The Evolution, Current Status, and Future Prospects of Using Biotechnologies in the Mineral Extraction and Metal Recovery Sectors
Abstract
:1. Introduction
2. History and Current Status of Biomining
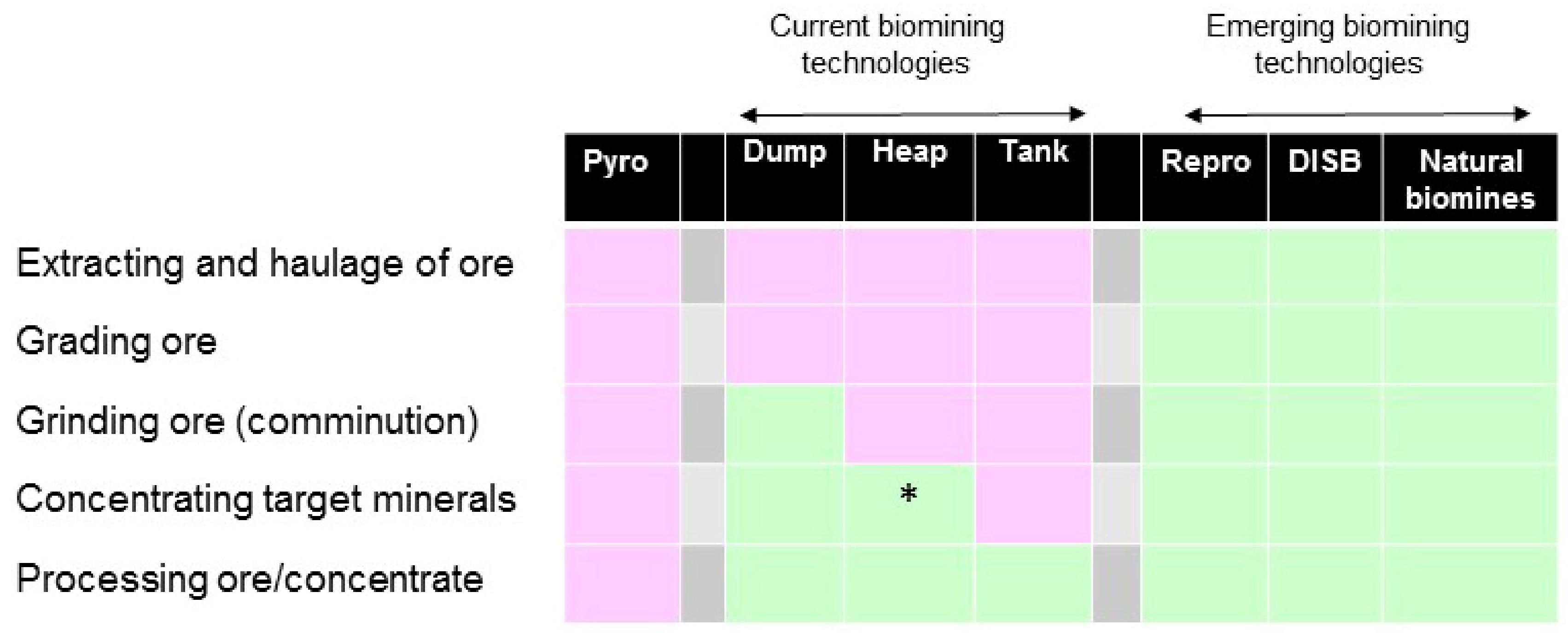
3. Biomining: A Niche Technology
4. New Developments in Biomining Technologies
4.1. Sulfur-Enhanced Bioleaching (SEB)
4.1.1. Reductive Bio-Processing of Oxidised Mineral Ores
4.1.2. Using SEB to Enhance Oxidative Processing of Sulfidic Ores and Tailings
4.2. Deep In Situ Biomining
4.3. Exploiting “Natural Biomines”
5. Retrospective Critique and Potential Future Developments in Mining Biotechnologies
Conflicts of Interest
References
- Elshkaki, A.; Graedel, T.E.; Ciacci, L.; Reck, B.K. Resource demand scenarios for the major metals. Environ. Sci. Technol. 2018, 52, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Brierley, C.L.; Brierley, J.A. Progress in bioleaching: Part B: Applications of microbial processes by the minerals industries. Appl. Microbiol. Biotechnol. 2013, 97, 7543–7552. [Google Scholar] [CrossRef] [PubMed]
- Johnson, D.B. Biomining-biotechnologies for extracting and recovering metals from ores and waste materials. Curr. Opin. Biotechnol. 2014, 30, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.T.L. Biotechnologies that utilize acidophiles. In Acidophiles: Life in Extremely Acidic Environments; Quatrini, R., Johnson, D.B., Eds.; Caister Academic Press: Haverhill, UK, 2016; pp. 265–284. [Google Scholar]
- Brierley, J.A.; Brierley, C.L. Present and future commercial applications of biohydrometallurgy. Hydrometallurgy 2001, 59, 233–239. [Google Scholar] [CrossRef]
- Johnson, D.B. Development and application of biotechnologies in the metal mining industry. Environ. Sci. Pollut. Res. 2013, 20, 7768–7776. [Google Scholar] [CrossRef] [PubMed]
- Colmer, A.R.; Temple, K.L.; Hinkle, M.E. An iron-oxidizing bacterium from the acid drainage of some bituminous coal mines. J. Bacteriol. 1950, 59, 317–328. [Google Scholar] [PubMed]
- Kelly, D.P.; Wood, A.P. Reclassification of some species of Thiobacillus to the newly designated genera Acidithiobacillus gen. nov., Halothiobacillus gen. nov., and Thermothiobacillus gen. nov. Int. J. Syst. Evol. Microbiol. 2000, 50, 511–516. [Google Scholar] [CrossRef] [PubMed]
- Schippers, A.; Sand, W. Bacterial leaching of metal sulfides proceeds by two indirect mechanisms via thiosulfate or via polysulfides and sulfur. Appl. Environ. Microbiol. 1999, 65, 319–321. [Google Scholar] [PubMed]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: Fundamentals and mechanisms of bacterial metal sulfide oxidation-part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Dopson, M. Physiological and phylogenetic diversity of acidophilic bacteria. In Acidophiles: Life in Extremely Acidic Environments; Quatrini, R., Johnson, D.B., Eds.; Caister Academic Press: Haverhill, UK, 2016; pp. 79–92. [Google Scholar]
- Agricola, G. De Re Metallica Libri XII; Froben: Basle, Switzerland, 1556. [Google Scholar]
- Wadden, D.; Gallant, A. The in-place leaching of uranium at Denison mines. Can. Metall. Q. 1985, 24, 127–134. [Google Scholar] [CrossRef]
- Brierley, J.A.; Kuhn, M.C. From laboratory to application heap bioleach or not. Adv. Mater. Res. 2009, 71–73, 311–317. [Google Scholar] [CrossRef]
