Abstract
In this study, four samples of recycled aggregates from the construction and demolition waste of Mexico City were characterized in order to find innovative uses for these types of materials. Gravel and sand from a recycling plant were analyzed, as well as aggregates produced in the laboratory from demolished concrete collected from landfills. The characterization was carried out by means of XRD (X-ray Diffraction), chemical microanalysis (EDS), X-ray fluorescence (XRF), pH measurement, and sieve analysis. The minerals present in the analyzed materials were feldspars, cristobalite and pyroxene, which corresponded to the natural aggregates, as well as variable amounts of calcite, a product of the carbonation of the cement paste adhered to these aggregates, and in a smaller proportion, calcium hemicarboaluminate, rosenhanite, and tobermorite. The quality (amount of cement) of the original concrete has a great influence on the granulometry and the chemical–mineralogical composition of the aggregates, since there will be different quantities and qualities of the cement paste adhered to the aggregates depending on their size. Finally, the pH values measured in all samples fluctuated between 10.15 and 12.08, suggesting that these materials can be used in soil stabilization or in agricultural applications.
1. Introduction
According to the Mexico City NADF-007-RNAT-2013 [1] environmental norm, the amount of construction and demolition waste (CDW) generated by the city is approximately 7000 tons/day; 25% of such waste consists of demolished concrete that is usually disposed in illegal landfills, despite the norm recommending that these materials have to be recycled through a process involving selection, crushing, sieving, and storage in order to reuse them in the construction cycle.
Numerous definitions have been suggested for these recycled materials. This study used the definition proposed by Zhao et al. [2] who defined recycled concrete aggregates as an intimate mix between original natural aggregates (gravel and sand) and hardened cement paste adhered to them.
The environmental benefits of the use of recycled aggregates can play a key role in reducing the need for landfill waste disposal, and limiting the exploitation of natural aggregates [3]. In Mexico City, Rivera-Mera [4] characterized recycled aggregates of the Concretos Reciclados S.A. plant from a physical viewpoint, considering the regulations for roads established by the Mexican Transportation Institute (IMT). Although these aggregates complied with these regulations, there are no mineralogical and chemical studies to further constrain the use of these recycled aggregates in geotechnical applications such as sub-bases, bases, embankments, fillings, etc., in the manufacture of concrete or in other applications.
Hence, the objective of this work is to characterize the mineralogy and chemical composition of four samples of recycled aggregates of demolished concrete from Mexico City, to demonstrate the possibility of using this recycled material, and eventually generating new market opportunities [5]. Sand and gravel were sampled from Concretos Reciclados S.A., a plant located in Iztapalapa (Mexico City) as well as from sand and gravel crushed and sieved in the laboratory, collected from illegal landfills located to the north of the city.
2. Experimental Procedure
2.1. Materials
Plant-recycled aggregates were obtained by sampling materials directly from the processed mounds (¼“-grain to fine grain sand and 1” gravel) formed by the McCloskey movable recycling unit, model I44R (McCloskey International, Peterborough, ON, Canada) at the Concretos Reciclados plant (Figure 1a). On the other hand, laboratory-recycled aggregates were obtained by crushing and sieving demolished concrete collected from two illegal landfills (empty lots) located to the north of Mexico City (Figure 1b). Only simple concrete fragments without surface coatings were collected by manual selection. Table 1 shows the sample quantities that were used in this study.

Figure 1.
(a) Mounds of recycled gravel at the Concretos Reciclados S.A. plant; (b) a landfill containing construction and demolition waste (CDW) where the demolished concrete was collected.

Table 1.
Recycled concrete samples obtained in Mexico City.
To produce the laboratory-recycled aggregates, fragments of demolished concrete collected from the landfills were processed using a laboratory jaw crusher (Allis Mineral System, York, PN, USA) and were manually classified using #4 mesh ASTM (4.75 mm) to separate the gravel from the sand.
In order to obtain adequate samples of the size required for the tests, the aforementioned recycled aggregates (gravel and sand) were homogenized and reduced manually as per the NMX C-170-ONNCCE-1997 norm [6]. This norm, which does not coincide with any international standard, provides specifications for the reduction of aggregate samples obtained in the field to the size required for tests.
