Synchrotron Radiation Based Study of the Catalytic Mechanism of Ag+ to Chalcopyrite Bioleaching by Mesophilic and Thermophilic Cultures
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain and Culture Medium
2.2. Mineral Samples
2.3. Bioleaching Experiment
2.4. Analytical Methods
2.4.1. Leaching Parameters Determination
2.4.2. Surface Morphology
2.4.3. Chalcopyrite Surface Compositions Analyses
3. Results
3.1. Leaching Characters of Chalcopyrite by Mesophilic Culture in the Presence of Ag+
3.2. Leaching Characters of Chalcopyrite by Thermophilic Culture in the Presence of Ag+
3.3. Surface Morphologies of Leaching Residues
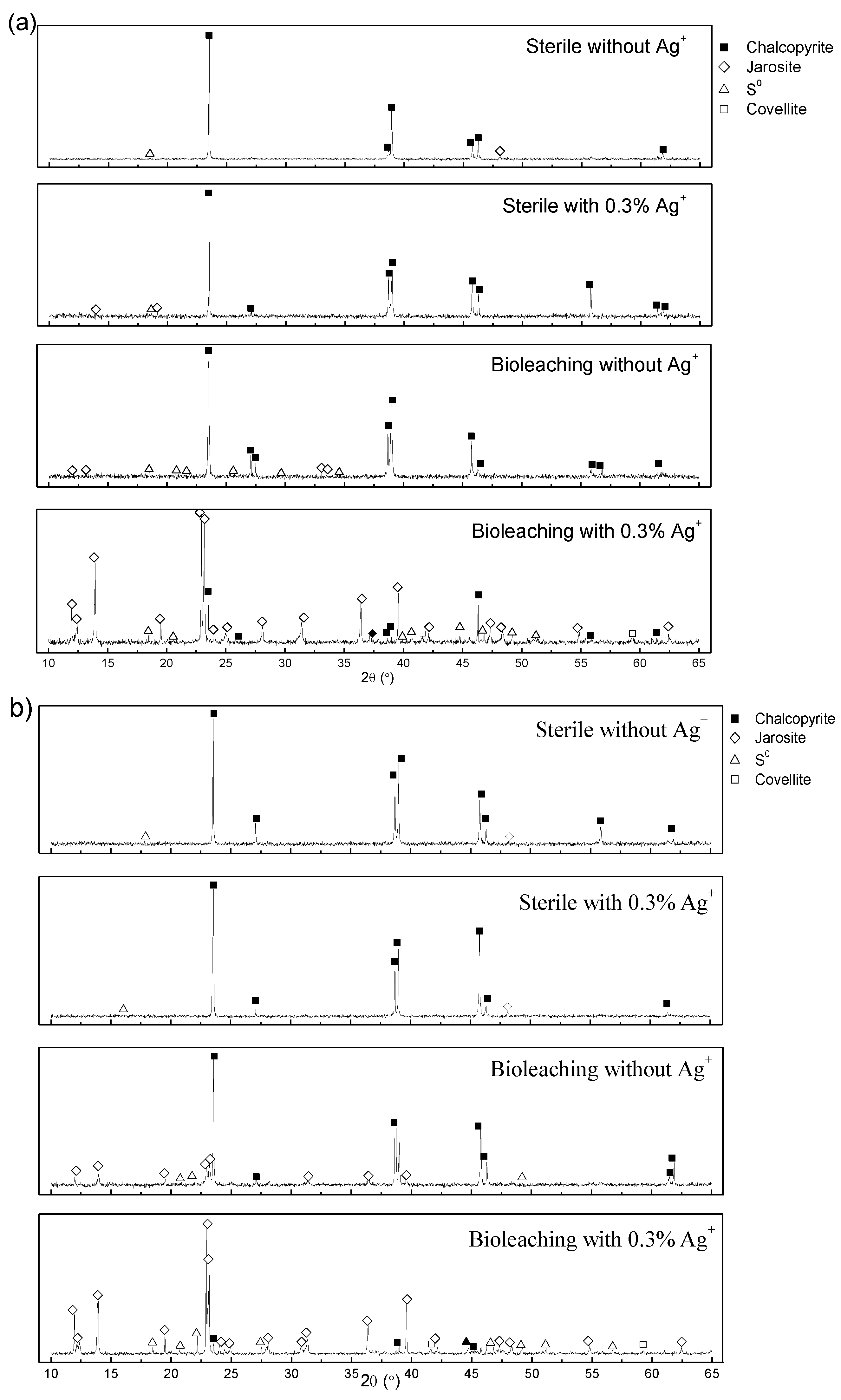
3.4. SR-XRD Analysis
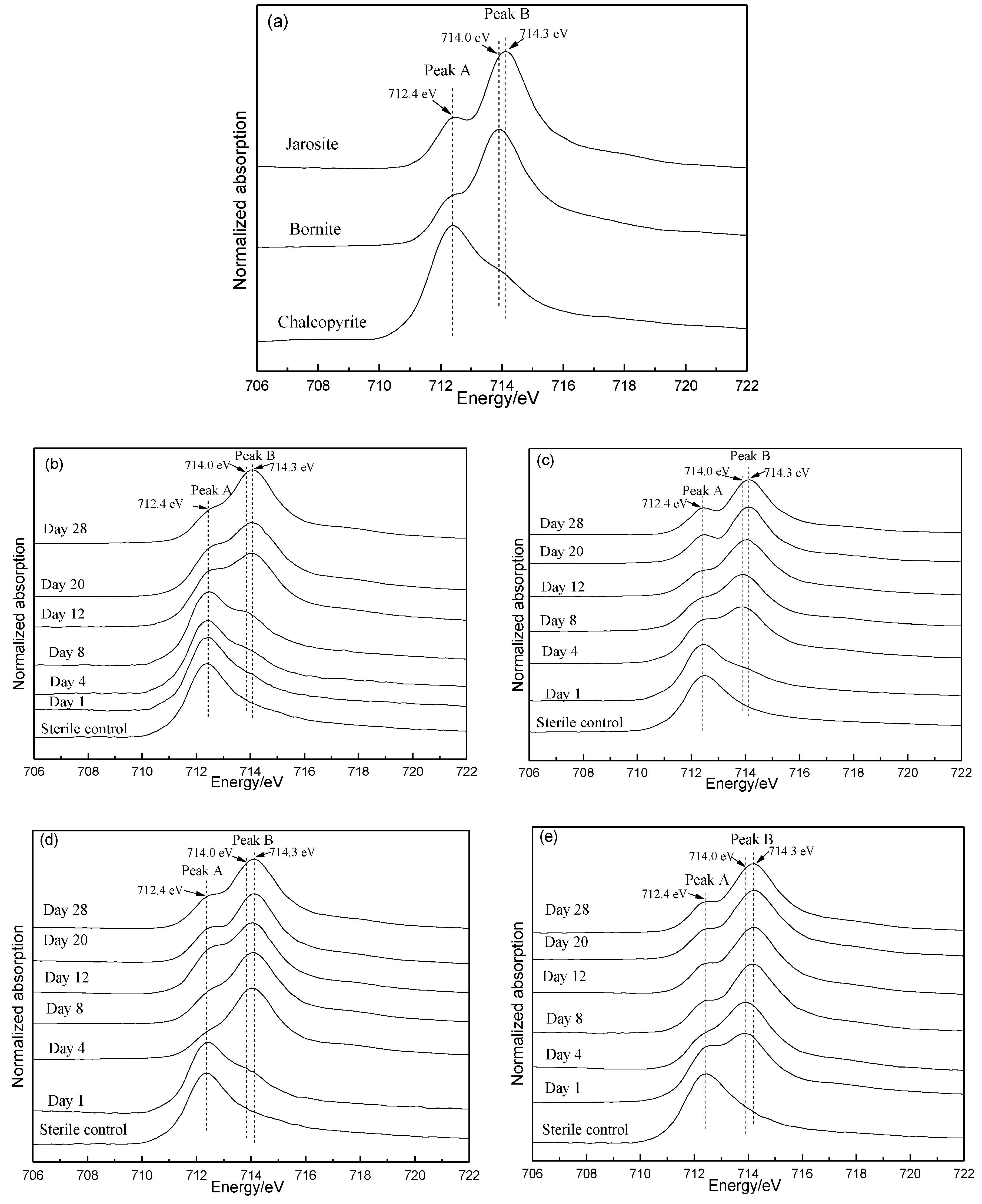
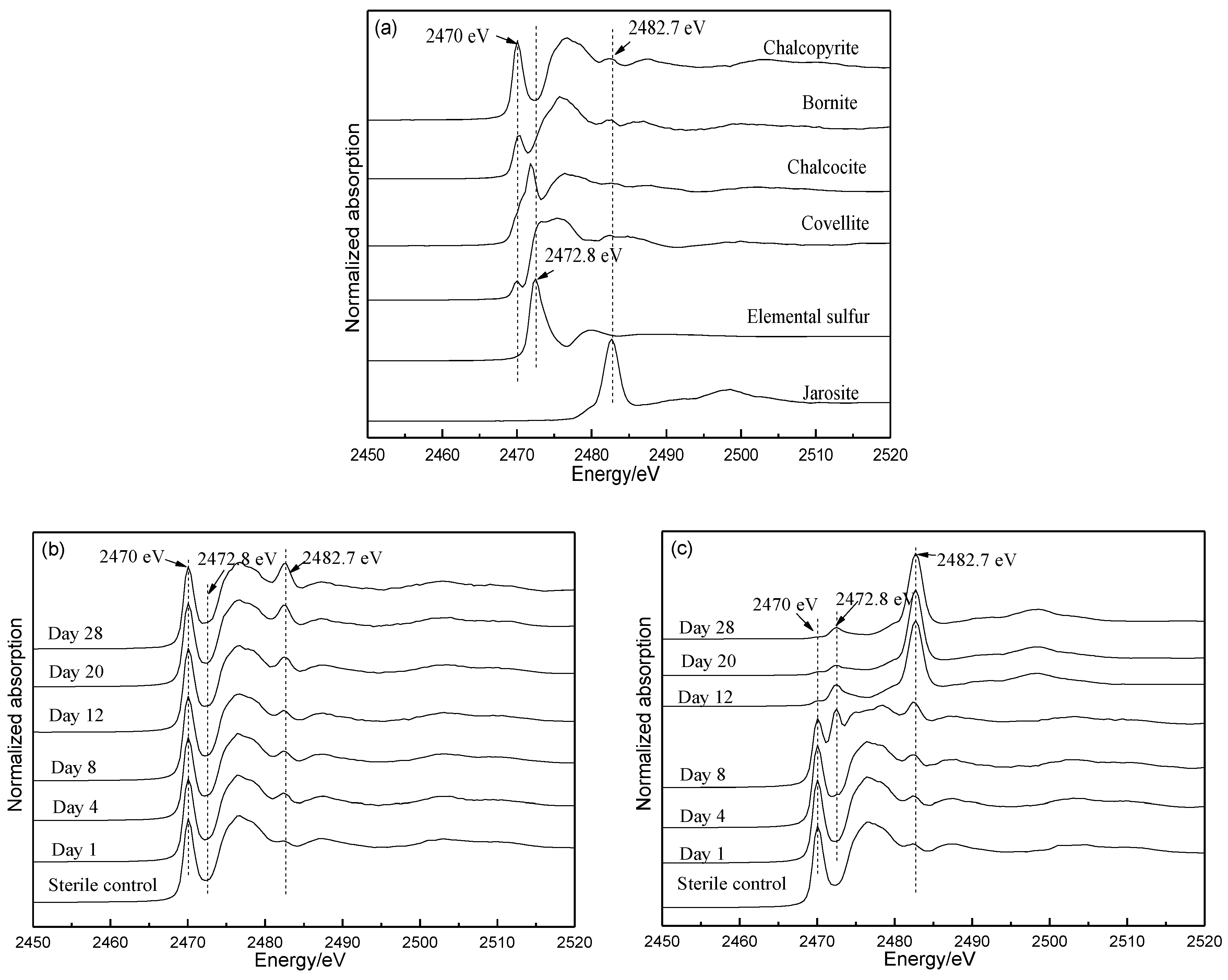
3.5. Fe L-Edge and S K-Edge XANES
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Pradhan, N.; Nathsarma, K.C.; Srinivasa, R.K.; Sukla, L.B.; Mishra, B.K. Heap bioleaching of chalcopyrite: A review. Miner. Eng. 2008, 21, 355–365. [Google Scholar] [CrossRef]
- Li, Y.; Kawashima, N.; Li, J.; Chandra, A.P.; Gerson, A.R. A review of the structure and fundamental mechanisms and kinetics of the leaching of chalcopyrite. Adv. Colloid Interface Sci. 2013, 197−198, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Watling, H.R. The bioleaching of sulphide minerals with emphasis on copper sulphides—A review. Hydrometallurgy 2006, 84, 81–108. [Google Scholar] [CrossRef]
- Dew, D.W.; Van, B.C.; Mcewan, K.; Bowker, C. Bioleaching of base metal sulphide concentrates: A comparison of high and low temperature bioleaching. J. S. Afr. Inst. Min. Metall. 2000, 100, 409–413. [Google Scholar]