- Riekkola-Vanhanen, M. Talvivaara mining company—from a project to a mine. Miner. Eng. 2013, 48, 2–9. [Google Scholar] [CrossRef]
- Morin, D.H.R.; d’Hugues, P. Bioleaching of a cobalt-containing pyrite in stirred reactors: A case study from laboratory scale to industrial application. In Biomining; Rawlings, D.E., Johnson, D.B., Eds.; Springer: Heidelberg, Germany, 2007; pp. 35–55. [Google Scholar]
- Dopson, M.; Johnson, D.B. Biodiversity, metabolism and applications of acidophilic sulfur-metabolizing micro-organisms. Environ. Microbiol. 2012, 14, 2620–2631. [Google Scholar] [CrossRef] [PubMed]
- Hallberg, K.B.; Grail, B.M.; du Plessis, C.A.; Johnson, D.B. Reductive dissolution of ferric iron minerals: A new approach for bioprocessing nickel laterites. Miner. Eng. 2011, 24, 620–624. [Google Scholar] [CrossRef]
- du Plessis, C.A.; Slabbert, W.; Hallberg, K.B.; Johnson, D.B. Ferredox: A biohydrometallurgical processing concept for limonitic nickel laterites. Hydrometallurgy 2011, 109, 221–229. [Google Scholar] [CrossRef]
- Marrero, J.; Coto, O.; Goldmann, S.; Graupner, T.; Schippers, A. Recovery of nickel and cobalt from laterite tailings by reductive dissolution under aerobic conditions using Acidithiobacillus species. Environ. Sci. Technol. 2015, 9, 6674–6682. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.L.; Grail, B.M.; Johnson, D.B. Reductive bioprocessing of cobalt-bearing limonitic laterites. Miner. Eng. 2017, 106, 86–90. [Google Scholar] [CrossRef]
- Johnson, D.B.; du Plessis, C.A. Biomining in reverse gear: Using bacteria to extract metals from oxidized ores. Miner. Eng. 2015, 75, 2–5. [Google Scholar] [CrossRef]
- Johnson, D.B.; Hedrich, S.; Pakostova, E. Indirect redox transformations of iron, copper and chromium catalyzed by extremely acidophilic bacteria. Front. Microbiol. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Falagán, C.; Grail, B.M.; Johnson, D.B. New approaches for extracting and recovering metals from mine tailings. Miner. Eng. 2017, 106, 71–78. [Google Scholar] [CrossRef]
- Batterham, R. In situ leaching-how far in the future? In Biohydrometallurgy: Biotech Key to Unlock Mineral Resources Value; Qui, G., Jiang, T., Qin, W., Liu, X., Yang, Y., Qang, H., Eds.; Central South University Press: Changsha, China, 2011; pp. 21–22. [Google Scholar]
- Johnson, D.B. Biomining goes underground. Nat. Geosci. 2015, 8, 165–166. [Google Scholar] [CrossRef]
- Pakostova, E.; Grail, B.M.; Johnson, D.B. Bio-processing of a saline, calcareous copper sulfide ore by sequential leaching. Hydrometallurgy 2018, 179, 36–43. [Google Scholar] [CrossRef]
- Carranza, F.; Iglesias, N.; Romero, R.; Palencia, I. Kinetics improvement of high-grade sulfides bioleaching by effects separations. FEMS Microbiol. Rev. 1993, 11, 129–138. [Google Scholar] [CrossRef]
- Seredkin, M.; Zabolotsky, A.; Jeffress, G. In situ recovery, an alternative to conventional methods of mining: Exploration, resource estimation, environmental issues, project evaluation and economics. Ore Geol. Rev. 2016, 79, 500–514. [Google Scholar] [CrossRef]
- Hiskey, J.B. Technical innovations spur resurgence of copper solution mining. Min. Eng. 1986, 38, 1036–1039. [Google Scholar]
