Abstract
Measures to counteract Acid Rock Drainage (ARD) generation need to start at the mineral surface, inhibiting mineral-oxidizing, acidophilic microbes. Laboratory and long-term field tests with pyrite-containing mining wastes—where carbonaceous phosphate mining waste (CPMW) was added—resulted in low acidity and near neutral drainage. The effect was reproducible and confirmed by several independent research groups. The improved drainage was shown to involve an organic coating, likely a biofilm. The biofilm formation was confirmed when CPMW was added to lignite coal waste with an initial pH of 1. Forty-five days after the addition, the coal waste was dominated by heterotrophic microorganisms in biofilms. Reviewing the scientific literature provides ample support that CPMW has physical and chemical characteristics which can induce a strong inhibitory effect on sulphide oxidation by triggering the formation of an organic coating, a biofilm, over the mineral surface. CPMW characteristics provide the cornerstone of a new technology which might lead to reduction of sulphide oxidation in mine wastes. A hypothesis for testing this technology is presented. The use of such a technology could result in an economical and sustainable approach to mine waste and water management.
1. Introduction
As early as 1556, Agricola [1] declared that acid mine drainage (AMD) is a curse of mining. To date, no sustainable options exist to curtail the curse. Global estimates of tailings production from milling ore are several hundred thousand tons per day [2]. In addition, open pit mining generates rock waste covering areas that can be hectares or square kilometers in extent. This has led to potential conflicts with available, arable land. Public concern about AMD, in connection to water shortages and land demands, requires serious attention [3].
1.1. General Mine Waste Management Practices
The present approaches to mine waste management focus on keeping water and oxygen away from waste depositories, where they are considered the main drivers of weathering. To slow the infiltration of water and oxygen, waste rock is stacked and compacted and/or sealed with engineered covers. For tailings, water covers have provided some reduction of oxygen penetration, slowing the oxidation of sulphides. Vegetation covers assisted by adding organics and bactericide may have a longer-lasting effect, as recently assessed with aerial photography [4]. Control over mineralized rock surface or weathering has also been pursued by adding bactericides, phosphate materials, and other chemicals to develop a coating over the mineral surface. These additions have worked for a while, and are evaluated in detail in the GUARD guide, most not tested or not lasting in the long term [5].
One of the most intensely pursued measures was the use of phosphate-containing materials, such a natural phosphate rock (commonly referred to as NPR) from a North Carolina, USA. phosphate mine. This material was tested in coal waste piles outdoors and in the laboratory along with some other phosphate-containing materials. An iron-phosphate coating was expected to form on the mineral surface, reducing oxygen access and thereby limiting oxidation. The dosage of NPR ore added was based on the expected stoichiometric reaction between the iron released from pyrite weathering and the phosphate concentration in the phosphate ore. By measuring the acidity/alkalinity of the effluent, the effect of the addition was determined. The additions reduced the acidity generated, but the lowest dosage of NPR produced better results than higher dosages. The approach was abandoned by industry [6,7,8,9,10,11,12,13,14,15,16,17] for various reasons. The results, though, were intriguing to ecologists, possibly indicating microbial involvement. If a heterotrophic, microbial biofilm were present, it might explain the results. This concept would fit well into an ecological engineering approach which is being pursued for decommissioning mine waste and water management areas [18]. Reducing the oxidation rate within tailings and waste-rock depositories would boost the effectiveness of producing an economically-sustainable decommissioning technology.
1.2. Challenges in Mine Waste Management
Wilson [19] summarized the physical, chemical, and geotechnical limitations of handling mine wastes, given the vast accumulations of waste rock and tailings. However, he failed to acknowledge the role of microbes which increase the oxidation rate by orders of magnitude, which is well documented. The ubiquitous nature of these microbes, along with the dramatically increased surface area of exposed sulphides in mine wastes, guarantees that oxidation reactions will overwhelm any potential reductive microbial heterotrophic metabolism. In addition, the sulphide oxidation reaction is exothermic, which ultimately leads to steaming or even burning waste rock piles [20,21].
Another challenge is the scale (square km, m, µm, or even nm) at which different processes operate. This was highlighted by Lüttge and Arvidson [22] in their book on kinetics of water–rock interactions. The authors suggest that looking at only one scale can easily lead to misinterpretations of the reactivity of processes on the mineral surface. Microbial metabolism alters the reaction kinetics of weathering, as well as altering the topography of the mineral surface. Microbes and their exudates act on mineral surfaces by generating corrosion pits and/or biofilms, which can change the electrical charge of the surface.
For mine waste management, the scales can span 6 to 12 orders of magnitude from the mineral surface with its corrosion pits to the vast expanses of waste rock and tailings deposits (ha or km2). At each level, interactions between water and rock are taking place. The water characteristics within the piles are altered by precipitation and dissolution reactions while passing through the wastes, hence they may alter the interaction and processes. From a practical perspective, given that microbes are a major accelerating factor, these powerful geo-microbiological processes must be of primary concern in mine waste management.
1.3. From an Iron Phosphate Coating to a Biofilm or from Stoichiometry to Geomicrobiology
This review is not a classical review as it does not include all research on sulphide mineral coatings intended to reduce iron sulphide oxidation. The literature of the mid-1980s is reviewed as it pertains to the formation of iron phosphate coatings on the mineral surface. This avenue was intensely pursued with various phosphate containing materials to limit sulphide oxidation [6,7,8,9,10,11,12,13,14,15,16,17]. Among these research efforts, one using NPR ore from a phosphate mine in North Carolina caught our attention. Their results appeared, to us, to involve the action of microbes. Over the past 30+ years, we have carried out field and laboratory experiments on several sulphidic wastes from different mines (both field and laboratory tests) with NPR, not ore, wastes from the same mine. We repeatedly obtained improved effluents and documented an organic coating. The coating contained inorganic, secondary precipitates, but only traces of phosphate, i.e., no phosphate iron coating. We elucidated the physical and chemical characteristics of the NPR wastes. What was lacking is the proof that indeed the improved effluent is directly connected to the growth of a neutrophilic biofilm previously only observed as an organic coating.
Several decades later, we identified the groups of microbes which form the organic coating on sulphide minerals [23]. Here, we extract the relevant results—the effluent improvements achieved with the addition of NPR to sulphidic wastes and the chemical and physical characteristics of NPR. This review examines the question: Do the characteristics of the NPR relate to the geomicrobiological factors which stimulate biofilm development and growth, and influence its structure? We integrate the biogeochemical literature on heterotrophic neutrophilic biofilms which compete with chemo-lithotrophs forming the organic coatings. This work facilitates the formulation of a hypothesis on the mode of action of NPR, now named CPMW “Carbonaceous Phosphate Mining Waste” to reflect its relevant characteristics.
2. Approaching the Geo-Microbiological Challenge
Ecologists view mine waste areas as extreme ecosystems. These ecosystems are defined as areas where most life forms find it hard to survive. Chemo-lithotrophic microbes, however, are a dominant feature of these habitats. They survive and even flourish under harsh physical and chemical conditions. Given these challenges, the fundamental question is, can these oxidative habitats be gradually altered to more normal reductive environments? Ecological principles such as niche construction [24,25] might hold the answer. Niche construction can be defined as the process by which organisms modify their own (and other organisms’) environments.
If niche construction was operating in extreme habitats such as mining wastes, then it would probably be working naturally in metal leach piles. We searched the mining literature and found an example. The Gibraltar heap leach pile in British Columbia, Canada reported cessation of their leach dump [26]. What caused the oxidation processes to stop? This event was an opportunity for us to investigate possible microbial niche construction in a primarily oxidizing habitat. Rocks from the dump (Figure 1) were obtained and investigated with scanning electron microscopy (SEM) with energy dispersive spectroscopy (EDS).

Figure 1.
One of the rocks from the sections of the Gibraltar leaching dump.