- Gu, G.; Hu, K.; Zhang, X.; Xiong, X.; Yang, H. The stepwise dissolution of chalcopyrite bioleached by Leptospirillum ferriphilum. Electrochim. Acta 2013, 103, 50–57. [Google Scholar] [CrossRef]
- Feng, S.; Yang, H.; Xin, Y.; Gao, K.; Yang, J.; Liu, T.; Zhang, L.; Wang, W. A novel and highly efficient system for chalcopyrite bioleaching by mixed strains of Acidithiobacillus. Bioresour. Technol. 2013, 129, 456–462. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Xia, J.; Nie, Z.; Yang, Y.; Ma, C. Effect of sodium chloride on sulfur speciation of chalcopyrite bioleached by the extreme thermophile Acidianus manzaensis. Bioresour. Technol. 2012, 110, 462–467. [Google Scholar]
- Wang, J.; Liao, R.; Tao, L.; Zhao, H.; Zhai, R.; Qin, W.; Qiu, G. A comprehensive utilization of silver-bearing solid wastes in chalcopyrite bioleaching. Hydrometallurgy 2017, 169, 152–157. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Zhu, P.; Chen, A.; Liu, H.; Yin, H.; Qiu, G.; Liang, Y. Carbon material with high specific surface area improves complex copper ores’ bioleaching efficiency by mixed moderate thermophiles. Minerals 2018, 8, 301. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Nie, Z.; Liu, L.; Wang, L.; Ma, C.; Zheng, L.; Zhao, Y.; Wen, W. Comparative study of S, Fe and Cu speciation transformation during chalcopyrite bioleaching by mixed mesophiles and mixed thermophiles. Miner. Eng. 2017, 106, 22–32. [Google Scholar] [CrossRef]
- Miller, J.D.; Portillo, H.Q. Electrochemistry in silver catalysed ferric sulfate leaching of chalcopyrite. J. Macromol. Sci. 1981, 27, 327–338. [Google Scholar]