2.2. Methods
2.2.1. Granulometric Characterization
To determine the size of the recycled gravel and sands, samples of 10 kg for coarse aggregates and 1 kg for fine aggregates [7], were dried at 100 °C to a constant weight prior to sieving. The size distribution was analyzed in accordance with the NMX C-077-ONNCCE-1997 norm [8] (EN 933-1:1997), which describes sieve analyses and test methods for concrete aggregates.
2.2.2. Chemical and Mineralogical Characterization
These analyses were carried out using representative samples of the recycled sand and gravel (plant and laboratory), which were ground until 100% of the sample passed through the #200 ASTM (<75 μ) mesh and dried at 100 °C for 6 h in a laboratory oven with a digital controller.
The mineralogical composition was determined by X-ray diffraction (XRD) using an Equinox 2000 diffractometer (INEL, Artenay, France) with CoKα1 radiation. Phase identification was based on the COD Inorganics 2015 and Cements 2014 databases included in the Crystallography Open Database; Match! software was used in this procedure (v.1.10, Crystal Impact, Bonn, Germany).
Different techniques were used for the chemical characterization. Representative samples were examined through energy-dispersive X-ray microanalysis (EDS) using a JEOL scanning electron microscope model JSM-IT300 (JEOL Ltd., Tokyo, Japan) and an OXFORD X-ray detector (OXFORD Instrument, Oxfordshire, UK) using an acceleration voltage of 30 kV.
In order to obtain a representative (bulk) analysis, the dry powders of the samples were placed on the specimen stub in uniform layers; quantitative routines were performed over large scanning areas (4.75 mm2).
It should be made clear that even though EDS (micro-chemical method) is not appropriate for assessing bulk composition, it is useful, and may lead to validation of the XRD results.
In addition, X-ray fluorescence (XRF) chemical analysis was carried out by a BRUKER handheld XRF spectrometer, model S1 TITAN (BRUKER, Kennewick, WA, USA).
To complement the results of the chemical analysis, pH measurements were made at room temperature with an OAKTON 700 series pH meter (OAKTON, Vernon Hills, IL, USA) equipped with an OAKTON electrode. For this purpose, 10 g of pulverized recycled aggregates was mixed with 20 mL of distilled water; this mixture was stirred with a glass rod for several minutes and then the pH measurement was made.
3. Results and Discussion
3.1. Particle Size Distribution
The granulometric curves were determined for both types of recycled aggregates (plant and laboratory) and were graphed considering the granulometric limits established in the NMX C-111-ONNCCE-2014 standard [9] (EN 12620:2008), which provides specifications and test methods for hydraulic concrete aggregates in the construction industry. The main objective of this granulometric analysis was to understand the size distribution of the aggregates and relate it to the chemical analysis.
As already stated, the objective is to find alternative uses (nonstructural applications) for these aggregates. Given that in the literature there are many works about the incorporation of recycled aggregates into new concrete mixtures (structural applications), this indicates that it is possible to replace up to 50% of natural gravel with recycled one from demolished concrete, without compromising the quality of the concrete [10].
Figure 2 shows that both plant-recycled gravel and laboratory-recycled gravel have a maximum nominal size of 25 mm. However, in the plant aggregate curve, a deficiency of particles less than 10 mm was observed, mirroring a high resistance to fragmentation of the plant aggregates obtained from high-resistance or reinforced concretes. The laboratory-recycled gravel curve is similar to the former one, with a similar slope, but shifted towards tinier clast size, i.e., it is richer in fine grains than plant CDW (Figure 2). This probably reflects that illegal CDW comes from low-quality concrete, which is relatively easy to grind.

Figure 2.
Recycled gravel particle size distribution; dotted lines indicate granulometric limits per the NMX C-111-ONNCCE-2014 standard [9].
These results are in line with a study by Kobayashi and Kawano [11], who determined that aggregates recycled from high-resistance concrete presented higher granulometric modules when compared with lower resistance concretes.
In addition, it must be highlighted that a larger number of particles (fragments) composed entirely of mortar (sand and cement paste) were readily apparent in plant-recycled gravel, in contrast to illegal CDW, where original gravel or rock are evident (Figure 3) [12]; again, high-quality demolished concrete has a high amount of cement and sand aggregate. The interpretation of these results suggests the influence of the quality of the original concrete on the granulometry of the recycled gravel.