- Ballantyne, G.; Marsh, T.; Hehnke, C.; Andrews, D.; Eichenlaub, A.; Krahulec, K. The Resolution copper deposit, a deep, high-grade porphyry copper deposit in the Superior district, Arizona. In Proceedings of the Marco T, Einaudi Synposium, Colorado School of Mines, Golden, CO, USA, 3–4 April 2003. [Google Scholar]
- Matthies, R.; Hejny, H.; Hirsch, K.; Kahnt, R.; Märten, H.; Johnson, D.B. In-situ bioextraction of industry metals from deep ore deposits to secure EU resource supply. GeoResour. J. 2017, 2, 37–40. [Google Scholar]
- Coupland, K.; Johnson, D.B. Geochemistry and microbiology of an impounded subterranean acidic water body at Mynydd Parys, Anglesey, Wales. Geobiology 2004, 2, 77–86. [Google Scholar] [CrossRef]
- Ňancucheo, I.; Johnson, D.B. Selective removal of transition metals from acidic mine waters by novel consortia of acidophilic sulfidogenic bacteria. Microb. Biotechnol. 2012, 5, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.; Johnson, D.B. Design and application of a low pH upflow biofilm sulfidogenic bioreactor for recovering transition metals from synthetic waste water at a Brazilian copper mine. Front. Microbiol. submitted.
- Sánchez España, F.J.; López Pamo, E.; Santofimia, E.; Aduvire, O.; Reyes, J.; Martín Rubí, J.A. The Tintillo acidic river (Rio Tinto mines, Huelva, Spain): An example of extreme environmental impact of pyritic mine wastes on the environment or an exceptional site to study acid-sulphate mine drainage systems? In Proceedings of the Volume of the Securing the Future International Conference on Mining, Metals and the Environment, Skellefteä, Sweden, 27 June–1 July 2005; pp. 278–287. [Google Scholar]






| Parameter | Oxidative Mineral Dissolution | Reductive Mineral Dissolution |
|---|---|---|
| Key reaction | iron oxidation | iron reduction |
| Acidity | often generated | consumed |
| Aeration required | yes (aerobic process) | no (anaerobic process) |
| Additional energy source required? | no (Fe2+, reduced S in minerals) | yes (elemental sulfur) |
| Electron acceptor | Oxygen | Fe3+ (in minerals) |
| PLS generated | Oxidized | reduced |
| (a) | |||
| Parameter | Afon Goch | Post Cu Removal | Post Zn Removal |
| pH | 2.37 | 2.38 | 4.05 |
| Total Fe | 512 | 510 | 506 |
| Cu | 42 | 0 | 0 |
| Zn | 58 | 53 | 0 |
| Mn | 14 | 14 | 14 |
| (b) | |||
| Metal | Consumable Costs/Year $ | Metal Value/Year $ | |
| Cu | 10,500 | 88,000 | |
| Zn | 34,500 | 56,500 | |
| Total | 45,000 | 144,500 | |
| Iron | Zinc | Copper | Cobalt | |
|---|---|---|---|---|
| Concentration (mg/L) | ~1750 | ~550 | ~180 | ~45 |
| Price ($/kg) | 0.07 | 3.07 | 6.78 | 88.9 |
| Value ($/m3) | 0.12 | 1.69 | 1.23 | 3.99 |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, D.B. The Evolution, Current Status, and Future Prospects of Using Biotechnologies in the Mineral Extraction and Metal Recovery Sectors. Minerals 2018, 8, 343. https://doi.org/10.3390/min8080343
Johnson DB. The Evolution, Current Status, and Future Prospects of Using Biotechnologies in the Mineral Extraction and Metal Recovery Sectors. Minerals. 2018; 8(8):343. https://doi.org/10.3390/min8080343
Chicago/Turabian StyleJohnson, D. Barrie. 2018. "The Evolution, Current Status, and Future Prospects of Using Biotechnologies in the Mineral Extraction and Metal Recovery Sectors" Minerals 8, no. 8: 343. https://doi.org/10.3390/min8080343