- Parker, A.; Klauber, C.; Kougianos, A.; Watling, H.R.; Van Bronswijk, W. An X-ray photoelectron spectroscopy study of the mechanism of oxidative dissolution of chalcopyrite. Hydrometallurgy 2003, 71, 265–276. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, Y.; Deng, T.; Wang, K. Kinetic modeling for the bacterial leaching of chalcopyrite catalyzed by silver ions. Miner. Eng. 2004, 17, 943–947. [Google Scholar] [CrossRef]
- Nazari, G.; Dixon, D.G.; Dreisinger, D.B. The mechanism of chalcopyrite leaching in the presence of silver-enhanced pyrite in the Galvanox™ process. Hydrometallurgy 2012, 113–114, 122–130. [Google Scholar] [CrossRef]
- Ghahremaninezhad, A.; Radzinski, R.; Gheorghiu, T.; Dixon, D.G.; Asselin, E. A model for silver ion catalysis of chalcopyrite (CuFeS2) dissolution. Hydrometallurgy 2015, 155, 95–104. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gan, X.; Hu, M.; Zhang, E.; Qin, W.; Qiu, G. Cooperative bioleaching of chalcopyrite and silver-bearing tailing by mixed moderately thermophilic culture: An emphasis on the chalcopyrite dissolution with XPS and electrochemical analysis. Miner. Eng. 2015, 81, 23–29. [Google Scholar] [CrossRef]
- Xia, J.; Song, J.; Liu, H.; Nie, Z.; Shen, L.; Yuan, P.; Ma, C.; Zheng, L.; Zhao, Y. Study on catalytic mechanism of silver ions in bioleaching of chalcopyrite by SR–XRD and XANES. Hydrometallurgy 2018, 180, 26–35. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Nie, Z.; Zhu, H.; Zhao, Y.; Ma, C.; Zheng, L. Evolution of leaching products on the surface of chalcopyrite by mesophiles and thermophiles based on SR–XRD and XANES spectroscopy. Adv. Mater. Res. 2015, 1130, 183–187. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Nie, Z. Relatedness of Cu and Fe speciation to chalcopyrite bioleaching by Acidithiobacillus Ferrooxidans. Hydrometallurgy 2015, 156, 40–46. [Google Scholar] [CrossRef]