Figure 3.
(Top) Plant-recycled gravel showing fragments entirely composed of mortar (sand and cement paste). (Bottom) Laboratory-recycled gravel with aggregates composed of rock with adhered mortar.
Figure 4 compares the sieving results of sands recycled in the plant and in the laboratory. The graph shows that both sands failed to meet the Mexican norm, although their curves displayed continuous behavior and lacked sudden changes of slope. In both sands, a scarcity of particles close to 1 mm was evident. This behavior could be attributed to the fact that the cement paste and the mortar (cement paste and micro aggregates) adhered to millimeter particles, increasing the retention in meshes of greater aperture. This trend was also observed by Bianchini et al. [13], who analyzed different size fractions of recycled fine aggregates by XRF, and determined that there was a high CaO content in fractions sized between 2 and 4 mm.

Figure 4.
Recycled sand particle size distribution; dotted lines indicate granulometric limits per the NMX C-111-ONNCCE-2014 standard [9].
Curiously, plant-recycled sand presented a higher content of fine particles (<75 µ) than laboratory-recycled sand, which contradicts the argument that when the quality of the demolished concrete is high, the recycled aggregates will be larger and more resistant due to the resistance of adhered material. However, the plant-processed sands underwent a more complex grinding and sieving process entailing higher fragmentation as a result of agitation and transportation [14], as opposed to laboratory sands, which were crushed in a single cycle and sieved by hand.
The recycled sands (plant and laboratory) do not comply with the size distribution required to make concrete, but even when they did meet the tolerances indicated, there is a consensus in the literature that the use of recycled sand in concrete production has a negative effect, inducing high water absorption and low mechanical properties [15].
3.2. Mineralogical Composition
Figure 5 and Figure 6 illustrate the XRD results from the gravels and sands recycled in the laboratory and in the industrial plant. All of these patterns are very similar to each other, showing peaks and reflection overlapping typical of multiphase mixtures.
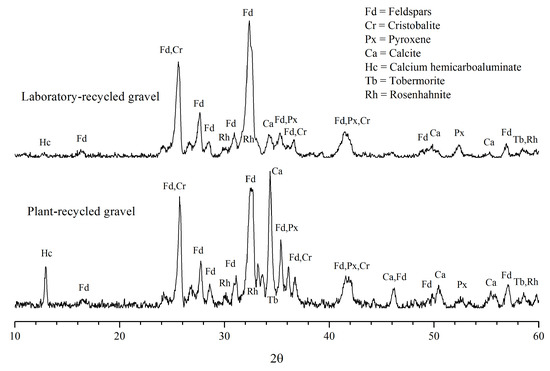
Figure 5.
X-ray diffraction patterns in the recycled gravel samples.
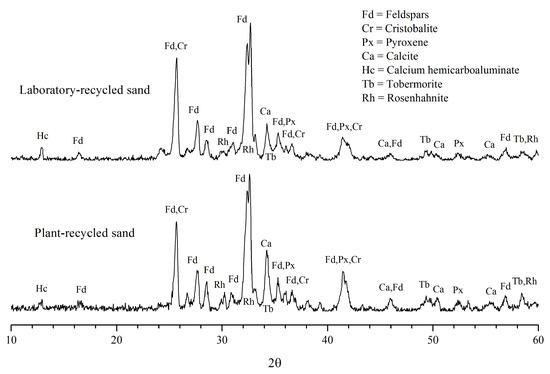
Figure 6.
X-ray diffraction patterns in the recycled sand samples.
Phase identification was carried out automatically by Match! Software, identifying the presence of different amounts of crystalline phases that corresponded to the original aggregates and to the cement paste adhered to them.
3.2.1. Minerals of the Original Aggregates
Normally, aggregates represent around 70–80% of the components of concretes [16], therefore the abundant minerals in all of the analyzed samples (plant and laboratory) belonged to the primary aggregates; here, we identified feldspars such as plagioclases (Na and Ca feldspars) and anorthoclase (Na and K feldspars).