The surface revealed a coating, comprised mainly of iron-hydroxide, with trace amounts of phosphate, and numerous microbial colonies. These observations confirmed our belief that microbiological niche construction might reduce sulphide oxidation.
2.1. Reproducable and Replicable Outdoor and Laoratory Experiments
A replicable, controlled experiment was set up outdoors with sulphidic waste rock from a northern Quebec Cu/Zn mine, basically hoping to induce a phosphate iron coating mixed with microbes. Two-and-a-half tonnes of sulphidic waste rock containing up to 15% sulphides were placed in fifteen 70 L plastic drums drained at the bottom. To the top of these drums, 8 L of gravel size CPMW (carbonaceous phosphate mining waste), was added. Several drums were left alone as controls. The CPMW originated from the same mine in North Carolina used by earlier investigators [6,7,8,9,10,11,12,13,14,15,16,17], with the same mineralogy as the ore, only lower in phosphate content.
The topical application simulates a onetime application on each completed waste rock lift, a practice easily implemented by a mine operator. Rain would transport small particles of this potentially niche-altering neutralizing and nutrient rich substance to the mineral surface, the location of origin of ARD or AMD. The CPMW dosage for waste rock was dictated by mine operations ($0.05/t of ore mined) and not by the stoichiometry of the sulphide/iron content of wastes as in the earlier investigations.
Effluent from the drums was collected intermittently for 2.7 years, after which time the experiment was dismantled. The last effluents collected were submitted for elemental analysis [27]. About 3.2 L of the 8 L CPMW added was recovered as gravel which was stained in part with iron, but essentially unreacted. Based on CMPW dissolution experiments, the carbonate neutralization was completely insufficient to account for the effluent improvements of the drums [28]. The rocks were stored in an industrial basement awaiting microscopic investigations of the mineral surfaces.
The difference between the mineral surfaces with CPMW and without CPMW was striking (Figure 2a,b). Given the pronounced desiccation cracks (Figure 2b) it was expected that the coating would decompose during the 4.5 years’ storage, even though some microbes can deal well with desiccation stresses. We re-exposed the rocks, stored for 4.5 years indoors, again outdoors for one year, covering all four seasons. We did not add any new CPMW and provided intense acid-generating conditions collecting—rather than draining—the drums, creating humid conditions altering between dry or submerged, as all water was drained when sampling took place. Initially, some acidic effluent was generated, but shortly thereafter, the effluent pH increased to circum-neutrality with low acidity. The results of both exposures and the elemental composition of the final effluents are summarized in [27].
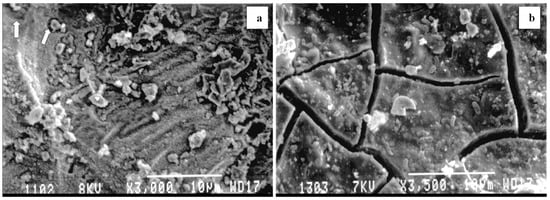
Figure 2.
(a) SEM images of waste rock surfaces in the absence or presence of CPMW (formerly named NPR; (b) both rocks were part of the 989 days of outdoor exposure and 4.5 years of indoor storage (scale bars: 10 μm). Surface corrosion by microbes was observed in the experiment (see arrows on (a) effluents with low pH. In the presence of the CPMW coatings with classical desiccation cracks in the organic coating were recognized on the surface (b) effluents with circumneutral pH. SEM observations from University of Toronto [29].
Rocks from the experiment were shipped to the University of Ottawa where the desiccated coating was revived (Figure 3a,b) by immersing the rocks in a PTYP medium (peptone trypticase yeast extract pyruvate) adjusted at pH 7.2. On day 1 Figure 3a and after seven days Figure 3b demonstrate the disappearance of the dry coating. The selected medium provided nutrients for the revival of the microbes present within the dry biofilm. The surfaces were observed using an ESEM (environmental scanning electron microscope) facilitating observation in a moist state [29].
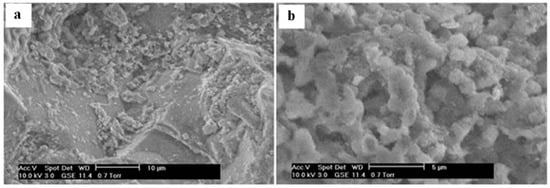
Figure 3.
ESEM images of the revived organic coating in a PTYP media (at pH 7) on the surface of pyrite-rich waste rocks from drum in outdoor experiments for 989 days and 4.5 years indoor storage at day one (a). After seven days, microbes (cocci) originally present on the rocks were revived and they reformed a coating on the waste rock surface (b). Mineral precipitates could also be observed on the rock surface in close association with the microbial cells, suggesting that biomineralization could occur on the microbial cell walls and extra polymeric substances (EPS; (b)). ESEM observations from University of Ottawa [29].
Rocks from the same experiment were also shipped to the University of Western Australia as part of an AMIRA International Ltd. project P933 [30,31]. There, the mineral surfaces were intensely investigated with electron microscopy, quantifying the thickness of the coating, oxygen content, and composition of the inorganic precipitates. In addition, CPMW was added to Red Dog (AK, USA) mining wastes which resulted in similar findings, the coating was organic and the effluent had improved [27,28,29,30,31].
2.2. LUNEs, Large Un-Replicable Natural Experiments
As the independent investigations proceeded over several years, sufficient evidence emerged that the organic coatings are indeed of microbial nature generated with CPMW additions we had gradually set up field plots with CPMW. As highlighted in Section 1.2, laboratory investigations are not necessarily representative of the conditions in waste depositories. Hence, experimentation on a larger scale in the field and under different field conditions is necessary. Field experiments provide essential information on the viability of any environmental technologies and scale up potential is essential for bioleaching and bioremediation techniques. Mine waste environments are not static ecosystems and the composition of indigenous microbial populations is a dynamic—albeit slow—process, adapting to chemical and physical changes in the wastes. Niche construction might be different at scale up in the field from the controlled laboratory conditions [24,25]. In mining wastes, the addition of CPMW represents the chemical and physical basis that drives niche construction.
Larger-scale field experiments with CPMW were set up, although they are strictly not replicable. The in-situ tests had control plots, but conditions in northern Ontario are different from Newfoundland with respect to climate, weather, ore (minerals), degree of grinding, and the methods of deposition. All these factors contributed to the irreproducibility of field trials. These types of experiments have been called “Large-scale Un-Replicated Natural Experiments” or LUNEs. While they have not been well received in some areas of science, they have gained recognition in ecology. Barley and Meeuwig [32] state that these LUNEs have aided in the formulation of hypotheses at ecologically-realistic scales. These LUNEs have made positive contributions to conservation policy, providing powerful insights into cosmology, evolution, and geology. Hence our field experiments provide some support to the applicability on a large scale.
Our field tests or LUNEs in tailings ranged in size from 0.5 to 1.5 ha, an area, large enough to be ecologically relevant. Plots were set up on old uranium tailings in Elliot Lake, a fresh installed pyrrhotite area of the nickel/copper tailings in Copper Cliff, both Ontario Canada, and on a spill area of zinc concentrate from a polymetallic mine in Buchans, Newfoundland (Table 1). Sulphide content ranged from 2% to 85%. CPMW was ploughed 10 to 20 cm into the tailings surface. The plots were stabilized by folding straw chips or horse manure into the tailings and seeded to prevent erosion during rain events, but not on the spill area in Buchans. Details of all the field tests and the methodology by which the pore water of the tailings samples were assessed are given in [33,34,35,36,37,38,39]. After the one-time application of CPMW, 3.1–3.8 years elapsed before sampling. Sampling consisted of profiling 5 to 25 cm into the tailings with a spade, reaching below the root zone. Dosage for the tailings was based on the cost of trucking lime used in the standard development of a grass cover on the tailings. It was expected that the CPMW would lead to a hardpan below the seeded surface and below the root zone. A hardpan would reduce volume of seepage by reducing the infiltration of atmospheric precipitation and by promoting runoff. All experimental parameters and the characteristics of the tailings are given in Table 1, along with the same parameters for the controlled, reproducible outdoor waste rock experiment for comparison.