- Ide–Ektessabi, A.; Kawakami, T.; Watt, F. Distribution and chemical state analysis of iron in the Parkinsonian substantia nigra using synchrotron radiation micro beams. Nucl. Instrum. Methods. B 2004, 213, 590–594. [Google Scholar] [CrossRef]
- Ravel, B.; Newville, M. ATHENA. ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. J. Synchrotron Radiat. 2005, 12, 537–541. [Google Scholar] [CrossRef] [PubMed]
- Prange, A. Speciation analysis of microbiologically produced sulfur by X-ray absorption near edge structure spectroscopy. In Microbial Sulfur Metabolism; Dahl, C., Friedrich, C.G., Eds.; Springer: Heidelberg, Germany, 2008; pp. 259–272. [Google Scholar]
- Vera, M.; Schippers, A.; Sand, W. Progress in bioleaching: fundamentals and mechanisms of bacterial metal sulfide oxidation—Part A. Appl. Microbiol. Biotechnol. 2013, 97, 7529–7541. [Google Scholar] [CrossRef] [PubMed]
- Raheb, J.; Sharoknyan, S.; Nazari, F.; Rakhshany, Y. The studying of silver nanoparticle effect on the copper bioleaching output from low grade sulfidic ores. Am. J. Nano Res. Appl. 2015, 3, 6–11. [Google Scholar]
- Klauber, C. A critical review of the surface chemistry of acidic ferric sulphate dissolution of chalcopyrite with regards to hindered dissolution. Int. J. Miner. Process. 2008, 86, 1–17. [Google Scholar] [CrossRef]
- Pan, H.; Yang, H.; Tong, L.; Zhong, C.; Zhao, Y. Control method of chalcopyrite passivation in bioleaching. Trans. Nonferrous Met. Soc. China 2012, 22, 2255–2260. [Google Scholar] [CrossRef]