The widening of the feldspar peaks (Fd in Figure 5 and Figure 6) is attributable to the presence of close XRD reflections of similar crystallographic planes of different feldspar minerals.
Besides feldspars, all the samples contained small proportions of cristobalite and pyroxene. These three mineral species are common constituents of rocks; therefore, they are assumed to correspond to the original rock aggregates, in both the laboratory and at the plant.
The minerals identified in all recycled aggregate samples reflected the local geological context, which suggests that these recycled aggregates contained rock materials extracted in the region [17]. According to the Mexico City geological and mining chart [18], there are numerous andesite–dacite, andesite, and basaltic andesite rocks, rich in feldspar, pyroxene and cristobalite, from which gravel is extracted.
3.2.2. Minerals of the Adhered Cement Paste
According to peak intensity, calcite (CaCO3) appears in medium to minor proportions in all of the studied recycled aggregates; faint diffraction peaks of calcium hemicarboaluminate and crystalline hydrated calcium silicates such as rosenhanite and tobermorite have also been detected (Figure 5 and Figure 6).
Limestone aggregate is not used in the region. Therefore, the significant presence of calcite is predominantly due to the carbonation of cement hydration products in the paste adhered to recycled aggregates, which consists mainly of portlandite Ca(OH)2 and C-S-H hydrated calcium silicates (CaO-SiO2-H2O). These phases are carbonated as described by Reactions (1) and (2) [19]:
Ca(OH)2 + CO2 → CaCO3 + H2O
C-S-H + CO2 → CaCO3 + SiO2 ∙ nH2O
It is important to note that no portlandite Ca(OH)2 peaks were detected in any of the analyzed materials, although it should be present according to Liu et al. [20], who determined that calcium hydroxide Ca(OH)2 undergoes accelerated carbonation when the samples are ground into fine particles for analysis. During this grinding process, the Ca(OH)2 is released from the cement paste and is exposed directly to the CO2 in the air, therefore part of the CaCO3 detected in the diffractogram belongs to the carbonate generated after the grinding necessary for the powder diffraction technique. Mymrin et al. [21], mentioned that the peaks of portlandite Ca(OH)2 became lower than the detection limit of the XRD method (approximately 5%), in the same way, Evangelista et al. [14] indicated that concrete waste presents low Ca(OH)2 amounts (below 2.6 wt %).
It should be emphasized that an unknown amount of calcite is generated by the carbonation of the hardened cement paste during the useful life of the original concrete, and during the storage time of recycled concrete. In addition to carbonation, the composition can include a minor portion of CaCO3 from the limestone filler used as a partial cement replacement [22]. Limestone fillers are generally added directly at the cement production plant [23].
On the other hand, the calcium hemicarboaluminate detected was a phase formed in the first stages of the carbonation of cementitious materials [24]. Specifically, it is the product of the decomposition of calcium monosulfoaluminate [25], a minor component of cement paste.
Another tentative explanation for the presence of this compound is that it was formed during the cement hydration due to the amount of CaCO3 used as a filler in cement production [26,27]. The calcium carbonate in the form of pulverized limestone, which is an additive allowed in the production of Portland cement, favors the formation of calcium hemicarbolauminate during the hydration of cement [26]. In this case, it is possible that the mineral had been in the concrete before the carbonation and recycling process.
Regarding the identification of the crystalline calcium-silicate-hydrates (C-S-H), rosenhahnite and tobermorite, although the main peaks of these species were not evident, as they are overlapped by the intense peaks of the feldspars and calcite, they were identified by their secondary peaks close to 60° (Figure 5 and Figure 6). These minerals, resulting from the cement hydration reactions, were found in small proportions in the four samples, in agreement with Mymrin et al. [21], who stated that tobermorite is an inevitable component of concretes.
It should be mentioned that none of the diffraction patterns presented the typical signal (wide bands) of the amorphous C-S-H gel, nor of the semicrystalline C-S-H, whose signal is a double peak close to 30° [28]. These results agreed with those obtained by Evangelista et al. [14], who did not detect the presence of C-S-H by this technique.