Table 1.
Summary of experimental timelines and characteristics of sulphidic tailings and waste rock (both data extracted from [34,37,38]).
In Table 2 the chemical characteristics and the elemental concentrations are presented for the tailings slurries and the effluents of the drum waste rock experiment. The difference in acidity between effluents from CPMW samples and control samples (no CPMW) was remarkable. Furthermore, the higher pH values in CPMW effluents reflect the reductions in metal concentrations. The importance of these empirical results was that both the un-replicable and replicable experiments showed order of magnitude differences in nearly all parameters measured in most of the concentrations of the elements.

Table 2.
Results of LUNE tailings slurries and effluents from the waste rock drums.
The tailings samples were stored for 0.5 to 6.5 years in coolers with access to air. Afterwards, slurries (20% w:v) were prepared with a magnetic mixer. The Eh, pH, and electrical conductivity were measured in the supernatant. As the determination of acidity/alkalinity consumed supernatant, the volume used was replaced to keep the supernatant volume the same throughout the monitoring period, which lasted up to 2.8 years (Table 1). A long monitoring period was selected as through the exchange of porewater along with oxygen access to the slurries, it was anticipated that acid generation would resume. In most samples, this occurred, but not in all of them. On termination of monitoring, tailings were dried and subjected to elemental analysis [38].
2.3. Characteristics and Composition of CPMW
As some of the CPMW grains had different colors, a size fractionation and elemental composition was carried out to determine if the particles had different compositions. All fractions were analyzed for their elemental composition, but no relevant differences between the grain sizes were evident. All elements measured were above the detection limits, except for cobalt in the two finest fractions (Table 3). These characteristics are relevant, as only the small particles would be expected to be transported by the rain to the mineral surface.

Table 3.
Particle size distribution and elemental content of CPMW (µg/g).
The dissolution and solubility of the elements contained in the CPMW were assessed by leaching the gravel in 0.1 N sulfuric acid, distilled and rain water (Table 4). In this table, the elements are classified as major nutrients or as co-factors in enzyme catalytic reactions. The smallest particles (Tyler mesh passing 270–400 or 0.053 to 0.037 mm) contained lower nutrient concentrations. However, the concentrations released were still above detection limits and likely sufficient to support growth of the microbes. The leachability of the CPMW was addressed by decanting and refilling with acid, rain, and distilled water, collecting each decant solution throughout the eight cycles. Ten grams of CPMW gavel was stirred for 1 min with a magnetic stirrer in a beaker in 100 mL of the leachate solutions. The slurry was allowed to settle for a minimum of 0.5 h after which time the supernatant was decanted (first three decant cycles). The settlement time was increased to 17 h for the remainder of the experiment. A stable, but increased pH value after addition of the leach solution was used to signal the end of the decanting experiment and the collected mixture was analyzed for elemental content (Table 4). Details of the methodology are given in [39] from which the data of Table 3 and Table 4 have been extracted.

Table 4.
Release of elements from 10 g CPMW after stirring in 0.1 M sulphuric acid, distilled water or rain water.
2.4. Which Microbial Groups Form the Organic Coating or Improve the Biofilm?
The next step was to identify the groups of microbes forming the biofilm, and possibly document their growth and development in conjunction with the improved effluent. A standard bioleach procedure was used where CPMW was added to lignite coal in laboratory columns [23]. The coal columns were sterilized and inoculated with an acidophilic enrichment culture originating from Rio Agrio, Argentina. Before inoculation the microbes were maintained using pyrite as sole energy source. The microbial populations were enumerated by “most probable number” before CPMW addition and 2, 4, and 10 weeks later. In those columns without CPMW, strong bioleaching was evident as measured by electrical conductivity, pH and Eh. In those columns with CPMW, there was a shift in the microbial populations after day 45. About 90% of populations consisted of neutrophilic heterotrophs covering the pyrite surfaces with a 10 µm thick biofilm, whereas without CPMW, 99% of the populations were iron-oxidizing acidophiles in mono-layered biofilms. The elemental content of the column effluents was not chemically analyzed due to economic constraints, but after 213 days CPMW, the effluent was still clear, which was not the case in its absence. The pH improved by about 0.5 units and the redox value and electrical conductivity were lower (Figure 4).
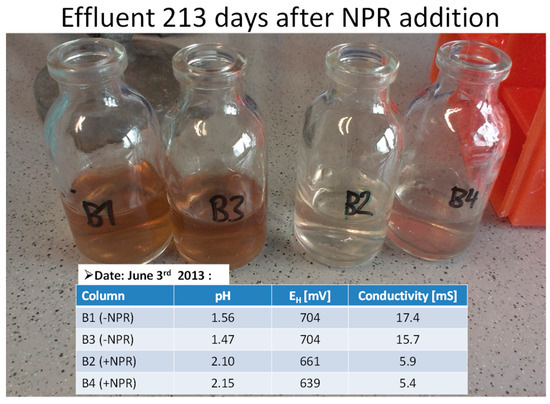
Figure 4.
Effluents from coal columns after 213 days since onset of the leach experiment. Carbonaceous phosphate mining wastes (CPMW = NPR) were added to columns B2 and B4, but not to B1 and B3. Since we know that biofilm formation is stimulated by NPR we changed the designations used in all previous publications, as we suspect the carbonaceous nature is more important than the phosphate content.
Microbial ecology describes one aspect of biofilms as spatially-organized microbial consortia consisting of many metabolic groups, which alter their activity depending on environmental conditions. It is hypothesized that atmospheric precipitation carries small particles of the added phosphate mining waste material to the mineral surface where it promotes the proliferation and growth of protective, heterotrophic, neutrophilic microorganisms. The pH within the microenvironment on the mineral surface with nutrient-bearing CPMW (P, Ca, K) is raised and organic matter is introduced, probably partially from dead chemolitho-autotrophic acidophiles. The formation of a protective biofilm by neutrophilic and heterotrophic microorganisms is induced. To date, this hypothesis has not been confirmed.
The experiments detailed here provide evidence for the longevity of the biofilm on the pyrite mineral surfaces. However, factors which lead biofilm initiation, formation, and longevity are documented in the geo-microbiological literature. They must be reviewed and evaluated in the context of the CPMW additions and its effects. Specifically, we examined if CPMW might indeed trigger niche alteration under bioleaching or weathering conditions. This niche alteration, by the construction of heterotrophic microbial biofilms, might outcompete or overgrow the acidophilic chemo-autotrophic monolayer on sulphide wastes. Can bad bugs be beaten by good bugs?
3. General Characteristics of Biofilms and Functions
3.1. Fundamentals of Biofilm Formation
Microorganisms populate every habitable environment on Earth [40,41]. They attach to surfaces and form biofilms virtually on all types of materials [41]. Through their metabolic activity, they affect the chemistry and physical properties of their surroundings [40,41,42,43]. In an evolutionary context, these alterations are known as niche construction, well documented for microbes. Life at the atmosphere–lithosphere boundary is a niche that is sparsely covered by thin subaerial microbial communities, which:
- have adapted to all types of terrestrial/subaerial stresses such as desiccation, extreme temperatures, low nutrient availability, and intense solar radiation;
- interact with minerals that serve both as a dwelling and a source of nutrients and trace elements, and;
- enhance weathering of rocks and soil formation.
Microbial biofilm communities are ubiquitous in aquatic and terrestrial ecosystems as well as on man-made materials. There are initial colonizers and other species more likely to attach to existing biofilms [42,43] and take part in biogenic weathering on natural rocks as well as on construction materials, such as stone, steel, and concrete [44,45,46]. Subaerial biofilm microorganisms have adapted to desiccation, solar radiation, and other environmental challenges by developing protective, melanized cell walls, assuming micro-colonial architectures and symbiotic lifestyles [47].