- Majuste, D.; Ciminelli, V.S.T.; Osseo-Asare, K.; Dantas, M.S.S.; Magalhães-Paniago, R. Electrochemical dissolution of chalcopyrite: Detection of bornite by synchrotron small angle X-ray diffraction and its correlation with the hindered dissolution process. Hydrometallurgy 2012, 111−112, 114–123. [Google Scholar] [CrossRef]
- Harmer, S.L.; Thomas, J.E.; Fornasiero, D.; Gerson, A.R. The evolution of surface layers formed during chalcopyrite leaching. Geochim. Cosmochim. Acta 2006, 70, 4392–4402. [Google Scholar] [CrossRef]
- Li, Y.; Qian, G.; Li, J.; Gerson, A.R. Kinetics and roles of solution and surface species of chalcopyrite dissolution at 650 Mv. Geochim. Cosmochim. Acta 2015, 161, 188–202. [Google Scholar] [CrossRef]
- Peng, A.; Liu, H.; Nie, Z.; Xia, J. Effect of surfactant Tween-80 on sulfur oxidation and expression of sulfur metabolism relevant genes of Acidithiobacillus ferrooxidans. Trans. Nonferrous Met. Soc. China 2012, 22, 3147–3155. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, J.; Gan, X.; Hu, M.; Zhang, E.; Qin, W.; Qiu, G. Comparison of electrochemical dissolution of chalcopyrite and bornite in acid culture medium. Trans. Nonferrous Met. Soc. China 2015, 25, 303–313. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Nie, Z.; Ma, C.; Zheng, L.; Hong, C.; Zhao, Y.; Wen, W. Bioleaching of chalcopyrite by Acidianus manzaensis under different constant pH. Miner. Eng. 2016, 98, 80–89. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Tsunekawa, M.; Okamoto, H.; Nakayama, R.; Kuroiwa, S. Improved chalcopyrite leaching through optimization of redox potential. Can. Metall. Q. 2008, 47, 253–258. [Google Scholar] [CrossRef]