Likewise, when visually comparing the diffractograms of the four samples analyzed (Figure 5 and Figure 6), it is noticeable that the diffraction peaks of calcite are more intense (high) in the recycled gravel sample from the Concretos Reciclados plant. This reflects a greater quantity of fragments formed entirely by mortar; hence, they will have a greater quantity of carbonated cement paste. This abundance of calcite was not observed in laboratory-recycled gravels as only simple concrete was collected during the sampling in the landfills (which may have a lower content of hardened cement paste), in contrast to the gravel sample from the plant, which contained fragments from reinforced concrete of higher quality, i.e., with more cement.
3.3. Chemical Composition and pH
Figure 7 and Table 2 report the results of the SEM-EDS analysis; scanned areas and elemental chemical analysis are expressed in percentage by weight to assess whether the compositional variation was consistent with the results of XRD.
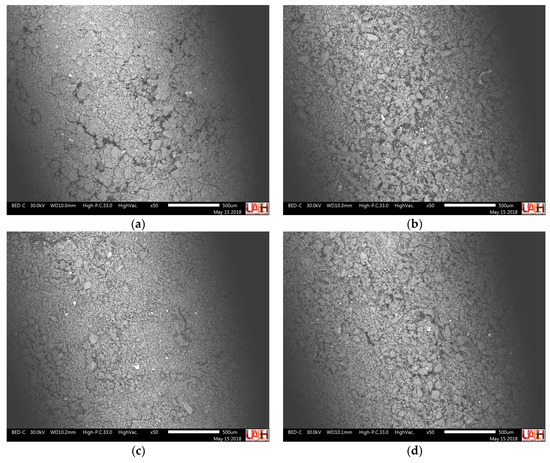
Figure 7.
SEM images showing areas of EDS analysis in the recycled aggregate samples: (a) plant-recycled gravel; (b) plant-recycled sand; (c) laboratory-recycled gravel; (d) laboratory-recycled sand.

Table 2.
Results of the chemical analyses by EDS of the recycled aggregate samples expressed as a weight percentage.
Figure 7 shows SEM images of scanned areas in the EDS analysis; these micrographs do not provide information on the morphology of the samples of recycled aggregates, because the particles have very small dimensions (<75 μ) that are only observed at high magnification. However, the images reveal a uniform distribution of the fine powders, a necessary condition for a representative analysis of the samples.
The data from the microanalysis (Table 2) showed that the variation in the weight percentage of calcium, attributed mostly to the cement paste adhered to the gravel and recycled sand, was consistent with the intensity of the calcite peaks in XRD. In other words, higher Ca percentages in the chemical analysis (EDS) corresponded to more intense calcite peaks in the diffractogram, and vice versa.
On the other hand, the amount of silicon remained the same, as did the intensity of the diffraction peaks of the feldspars (silicon-rich), with no significant changes in the four samples. Therefore, it can be affirmed that from a chemical–mineralogical viewpoint, the recycled aggregates had a composition inherent to their origins (igneous), which was enriched with calcium by the amount of adhered mortar.
Only main oxides of the XRF characterization are shown (Table 3), because the purpose of using this technique (bulk chemical analysis) is to enable a level of validation of the EDS and XRD results by comparing them with an independent chemical analysis. The content of minor and trace elements is not discussed in this paper.

Table 3.
X-ray fluorescence (XRF) chemical analysis of the recycled aggregate samples expressed as a weight percentage (main oxides).
XRF results are consistent with chemical information provided by EDS. To give an example, Ca (EDS) and CaO (XRF) contents, which originate from the binder, are well correlated, and it can be seen that the Ca concentration in plant-recycled gravel is twice that of the concentration in laboratory-recycled gravel (Table 2). The same relation is observed in the CaO contents measured by XRF (Table 3).
In summary, the chemical characterization (EDS and XRF) reveals the variation in content of calcium compounds between the four samples.
From another perspective, to adequately address the pH measurement section, it was necessary to consider that a non-carbonated concrete had a pH value of approximately 12.5, mainly due to the presence of calcium hydroxide formed during the process of cement hydration, and this value decreased below 9.5 during carbonation [29].
In the same way, it should be considered that when talking about the pH of the concrete, in reality, this refers to the pH of the solution that fills the pores in the cement paste, so that the dissolved species in that solution can only come from the cement paste since the aggregates by themselves are considered inert.
