Google Scholar] [CrossRef]
- Lussier, A.J.; Aguiar, P.M.; Michaelis, V.K.; Kroeker, S.; Hawthorne, F.C. The occurrence of tetrahedrally coordinated Al and B in tourmaline: An 11B and 27Al MAS NMR study. Am. Mineral. 2009, 94, 785–792. [Google Scholar] [CrossRef]
- Du, L.; Stebbins, J.F. Nature of Silicon-Boron Mixing in Sodium Borosilicate Glasses: A High-Resolution 11B and 17O NMR Study. J. Phys. Chem. B 2003, 107, 10063–10076. [Google Scholar] [CrossRef]
- Grew, E.S.; Rossman, G.R. Coordination of Boron in Sillimanite. Mineral. Mag. 1985, 49, 132–135. [Google Scholar] [CrossRef]
- Stubican, V.; Roy, R. Boron substitution in synthetic micas and clays. Am. Mineral. 1962, 47, 9–10. [Google Scholar]
- Kim, Y.; Kirkpatrick, R.J. 11B NMR investigation of boron interaction with mineral surfaces: Results for boehmite, silica gel and illite. Geochim. Cosmochim. Acta 2006, 70, 3231–3238. [Google Scholar] [CrossRef]
- Hwang, S.J.; Chen, C.Y.; Zones, S.I. Boron sites in borosilicate zeolites at various stages of hydration studied by solid state NMR spectroscopy. J. Phys. Chem. B 2004, 108, 18535–18546. [Google Scholar] [CrossRef]
- Bernard, E.; Lothenbach, B.; Le Goff, F.; Pochard, I.; Dauzères, A. Effect of magnesium on calcium silicate hydrate (C-S-H). Cem. Concr. Res. 2017, 97, 61–72. [Google Scholar] [CrossRef]
- Hamburger, G.E.; Buerger, M.J. The Structure of Tourmaline. Am. Mineral. 1948, 33, 532–540. [Google Scholar]
- Quintas, A.; Charpentier, T.; Majérus, O.; Caurant, D.; Dussossoy, J.L.; Vermaut, P. NMR study of a rare-earth aluminoborosilicate glass with varying CaMo-Na2O ratio. Appl. Magn. Reson. 2007, 32, 613–634. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nozawa, S.; Sato, T.; Otake, T. Effect of Dissolved Silica on Immobilization of Boron by Magnesium Oxide. Minerals 2018, 8, 76. https://doi.org/10.3390/min8020076
Nozawa S, Sato T, Otake T. Effect of Dissolved Silica on Immobilization of Boron by Magnesium Oxide. Minerals. 2018; 8(2):76. https://doi.org/10.3390/min8020076
Chicago/Turabian StyleNozawa, Shoko, Tsutomu Sato, and Tsubasa Otake. 2018. "Effect of Dissolved Silica on Immobilization of Boron by Magnesium Oxide" Minerals 8, no. 2: 76. https://doi.org/10.3390/min8020076