Biofilms are the key to understanding how microbes adapted to these stresses influence and interact with the physical environment. After settling and attaching to the mineral surface, cells begin excreting extracellular polymeric substances (EPSs), which protect the cells from desiccation, and provide a three-dimensional structure linking cells into a community. This EPS matrix controls the environment around settled microbes in structural and functional manners [48].
The development of a biofilm involves at least five stages [40]. The first stage is the initial attachment of cells to the surface, which in most cases involves a conditioning film of organic molecules that have adsorbed to the material. In stage two, production of EPS results in more firmly adhered, irreversible attachment. In stage three, the biofilm develops, and characteristic biofilm architectures are formed. The developed biofilm architecture with stratification patterns emerges in matured biofilms (stage four). At the same time, the niche development of diverse metabolic groups of different microbial species and cell subpopulations within the biofilm occurs. Finally, in the last developmental stage (stage five) the dispersion of single planktonic cells from the mature biofilm is observed. Those, motile, planktonic cells can be thought of as ”pioneers” that seek new suitable habitats for growth and biofilm formation in their new environment. These stages apply most appropriately on single-species biofilms of heterotrophic microorganisms in laboratory experiments. This holds mainly for acidophilic, chemo-litho-autotrophic or bioleaching microorganisms. Those groups are responsible for the oxidation of metal sulfides in mining waste and hence lead to contaminated AMD and they display a simple, monolayer biofilm architecture, and a low production of EPSs.
In general, free-swimming, planktonic microorganisms in water bodies represent a minority of the total number of microorganisms in aquatic systems. The remaining microorganisms form biofilms and are attached to surfaces [40,41]. They are found in soils, on inanimate mineral and rock surfaces, on living tissues, and decaying biomass and all other interfaces, such as the air–liquid interphase or oil–water interphases [41]. EPSs, consisting of polysaccharides, proteins, lipids, and nucleic acids, are important for contact and binding of planktonic cells to material surfaces during the initiation of the biofilm formation process and shape the environment of cells in established biofilms. EPSs are functional components that control environmental parameters in biofilms to some extent. Microbes have evolved specific organic coatings that mediate attachment to the substratum, protect the cells from desiccation, firmly anchor the cells to the surface, and act as an extracellular nutrient reservoir, due to presence of biodegradable material. EPSs also represent a sorption site for dissolved organic molecules from the bulk solution. Due to sorption, scavenging, and diffusion-limited transport of biocides through EPS, biofilm microorganisms are well protected from numerous threats, including biocide application and sanitation measures [48].
Extracellular polymeric substances may also be considered as a digestion system, functionalized by anchoring extracellular enzymes in the self-generated EPS environment. Therefore, the EPS components, which are species-specific, shape the biofilm community structure and control the spatial organization of microorganisms. Those can be attributed to different metabolic guilds, which are involved in synergistic, cooperative but also competitive and inhibitory interactions. At the same time, biofilms and EPSs also promote gene-transfer. Overall, biofilms and EPSs protect bacterial populations, increase their fitness and gene pool and can enhance metabolism and growth of different species in various niches [48]. Consequently, biofilm organic coatings on surfaces are a form of life insurance, functionally adapted to their microbial members, specific niches, and places in the environment.
3.2. Acidophilic, Mineral-Oxidizing Biofilms on Metal Sulfide Surfaces
Microbes, such as acidophilic, mineral-oxidizing bacteria, are ubiquitous in the environment [49]. These chemo-lithotrophs—such as Acidithiobacillus ferrooxidans or Leptospirillum ferrooxidans—form biofilms on metal sulfide surfaces. These oxidative biofilms formed by chemo-lithotrophic microbes accelerate metal sulfide oxidation. Hence, acid rock drainage or acid mine drainage is accelerated by the presence of these organisms [49,50]. Oxidative biofilms are most likely the first ones formed on exposed mineral surfaces. Bioleaching of pyrite often correlates with attached cells in the corrosion pits on the mineral surface [51]. Consequently, once attachment has occurred and the EPS matrix is developed, the weathering or corrosion of the metal sulfide surface is dependent on iron-oxidation activity and mass transfer of dissolution products within the EPS-matrix between the mineral and the cell [51,52].
The attachment of cells is the first and most important process, as with this the cells begin to colonize the mineral surface and persist on the metal sulfide. Motile species of acidophilic bacteria colonize metal sulfides once acidic conditions prevail due to chemical and physical weathering of the mineral surface and chemo-tactical attraction towards dissolution products of metal sulfides. Motile bacterial species have also been demonstrated to sense metal sulfides due to chemo-tactical sensing of dissolution products that arise due to the chemical oxidation or acidolysis of metal sulfides. A similar chemical sensing mechanism is hypothesized for Acidithiobacillus ferrooxidansT. This strain is not motile and does not have the genes associated with flagellar and chemotactical motility. However, it has a luxI/R-type quorum sensing (QS) cell–cell communication system, which allows for concerted control of gene expression in a cell-density dependent manner [52]. The corresponding acyl-homoserine lactone (AHL) signaling molecules were detected in pyrite and elemental sulfur cultures of this and related strains. This finding, in combination with commonly high cell densities in biofilms on mineral surfaces, suggested that cell–cell communication is involved in regulation of biofilm formation of several acidophilic leaching bacteria, as it has been described for other bacterial species [53,54,55,56,57,58].
This hypothesis has been confirmed since EPS production in Acidithiobacillus ferrooxidansT is enhanced when exposed to synthetic AHLs and attachment of this species and several other strains is specifically affected by the presence of AHLs with different acyl chain lengths [57,59,60]. Consequently, planktonic cells may attach passively due to electrostatic interaction. More specifically, motile cells may actively sense pyrite surfaces chemotactically due to the release of dissolution products at pitting sites, such as ferrous iron and reduced sulfur compounds. These chemicals represent nutrients for the microbial cells. Cell–cell communication may be involved in sensing established bacterial cell populations on mineral surfaces. In that context it is important to note that inter-species cell–cell-communication in bioleaching bacteria was demonstrated to exert effects that influence the microbial community composition in leaching habitats. Mineral colonization is especially influenced in many species of acidophilic leaching bacteria by presence of AHL molecules [57]. In summary, chemo-tactical attraction or repulsion is a possible mechanism influencing attachment. However, the expression of EPS-related genes, due to sensory mechanisms and cell–cell communication, also influences cell abilities to attach. This observation highlights cell–cell-communication as an important factor in niche generation and competition between iron-oxidizing acidophiles. Some forms of these processes are most certainly involved in the mechanism of bioleaching prevention after CPMW addition to mining waste.
The process of attachment has been studied extensively in pure- and mixed-cultures [59,60,61,62,63,64,65]. It has been hypothesized that the attached microorganisms are presumably the ones which start and enhance the leaching process. It has become evident that, at low concentrations of iron ions (<200 mg/L), biofilm cells on pyrite surfaces are exclusively relevant for the oxidation [59], since their EPS accumulate the oxidative agent, ferric iron. This situation is common at the initiation of AMD generation from waste rocks, when mining wastes are exposed to rainwater. With elevated levels of iron ions, the activity of free-swimming, iron-oxidizing cells also becomes important. However, for attached biofilm cells, the EPSs act as an enlarged reaction space between the metal sulfide surface and the attached cells. In the case of mineral-oxidizing, acidophilic bacteria, such as Acidithiobacillus ferrooxidans or Leptospirillum ferrooxidans on sulfide mineral surfaces, their EPSs are functionalized by presence of glucuronic acid residues [61,62,63]. Chemical analyses of the EPSs of A. ferrooxidans, A. thiooxidans, and Leptospirillum ferrooxidans indicated a common composition of neutral sugars, fatty acids, and uronic acids, but differed with the strain and the growth substrate [60]. Iron ions were only detectable in the EPSs of iron- or pyrite-grown cells, but not in EPSs of sulfur-grown cells. Pyrite oxidation rates correlated with the amount of complexed iron ions in the case of A. ferrooxidans and L. ferrooxidans [62,63,64,65].