Taking into account the above, the pH measurements of the pulverized samples of recycled aggregates mixed with water (Table 4) demonstrated the alkaline character of these materials with values ranging from a very alkaline pH of 12.08 for the plant-recycled gravel sample to 10.15 for the laboratory-recycled gravel sample.

Table 4.
pH values measured in water from the samples of the recycled aggregates.
There are several possible explanations for the high alkalinity of the four samples of recycled aggregates, which were considered to be mainly carbonated according to the diffraction data. The first reason is that there were soluble alkalis (Na and K) and quantities of portlandite Ca(OH)2 below the limit of detection by XRD that dissolved and released Ca2+ and OH− ions, thus maintaining a high pH. Although it is not possible to identify the presence of portlandite by XRD, it is here finally assumed its presence considering this very high pH in all of the CDW.
The second possible explanation is that in addition to the dissolution of alkalis and Ca(OH)2, the dissolution/release of Ca ions and hydroxyls present in the calcium hemicarboaluminate Ca4Al2(CO3)0.5(OH)13∙5.5H2O also occurred [30]. The increase in surface area that the sample undergoes when ground for the experiment-facilitates its dissolution.
The pH values correlate well with the intensity of the calcium hemicarboaluminate diffraction peaks in the four samples (Figure 5 and Figure 6); in other words, the samples with the highest amount of hemicarboaluminate presented a high pH.
In general, the pH data suggested that a recycled aggregate with a greater amount of cement paste will be more reactive when mixed with water, because the concentration of Ca2+ and OH− will be increased by the dissolution/release of these ions present in certain phases of the hydrated cement (portlandite and possibly calcium hemicarboaluminate).
Finally, although the present study was only based on two types of aggregates (recycled in the laboratory and in an industrial plant), the results contribute to the use of recycled aggregates in different applications according to their chemical and mineralogical composition. In this case, considering the amount of Ca in the chemical analysis and the alkaline pH, it is suggested that these materials (especially the plant-recycled gravel) could be used in geotechnical applications such as the stabilization of clay soils, after accounting for the fact that the alteration of clay minerals in an alkaline medium occurs at pH values of 11.7 [31]. Furthermore, other uses might be found in agricultural applications, for example as permeable layers in stables, or as basal layers for hydroponic systems.
4. Conclusions
The mineralogical characterization by XRD revealed that the minerals present in the recycled aggregates analyzed were feldspars, cristobalite, and pyroxene (silicon-rich minerals), which corresponded to the original rock aggregates, as well as variable amounts of calcite (CaCO3), a product of the carbonation of the cement paste adhered to these aggregates, and smaller proportions of calcium hemicarboaluminate, rosenhahnite, and tobermorite, phases that are also composed of the agglutinating matrix.
Chemical characterization hints that only Ca varies significantly in all of the samples, according to variable amounts of the cement paste. Therefore, from a chemical–mineralogical viewpoint, it can be affirmed that the recycled aggregates possessed a composition inherent to their igneous origin, which was enriched with calcium by the amount of the adhered mortar.
The quality (quantity of cement) of the original concrete has a great influence on the granulometry and the chemical–mineralogical composition of the recycled aggregates, since there will be different quantities and qualities of cement paste adhered to these aggregates depending on their size. For example, the proportion of calcium increased in plant-recycled gravel, as its resistant cement paste did not easily fragment. Consequently, aggregates formed entirely by mortar were produced during recycling.
Finally, the alkaline pH values (above 10) in all of the recycled aggregate samples indicated that there were enough soluble species to maintain an alkaline pH even though the cement paste adhered to these aggregates was mostly carbonated. For this reason, it is suggested that these materials could be used in geotechnical applications such as the stabilization of clay soils or in agricultural applications, which would represent innovative applications for these types of materials.
Author Contributions
Conceptualization, E.M.-P., and J.H.-A.; Methodology, E.M.-P., and J.H.-A.; Investigation, E.M.-P., Y.R.-M., A.A.-F., and E.C.-S.; Writing-Original Draft, E.M.-P., J.H.-A., M.I.R.-V., and E.S.-R.; Writing-Review and Editing, E.M.-P., J.H.-A., and E.S.-R.
Acknowledgments
The authors would like to thank SEP-PRODEP. The first author also thanks to CONACyT for Doctoral Scholarship awarded (Grant 388858). Yamile Rangel Martínez would like to thank the Concretos Reciclados Company for supplying recycled aggregates.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Norma Ambiental NADF-007-RNAT-2013. Que Establece la Clasificación y Especificaciones de Manejo Para Residuos de la Construcción y Demolición, en el Distrito Federal. Secretaria del Medio Ambiente del Distrito Federal. Available online: www.ordenjuridico.gob.mx (accessed on 19 July 2016).
- Zhao, Z.; Remond, S.; Damidot, D.; Xu, W. Influence of fine recycled concrete aggregates on the properties of mortars. Constr. Build. Mater. 2015, 80, 179–186. [Google Scholar] [CrossRef]
- Blengini, G.A.; Garbarino, E. Resources and waste management in Turin (Italy): The role of recycled aggregates in the sustainable supply mix. J. Clean. Prod. 2010, 18, 1021–1030. [Google Scholar] [CrossRef]
- Rivera-Mera, C.J. Análisis de Impacto Ambiental Por la Inadecuada Disposición de Residuos de la Construcción y Demolición en el Valle de México y Propuestas de Solución. Ph.D. Thesis, Facultad de Ingeniería, UNAM, México City, México, 2007. [Google Scholar]
- Cardoso, R.; Vasco Silva, R.; de Brito, J.; Dhir, R. Use of recycled aggregates from construction and demolition waste in geotechnical applications: A literature review. Waste Manag. 2016, 49, 131–145. [Google Scholar] [CrossRef] [PubMed]
- Norma Mexicana NMX C-170-ONNCCE-1997. Agregados-Reducción de las Muestras de Agregados Obtenidos en el Campo al Tamaño Requerido de las Pruebas. Organismo Nacional de Normalización y Certificación de la Construcción y Edificación, S.C. Available online: www.onncce.org.mx (accessed on 18 February 2017).
- LS-602, (2001), Method of Test for Sieve Analysis of Aggregates. Ministry of Transportation, Ontario, Laboratory Testing Manual. Available online: www.roadauthority.com (accessed on 6 May 2017).
- Norma Mexicana NMX C-077-ONNCCE-1997. Agregados Para Concreto, Análisis Granulométrico, Métodos de Prueba. Organismo Nacional de Normalización y Certificación de la Construcción y Edificación, S.C. Available online: www.onncce.org.mx (accessed on 18 February 2017).
- Norma Mexicana NMX C-111-ONNCCE-2014. Agregados Para Concreto Hidráulico, Análisis Granulométrico, Métodos de Prueba. Organismo Nacional de Normalización y Certificación de la Construcción y Edificación, S.C. Available online: www.onncce.org.mx (accessed on 18 February 2017).
- Gonzalez-Fonteboa, B. Hormigones con áridos reciclados procedentes de demoliciones: Dosificaciones, propiedades mecánicas y comportamiento estructural a cortante. Ph.D. Thesis, Departamento de Tecnología de la Construcción, Universidad Da Coruña, A Coruña, España, 2002. [Google Scholar]
- Kobayashi, S.; Kawano, H. Properties and Usage of Recycled Aggregate Concrete. Demolition and Reuse of Concrete and Masonry: Reuse of Demolition Waste; Chapman and Hall: London, UK, 1988; pp. 547–556. [Google Scholar]
- Vázquez, E.; Barra, M. Reciclaje y Reutilización del Hormigón. In Monografía CIMNE No. 67: Desarrollo Sostenible del Cementy del Hormigón; Gettu, R., Ed.; International Center for Numerical Methods in Engineering: Barcelona, Spain, 2002; pp. 43–65. [Google Scholar]
- Bianchini, G.; Marrocchino, E.; Tassinari, R.; Vaccaro, C. Recycling of construction and demolition waste materials: A chemical-mineralogical appraisal. Waste Manag. 2005, 25, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Guedes, M.; De Brito, J.; Ferro, A.C.; Pereira, M.F. Physical, chemical and mineralogical properties of fine recycled aggregates made from concrete waste. Constr. Build. Mater. 2015, 86, 178–188. [Google Scholar] [CrossRef]
- Bustillo, M. Manual de RCD y Áridos Reciclados; Fueyo Editores: Madrid, Spain, 2010; pp. 641–649. [Google Scholar]
- Omary, S.; Ghorbel, E.; Wardeh, G. Relationships between recycled concrete aggregates characteristics and recycled aggregates concretes properties. Constr. Build. Mater. 2016, 108, 163–174. [Google Scholar] [CrossRef]
- Rodrigues, F.; Carballo, M.T.; Evangelista, L.; De Brito, J. Physical-chemical and mineralogical characterization of fine aggregates from construction and demolition waste recycling plants. J. Clean. Prod. 2013, 52, 438–445. [Google Scholar] [CrossRef]
- Carta Geológica-Minera Ciudad de México E14-2, Esc.1:250,000. Segunda Edición 1997. Servicio Geológico Mexicano. Available online: www.sgm.gob.mx (accessed on 07 August 2017).