The uronic acid residues in the polymeric matrix of the EPS bind metal ions and exhibit a preference for ferric iron ions. In turn, two moles of glucuronic acid residues bind one mole of ferric iron. It is therefore accumulated in the extracellular space, directly at the cell surface. These ions provide a positive charge to the cells, which results in a primary electrostatic attraction to metal sulfide minerals that exhibit a negative surface charge at pH < 2 [64]. In addition, the accumulation of the oxidative agent, ferric iron, in the reaction space between biofilm cells and the sulfide mineral provides an oxidative environment, coupling chemical oxidation of sulfide moieties of the mineral with biological oxidation of ferrous iron. However, in addition to electrostatic, hydrophobic interactions contribute to the firm attachment to metal sulfides and elemental sulfur that occurs as an intermediate during bioleaching [61,66,67]. Consequently, attachment of leaching bacteria to metal sulfides is influenced by pH and ionic strength.
3.3. Potential Effects of CPMW on Oxidative Biofilms on Metal Sulfides Surfaces
CPMW, due to pH-raising acidolysis and the associated release Ca and Mg ions, will affect subsequent attachment of bacteria. Temperature also affects attachment. Stress through the addition of chloride or copper ions to assays with un-adapted cells inhibited attachment. In contrast, the presence of 1 mM glucuronic acid, glucose or galactose enhanced EPS production and colonization of pyrite surfaces [60,67,68]. Hence, the EPS selects for a preferential attachment to metal sulfides. Likewise, the mineral surface characteristics play an equally important role. Crystal structure defect sites, surface imperfections and corrosion pits on the mineral surface are colonized first. In general, metal sulfides are often preferentially colonized compared to gangue minerals [69]. Furthermore, growth conditions and growth substrate influence cell attachment. In general, pyrite-grown cells, with enhanced amounts of EPS compared to cells grown with iron(II)-ions as sole energy source, attach more efficiently to metal sulfides [61,62,63].
In another study, 16 strains of acidophilic bacteria were screened for their abilities to adhere to pyrite ore, glass beads, and ferric hydroxysulfates [70]. These were strains of the iron- and sulfur-oxidizer, Acidithiobacillus ferrooxidans; the sulfur-oxidizer, Acidithiobacillus thiooxidans; and the iron-oxidizer, Leptospirillum ferrooxidans; heterotrophic acidophiles Acidiphilium spp. and Acidocella sp. and moderately thermophilic iron- and sulfur-oxidizing Sulfobacillus thermosulfidooxidans and Sulfobacillus acidophilus. Considerable variations were found between different species of acidophiles, and also between different strains of the same species, in how they attached to solids. Attachment generally increased with time (over 100 min) though 99% of one A. ferrooxidans isolate attached to pyrite after just a 10 min exposure. Also, it was found that most acidophiles attached more readily to pyrite than to glass beads. However, attachment to ferric hydroxysulfates was highly variable, though one A. ferrooxidans isolate and one heterotrophic acidophile (Acidocella) attached strongly to ferric iron precipitates. These minerals, namely jarosites and schwertmannite, occur in AMD/ARD environments and are also observed in cultures of A. ferrooxidans and other acidophilic, iron-oxidizing species [70].
The demonstrated inhibiting effect of CPMW on metal sulfide oxidation certainly has multiple causes. Chemical effects, due to acid consumption and formation of a mineral precipitate coating alone do not explain the efficacy of CPMW as only scarce phosphate signals were noted during the microscopic investigations of the organic coating. Instead, a definite cover of organics was repeatedly identified in all investigations. The microbiological implications of CPMW additions have proven to be important in several studies [36]. However, the mechanisms influencing microbial biofilms on mining waste and CPMW particles have not been thoroughly studied. We propose a mechanism that may explain the demonstrated dramatic shift of the microbial community composition on metal sulfides [36], due to stimulation of a neutral pH on the mineral surface followed by heterotrophic niche formation. This niche is readily provided when CPMW particles are in contact with the acid mineral surface or leachate. Once metal sulfide oxidation begins, acidic conditions prevail on the waste rock minerals. Consequently, particles of the CPMW start to dissolve due to acidolysis, releasing microbial nutrients—such as PO42−, Ca2+, Mg2+, and CO2—while consuming acid. Consequently, CPMW particles provide a micro-niche for neutrophilic bacteria in the vicinity of the acidic environments colonized by mineral-oxidizing acidophiles on the metal sulfides.
The development of reducing biofilms by neutrophilic bacteria on CPMW particles or in their surroundings is facilitated by the presence of the released nutrients. Simultaneously, heterotrophic bacteria and fungi may utilize biomass from acidophilic leaching bacteria. In that context, it is also interesting to note that pyrite oxidation in acidophilic mixed cultures of the chemo-lithotroph Acidithiobacillus ferrooxidans and the heterotrophic Acidiphilium cryptum is more efficient at higher initial chemolithotroph/heterotroph inoculation ratios [71]. High abundances of the heterotrophs may inhibit chemo-lithotrophs, due to utilization of EPS as a carbon source and release of inhibitory metabolites [72]. Likewise, there may be release of signaling molecules [53,54,55], toxins [73], biofilm dispersal factors [74], or other inhibitory or antimicrobial metabolites from the neutrophilic, heterotrophic microbial populations that are establishing in the micro-environmental niche provided by the CPMW particles. These factors might also efficiently inhibit, inactivate, or even utilize the biomass of the neighboring acidophilic cell population. Therefore, a self-enhancing shift in the microbial community composition, representing a niche construction, is promoted by CPMW due to the formation of a protective, reducing biofilm on waste rock material. Consequently, metal sulfide oxidation and acid production will stop and the observed improved effluent characteristics would be met.
In addition to this hypothesis, CPMW inevitably supports the formation of stabile, protective biofilms [23,29]. It is anticipated that this observation can be explained by the release of ample Ca2+-ions upon contact with rain water [28]. At elevated pH (>4), Ca2+-ions support salt-bridge binding of polysaccharides. Hence, CPMW supports coagulation of cells and the attachment of free-swimming cells to mineral-attached organic material, thereby promoting biofilms. Since heterotrophs cannot grow using metal sulfides as an energy source such as chemo-lithotrophs do, they must find nutrients from elsewhere after establishing a biofilm. This biofilm and the elevated pH reduce or prevent further oxidation of the metal sulfide by mineral-oxidizing, acidophilic microorganisms. These protective biofilms have been found in laboratory experiments with CPMW [36]. The microbial colonization of pyrite coupons, which were used in column experiments described in [23] is shown in Figure 5. Here, colonization of pyrite coupon surfaces was strongly enhanced and biofilms of up to 10 µm in thickness were observed after treatment with CPMW, while in control experiments without CPMW only thin, cell-monolayer biofilms were observed.
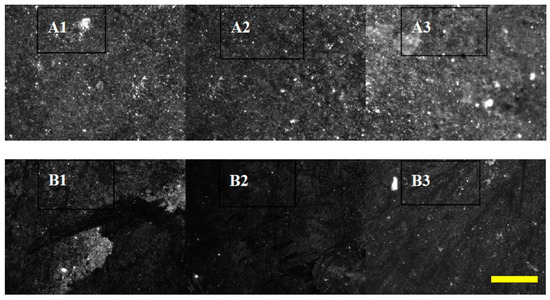
Figure 5.
Biofilm formation on pyrite coupon surfaces in lignite column leaching experiments is enhanced after treatment with CPMW. Epifluorescence images of DAPI-stained cells (white) on dark pyrite surfaces are shown. Coupons from experimental columns with (A1–A3) and without (B1–B3) CPMW addition were imaged 2 (1), 4 (2), and 10 weeks (3) after addition of 0.1% CPMW as a top layer on the columns. The size bar represents 100 μm. Experimental details are given in [23].