- Shi, C.; Li, Y.; Zhang, J.; Li, W.; Chong, L.; Xie, Z. Performance enhancement of recycled aggregate—A review. J. Clean. Prod. 2016, 112, 466–472. [Google Scholar] [CrossRef]
- Liu, Q.; Tong, T.; Liu, S.; Yang, D.; Yu, Q. Investigation of using hybrid recycled powder from demolished concrete solids and clay bricks as a pozzolanic supplement for cement. Constr. Build. Mater. 2014, 73, 754–763. [Google Scholar] [CrossRef]
- Mymrin, V.A.; Alekseev, K.P.; Catai, R.E.; Izzo, R.L.S.; Rose, J.L.; Nagalli, A.; Romano, C.A. Construction material from construction and demolition debris and lime production wastes. Constr. Build. Mater. 2015, 79, 207–213. [Google Scholar] [CrossRef]
- Angulo, S.C.; Ulsen, C.; John, V.M.; Khan, H.; Cincotto, M.A. Chemical-mineralogical characterization of C&D waste recycled aggregates from Sao Paulo, Brazil. Waste Manag. 2009, 29, 721–730. [Google Scholar] [PubMed]
- Catinaud, S.; Beaudoin, J.J.; Marchand, J. Influence of limestone addition on calcium leaching mechanism in cement-based materials. Cem. Concr. Res. 2000, 30, 1961–1968. [Google Scholar] [CrossRef]
- Runcevski, T.; Dinnebier, R.E.; Magdysyuk, O.V.; Pollmann, H. Crystal structures of calcium hemicarboaluminate and carbonated calcium hemicarboaluminate from synchrotron powder diffraction data. Acta Crystallogr. Sect. B 2012, B68, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Glasser, F.P.; Matschei, T. Interactions between portland cement and carbon dioxide. In Proceedings of the 12th International Congress on the Chemistry of Cement-ICCC, Montreal, QC, Canada, 8–13 July 2007. [Google Scholar]
- Matschei, T.; Lothenbach, B.; Glasser, F.P. The role of calcium carbonate in cement hydration. Cem. Concr. Res. 2007, 37, 551–558. [Google Scholar] [CrossRef]
- Bonavetti, V.L.; Rahhal, V.F.; Irassar, E.F. Studies on the carboaluminate formation in limestone filler-blended cements. Cem. Concr. Res. 2001, 31, 853–859. [Google Scholar] [CrossRef]
- Manzano, H.; González-Teresa, R.; Dolado, J.S.; Ayuela, A. Espectros de rayos X y propiedades elásticas teóricas de los silicatos cálcicos hidratados cristalinos: comparación con los geles de cemento. Mater. Constr. 2010, 299, 7–19. [Google Scholar] [CrossRef]
- Krajci, L.; Janotka, I. Measurement techniques for rapid assessment of carbonation in concrete. ACI Mater. J. 2000, 97, 168–171. [Google Scholar]
- Lagerblad, B. Carbon Dioxide Uptake during Concrete Life Cycle-State of Art, Report 2:2005; Swedish Cement and Concrete Research Institute: Stockholm, Sweden, 2005. [Google Scholar]
- Gaucher, E.C.; Blanc, P. Cement/clay interaction—A review: Experiments, natural analogues, and modeling. Waste Manag. 2006, 26, 776–788. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).