Biofilms have not been identified in the samples from LUNEs, but it can be assumed that the same microbiological processes take place in the field. Low acidity and the raised pH in the supernatant of the slurries obtained from the field tailings samples are indicative of some protective biofilm. An organic coating was documented on the waste rocks of the drum experiment from the northern Quebec Cu/Zn mine multiple times, after different, acid-generating, storage conditions [28]. These organic coatings were found in our studies to be persistent over several years. They were drought- and frost-resistant, and revivable as documented by [29,31]. It is evident that changes in the microenvironment at the surface of the mineral will bring about changes in biofilm metabolic activity. Consequently, influencing microbial metabolism can alter habitats over time, and these influences will then exert control over the evolution and structure of microbial communities [75]. EPS produced from one species will be shared with neighboring cells. This altruistic use of a shared resource may be an evolutionary advantage, as it pushes later generations of the same species up and out into better oxygen conditions, while limiting oxygen supply to others [76].
Those sharing cells are deemed to utilize less efficient alternative electron acceptors or use fermentative pathways for energy conservation. The maturation of the biofilm will therefore limit access of oxygen to the covered metal sulfide surface favoring reducing conditions at this critical interface. De Beer et al. [77] measured the concentration of oxygen at various points in a biofilm grown from an undefined consortium of microorganisms. The profiles of the concentration of oxygen within the biofilm indicated that there was a large depletion of oxygen within the bacterial clusters, while the voids between the clusters acted as conduits for the supply of oxygen to the lower regions of the clusters. Whether minute amounts of oxygen could still be available at the CPMW-treated metal sulfide surface or not is not critical, since the niche of acidophilic, metal-sulfide oxidizing microorganisms has been eradicated by CPMW. The slight local increase in pH and the manifested shift in the microbial community composition is evidence in this direction. A succession of this process with drainage water flowing through a waste rock pile that has been treated with CPMW particles on each lift is therefore likely to occur. This succession is likely supported by transport of fine CPMW particles with the water entering the wastes, along with the elements leached from CPMW and the suggested presence of biogenic signaling or antimicrobial substances.
4. Conclusions
Our knowledge of the microbial communities involved in bioleach operations has grown tremendously in the last few decades. The CPMW results presented here suggest that heterotrophic biofilms inhibit acidophilic bioleaching microorganisms, and by extension stop generation of acid mine drainage at its source. This approach of using CPMW as a stimulant, fostering stabile, heterotrophic, reducing biofilms by acting as a neutralizing agent and releasing nutrients for microorganisms in a gentle manner without risk of eutrophication, may signal a possible resolution to Agricola’s dilemma. We hope that this contribution assists others with bioleach operations, but it might also bring about an urgently needed paradigm shift in how the mining industry approaches the environmental burden associated with acid mine drainage. Acidophilic microbes have been around for millennia, interfering in man’s quest for metals. By accepting the role of microbes in mining, and approaching the wastes as microbial habitats, we can support natural, ecological repair processes, and shift mine effluents from acidic to neutral pH. Carbonaceous phosphate mining wastes may be an important tool in that competition, but other materials such as phosphate mine tailings likely have similar effects. Laboratory test work has shown success. It is now time to proceed with evaluating existing LUNEs and/or start large-scale LUNEs in an operating mine.
Author Contributions
W.N.W. recognized the potential of NPR as an approach to alter the mineral surface in 1991 at an ARD conference. He carried out the first field experiments and the needed literature searches relating to CPMW and assisted in writing the manuscript. S.B. designed and conducted the experiments on lignite coal and assisted in the literature review for this manuscript. M.K. is the research director and CEO of Boojum Research Ltd. She supervised and assisted in the design and analysis of all experiments.
Acknowledgments
Most of the work on CPMW was funded by Boojum Research Ltd. Some contributions were made by the Ontario Centre for Research in Earth and Space Technology as Projects LR01REM43-RPA/B-O 2002, NRC/IRAP Project No 22643U 1994; NRC/Tech Project # 257140 1996. AMIRA Projects 933, 933A also contributed to our knowledge base of biofilms on mining waste rocks. Contributions of waste rocks and permission to work on tailings areas were granted by Dennison Mines, Inco, BHP Billiton, and ASARCO. Their assistance with the development of this technology is gratefully acknowledged. This publication is paid for by Boojum Research Ltd.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Agricola, C. De Re Metallica, First English ed.; Hoover, H.; Hoover, H.L., Translators; Dover Publications, Inc.: New York, NY, USA, 1912; Available online: Available online: http://farlang.com/books/agricola-hoover-de-re-metallica (accessed on 3 May 2018).
- Jakubick, A.; McKenna, G. Stabilization of tailings deposits: International experience. In Proceedings of the Mining in the Environment III, Sudbury, ON, Canada, 25–28 May 2003; pp. 1–9. [Google Scholar]
- Kalin, M.; Wheeler, W.N.; Sudbury, M.P.; Harris, B. Mining, Ecological Engineering and Metals Extraction for the 21st Century. Subject: Environmental Issues and Problems, Environmental Engineering, Sustainability and Solutions. In Oxford Research Encyclopedias; Oxford University Press: Oxford, UK, 2018. [Google Scholar]
- Gusek, J.; Plocus, V.G. Case study 20 years of acid rock drainage chemistry improvements after bactericide application. J. Am. Soc. Min. Reclam. 2016, 67–85. [Google Scholar] [CrossRef]
- INAP. The International Network of Acid Prevention, Global Acid Rock Drainage Guide. Chapter 6.0, Prevention and Mitigation. 6.6.4. pp. 272–275. Available online: http://www.gardguide.com/images/5/5f/TheGlobalAcidRockDrainageGuide.pdf (accessed on 4 May 2018).
- Mauric, A.; Lottermoser, B.G. Phosphate amendment of metalliferous waste rocks, Century Pb–Zn mine, Australia: Laboratory and field trials. Appl. Geochem. 2011, 26, 45–56. [Google Scholar] [CrossRef]
- Meek, F.A. Research into the Use of Apatite Rock for Acidic Drainage Prevention. Available online: https://wvmdtaskforce.files.wordpress.com/2015/12/84-meek.pdf (accessed on 3 May 2018).
- Meek, F.A. Assessment of Acid Preventative Techniques Employed at the Island Creek Mining Company Tenmile Site. Available online: https://wvmdtaskforce.files.wordpress.com/2015/12/91-meek.pdf (accessed on 3 May 2018).
- Spotts, E.; Dollhopf, O.J. Evaluation of phosphate materials for control of acid production in pyritic mine overburden. J. Environ. Qual. 1992, 21, 627–634. [Google Scholar] [CrossRef]
- Renton, J.J.; Stiller, A.H.; Rymer, T.E. Use of phosphate materials as ameliorants for acid mine drainage. J. Am. Soc. Min. Reclam. 1987, 67–75. [Google Scholar] [CrossRef]
- Stiller, A.; Renton, J.; Rymer, T.E. An experimental evaluation of the use of rock phosphate (apatite) for the amelioration of acid-producing coal mine waste. Min. Sci. Technol. 1989, 9, 283–287. [Google Scholar] [CrossRef]
- Hart, W.; Stiller, A.; Rymer, T.; Skousen, J.; Sencindiver, J.; Samuel, D. The use of phosphate refuse as a potential AMD ameliorant. In Proceedings of the Mining and Reclamation Conference and Exhibition, Charleston, WV, USA, 23–26 April 1990; West Virginia University Publications Service: Morgantown, WV, USA, 1990; pp. 43–49. [Google Scholar]
- Ziemkiewicz, P. Advances in the prediction and control of acid mine drainage. In Proceedings of the Mining and Reclamation Conference and Exhibition, Charleston, WV, USA, 23–26 April 1990; pp. 51–54. [Google Scholar]
- Georgopoulou, Z.; Fytas, K.; Soto, H.; Evangelou, B. Feasibility and cost of creating an iron-phosphate coating on pyrrhotite to prevent oxidation. Environ. Geol. 1996, 28, 61–69. [Google Scholar] [CrossRef]
- Belzile, N.; Maki, S.; Chen, Y.-W.; Goldsack, D. Inhibition of pyrite oxidation by surface treatment. Sci. Total Environ. 1997, 196, 177–186. [Google Scholar] [CrossRef]
- Evangelou, V.P. Pyrite Oxidation and its Control; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Olson, G.J.; Clark, T.R.; Mudder, T.I.; Logsdon, M. Toward source control of acid rock drainage. In Proceedings of the Seventh International Conference on Acid Rock Drainage, St. Louis, MO, USA, 2006; Barnhisel, R.I., Ed.; American Society of Mining and Reclamation: Lexington, KY, USA, 2006; pp. 1435–1452. [Google Scholar]
- Kalin, M. Ecological Engineering and Biological Polishing: Methods to Economize Waste Management in Hard Rock Mining. In Ecological Engineering: An Introduction to Ecotechnology; Mitsch, W.J., Jorgensen, S.E., Eds.; Wiley & Sons, Inc.: New York, NY, USA, 1989; pp. 443–461. ISBN 0-471-62559-0. [Google Scholar]
- Wilson, G.W. Why are we still struggling with acid rock drainage? Geotech. News 2008, 26, 52–56. [Google Scholar]
- Kuenzer, C.; Stracher, G.B. Geomorphology of coal seam fires. Geomorphology 2012, 138, 209–222. [Google Scholar] [CrossRef]
- Rosenblum, G.; Finch, J.A.; Waters, K.E.; Nesset, J.E. A test apparatus for studying the effects of weathering on self-heating sulphides. In Proceedings of the Conference of Metallurgists, Canadian Institute of Mining, Metallurgy and Petroleum, Toronto, ON, Canada, 23–26 August 2015; p. 9. [Google Scholar]
- Lüttge, A.; Arvidson, R.S. The mineral-water interface. In Kinetics of Water-Rock Interaction; Brantley, S.L., Kubicki, J.D., White, A.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 73–107. [Google Scholar]
- Bellenberg, S.; Kalin, M.; Sand, W. Microbial Community Composition on Lignite before and after the Addition of Phosphate Mining Wastes. Adv. Mater. Res. 2013, 825, 42–45. [Google Scholar] [CrossRef]
- Erwin, D.H. Macroevolution of ecosystem engineering, niche construction and diversity. Trends Ecol. Evol. 2008, 23, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Laland, K.; Mathews, B.; Feldman, M.W. An introduction to niche construction theory. Evol. Ecol. 2016, 30, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Scott, D. Gibraltar’s dump leaching—An insight into acid effluent control. North. Min. 1991, 77, 1–7. [Google Scholar]
- Kalin, M.; Harris, B. Chemical precipitation within pyritic waste rock. Hydrometallurgy 2005, 78, 213–229. [Google Scholar] [CrossRef]
- Kalin, M.; Ferris, G.; Paulo, C. Reducing sulphide oxidation in pyritic mining wastes-phosphate mining wastes stimulate biofilm formation on mineral surface. In Proceedings of the “Securing the Future/8th ICARD”, Skellefteå, Sweden, 23–26 2009. [Google Scholar]
- Ueshima, M.; Fortin, D.; Kalin, M. Development of iron-phosphate biofilms on pyritic mine waste rock surfaces previously treated with natural phosphate rocks. Geomicrobiol. J. 2004, 21, 313–323. [Google Scholar] [CrossRef]
- AMIRA. 2017. Available online: http://www.amira.com.au/web/site.asp?section=projects&page=projectdetails&ProjectLink=2861&Source_ID=1 (accessed on 3 May 2018).
- Kalin, M.; Paulo, C.; Smart, R.; Wheeler, W. Phosphate mining reduces microbial oxidation of sulphidic minerals: A proposed mechanism. In Proceedings of the International Conference on Acid Rock Drainage, Ottawa, QC, Canada, 20–26 May 2012; pp. 585–593. [Google Scholar]
- Barley, S.; Meeuwig, J. The Power and the Pitfalls of Large-scale, Un-replicated Natural Experiments. Ecosystems 2017, 20, 1–9. [Google Scholar] [CrossRef]
- Kalin, M.; Smith, M.P.; Fyson, A. The use of natural phosphate rock to reduce drainage from pyritic waste rock. In Proceedings of the International Conference on Acid Rock Drainage, Vancouver, BC, Canada, 31 May–6 June 1997. [Google Scholar]
- Kalin, M.; Smith, M.P.; Fyson, A. The Role of Phosphate in Applied Biotechnology in Mine Waste Management: Reduction in AMD from Pyritic Waste Rock. In Proceedings of the International Symposium, the Metallurgical Society of the Canadian Institute for Mining, ‘Waste Processing and Recycling’, Calgary, AB, Canada, 16–19 August 1998; pp. 15–29. [Google Scholar]
- Kalin, M. Improving pore water quality in reactive tailings with phosphate mining wastes. In Proceedings of the “The Conference of Metallurgists”, COM 2004, Materials: The future of manufacturing in a sustainable environment, Hamilton, ON, Canada, 22–25 August 2004; pp. 427–437. [Google Scholar]
- Kalin, M.; Wheeler, W.N. A review of the role of phosphate mining waste: A chemical or biological reagent for AMD prevention. In Proceedings of the Twelfth Annual West Virginia Surface Mine Drainage Task Force Symposium, Morgantown, WV, USA, 29–30 March 2011; pp. 1–12. [Google Scholar]
- Kalin, M.; Paulo, C.; Sleep, B. Proactive prevention of acid generation: Reduction/inhibition of sulphide oxidation. In Proceedings of the International Mine Water Association Symposium, Sydney, NS, Canada, 5–9 September 2010; pp. 479–482. [Google Scholar]
- Kalin, M.; Fyson, A.; Smith, M.P.; Werker, A. Tailings surface cover development through integration of reactive phosphate and organic matter. In Proceedings of the Mining and Environment III and Annual Meeting of the CLRA, Sudbury, ON, USA, 25–28 May 2003. [Google Scholar]
- Kalin, M.; Paulo, C.; Sudbury, M.P.; Wheeler, W.N. Reducing sulphide oxidation in mining wastes by recognizing the geomicrobiology role of phosphate mining wastes. J. Am. Soc. Min. Reclam. 2015, 4, 102–121. [Google Scholar] [CrossRef]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef] [PubMed]
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Kolenbrander, P.E.; Andersen, R.N.; Moore, L.V. Intrageneric coaggregation among strains of human oral bacteria: Potential role in primary colonization of the tooth surface. Appl. Environ. Microbiol. 1990, 56, 3890–3894. [Google Scholar] [PubMed]
- Dang, H.; Lovell, C.R. Bacterial Primary Colonization and Early Succession on Surfaces in Marine Waters as Determined by Amplified rRNA Gene Restriction Analysis and Sequence Analysis of 16S rRNA Genes. Appl. Environ. Microbiol. 2000, 66, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Sand, W.; Bock, E. Concrete corrosion in the Hamburg Sewer system. Environ. Technol. Lett. 1984, 5, 517–528. [Google Scholar] [CrossRef]
- Kemmling, A.; Kämper, M.; Flies, C.; Schieweck, O.; Hoppert, M. Biofilms and extracellular matrices on geomaterials. Environ. Geol. 2004, 46, 429–435. [Google Scholar] [CrossRef]
- Kip, N.; van Veen, J.A. The dual role of microbes in corrosion. ISME J. 2015, 9, 542–551. [Google Scholar] [CrossRef] [PubMed]
- Gorbushina, A.A.; Broughton, W.J. Microbiology of the atmosphere-rock interface: How biological interactions and physical stresses modulate a sophisticated microbial ecosystem. Annu. Rev. Microbiol. 2009, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.C.; Wingender, J. The biofilm matrix. Nat. Rev. Microbiol. 2010, 8, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Nordstrom, D.K.; Alpers, C.N. Geochemistry of acid mine waters. Rev. Econ. Geol. 1999, 6, 133–160. [Google Scholar]
- Bennett, J.C.; Tributsch, H. Bacterial leaching patterns on pyrite crystal surfaces. J. Bacteriol. 1978, 134, 310–317. [Google Scholar] [PubMed]
- Farah, C.; Vera, M.; Morin, D.; Haras, D.; Jerez, C.A.; Guiliani, N. Evidence for a functional quorum-sensing type AI-1 system in the extremophilic bacterium Acidithiobacillus ferrooxidans. Appl. Environ. Microbiol. 2005, 71, 7033–7040. [Google Scholar] [CrossRef] [PubMed]
- Hammer, B.K.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae. Mol. Microbiol. 2003, 50, 101–104. [Google Scholar] [CrossRef] [PubMed]
- Parsek, M.R.; Greenberg, E.P. Sociomicrobiology: The connections between quorum sensing and biofilms. Trends Microbiol. 2005, 13, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Waters, C.M.; Lu, W.; Rabinowitz, J.D.; Bassler, B.L. Quorum sensing controls biofilm formation in Vibrio cholerae through modulation of cyclic di-GMP levels and repressio of vpsT. J. Bacteriol. 2008, 190, 2527–2536. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.; Bellenberg, S.; Mamani, S.; Ruiz, L.; Echeverria, A.; Soulère, L.; Doutheau, A.; Demergasso, C.; Sand, W.; Queneau, Y.; et al. AHL signaling molecules with a large acyl chain enhance biofilm formation on sulfur and metal sulfides by the bioleaching bacterium Acidithiobacillus ferrooxidans. Appl. Microbiol. Biotechnol. 2013, 97, 3729–3737. [Google Scholar] [CrossRef] [PubMed]
- Bellenberg, S.; Diaz, M.; Noel, N.; Sand, W.; Poetsch, A.; Guiliani, N.; Vera, M. Biofilm formation, communication and interactions of leaching bacteria during colonization of pyrite and sulfur surfaces. Res. Microbiol. 2014, 165, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Garrett, T.R.; Bhakoo, M.; Zhang, Z. Bacterial adhesion and biofilms on surfaces. Progress Nat. Sci. 2008, 18, 1049–1056. [Google Scholar] [CrossRef]
- Nöel, N.; Florian, B.; Sand, W. AFM & EFM study on attachment of acidophilic leaching organisms. Hydrometallury 2010, 104, 370–375. [Google Scholar]
- Bellenberg, S.; Barthen, R.; Boretska, M.; Zhang, R.; Sand, W.; Vera, M. Manipulation of pyrite colonization and leaching by iron-oxidizing Acidithiobacillus species. Appl. Microbiol. Biotechnol. 2015, 99, 1435–1449. [Google Scholar] [CrossRef] [PubMed]
- Gehrke, T.; Telegdi, J.; Thierry, D.; Sand, W. Importance of Extracellular Polymeric Substances from Thiobacillus ferrooxidans for Bioleaching. Appl. Environ. Microbiol. 1998, 64, 2743–2747. [Google Scholar] [PubMed]
- Gehrke, T.; Hallmann, R.; Kinzler, K.; Sand, W. The EPS of Acidithiobacillus ferrooxidans—A model for structure-function relationships of attached bacteria and their physiology. Water Sci. Technol. 2001, 43, 159–167. [Google Scholar] [PubMed]
- Harneit, K.; Göksel, A.; Kock, D.; Klock, J.H.; Gehrke, T.; Sand, W. Adhesion to metal sulphide surfaces by cells of Acidithiobacillus ferrooxidans, Acidithiobacillus thiooxidans and Leptospirillum ferrooxidans. Hydrometallurgy 2006, 83, 245–254. [Google Scholar] [CrossRef]
- Solari, J.A.; Huerta, G.; Escobar, B.; Vargas, T.; Badilla-Ohlbaum, R.; Rubio, J. Interfacial phenomena affecting the adhesion of Thiobacillus ferrooxidans to sulphide mineral surfaces. Colloids Surf. 1992, 69, 159–166. [Google Scholar] [CrossRef]
- Sampson, M.I.; Phillips, C.V.; Blake, R.C., II. Influence of the attachment of acidophilic bacteria during the oxidation of mineral sulfides. Min. Eng. 2000, 13, 373–389. [Google Scholar] [CrossRef]
- Schippers, A.; Jozsa, P.; Sand, W. Sulfur chemistry in bacterial leaching of pyrite. Appl. Environ. Microbiol. 1996, 62, 3424–3431. [Google Scholar] [PubMed]
- Barreto, M.; Gehrke, T.; Harneit, K.; Sand, W.; Jedlicki, E.; Holmes, D. Unexpected insights into biofilm formation by Acidithiobacillus Ferrooxidans revealed by genome analysis and experimental approaches. In Proceedings of the 16th International Biohydrometallurgy Symposium Compress, Cape Town, South Africa, 25–29 September 2005; pp. 817–825. [Google Scholar]
- Bellenberg, S.; Leon-Morales, C-F.; Sand, W.; Vera, M. Visualization of capsular polysaccharide induction in Acidithiobacillus ferrooxidans. Hydrometallurgy 2012, 129–130, 82–89. [Google Scholar] [CrossRef]
- Africa, C.-J.; Harrison, S.T.L.; Becker, M.; Hille, R.P. In situ investigation and visualisation of microbial attachment and colonisation in a heap bioleach environment: The novel biofilm reactor. Miner. Eng. 2010, 23, 486–491. [Google Scholar]
- Afzal Ghauri, M.; Okibe, N.; Johnson, D.B. Attachment of acidophilic bacteria to solid surfaces: The significance of species and strain variations. Hydrometallurgy 2007, 85, 72–80. [Google Scholar] [CrossRef]
- Bellenberg, S.; Florian, B.; Vera, M.; Rohwerder, T.; Sand, W. Comparative study of planktonic and sessile cells from pure and mixed cultures of Acidithiobacillus ferrooxidans and Acidiphilium cryptum growing on pyrite. Adv. Mater. Res. 2009, 71, 333–336. [Google Scholar] [CrossRef]
- Hallmann, R. Einfluss extrazellulärer, polymerer Substanzen von Leptospirillum ferrooxidans auf dessen Bioconenose mit Acidiphilium spec. RB8. Ph.D. Thesis, University of Hamburg, Hamburg, Germany, 1996. [Google Scholar]
- Caiazza, N.C.; O’Toole, G.A. Alpha-toxin is required for biofilm formation by Staphylococcus aureus. J. Bacteriol. 2003, 185, 3214–3217. [Google Scholar] [CrossRef] [PubMed]
- McDougald, D.; Rice, S.A.; Barraud, N.; Steinberg, P.D.; Kjelleberg, S. Should we stay or should we go: Mechanisms and ecological consequences for biofilm dispersal. Nat. Rev. Microbiol. 2012, 10, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, D.; McDermott, F.; Clipson, N. Understanding microbially active biogeochemical environments. Adv. Appl. Microbiol. 2007, 62, 81–104. [Google Scholar] [PubMed]
- Xavier, J.B.; Foster, K.R. Cooperation and conflict in microbial biofilms. Proc. Natl. Acad. Sci. USA 2007, 104, 876–881. [Google Scholar] [CrossRef] [PubMed]
- De Beer, D.; Stoodley, P.; Roe, F.; Lewandowski, Z. Effects of biofilm structures on oxygen and mass transport. Biotechnol. Bioeng. 1994, 43, 1131–1138. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).