- Hiroyoshi, N.; Arai, M.; Miki, H.; Tsunekawa, M.; Hirajima, T. A new reaction model for the catalytic effect of silver ions on chalcopyrite leaching in sulfuric acid solutions. Hydrometallurgy 2002, 63, 257–267. [Google Scholar] [CrossRef]
- Liu, H.; Xia, J.; Nie, Z.; Wen, W.; Yang, Y.; Ma, C.; Zheng, L.; Zhao, Y. Formation and evolution of secondary minerals during bioleaching of chalcopyrite by thermoacidophilic Archaea Acidianus manzaensis. Trans. Nonferrous Met. Soc. China 2016, 26, 2485–2494. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Chen, M. A copper and iron K-edge XANES study on chalcopyrite leached by mesophiles and moderate thermophiles. Miner. Eng. 2013, 48, 31–35. [Google Scholar] [CrossRef]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, Z.; Zhang, W.; Liu, H.; Xia, J.; Zhu, W.; Zhang, D.; Zheng, L.; Ma, C.; Zhao, Y.; Wen, W. Synchrotron Radiation Based Study of the Catalytic Mechanism of Ag+ to Chalcopyrite Bioleaching by Mesophilic and Thermophilic Cultures. Minerals 2018, 8, 382. https://doi.org/10.3390/min8090382
Nie Z, Zhang W, Liu H, Xia J, Zhu W, Zhang D, Zheng L, Ma C, Zhao Y, Wen W. Synchrotron Radiation Based Study of the Catalytic Mechanism of Ag+ to Chalcopyrite Bioleaching by Mesophilic and Thermophilic Cultures. Minerals. 2018; 8(9):382. https://doi.org/10.3390/min8090382
Chicago/Turabian StyleNie, Zhenyuan, Weiwei Zhang, Hongchang Liu, Jinlan Xia, Wei Zhu, Duorui Zhang, Lei Zheng, Chenyan Ma, Yidong Zhao, and Wen Wen. 2018. "Synchrotron Radiation Based Study of the Catalytic Mechanism of Ag+ to Chalcopyrite Bioleaching by Mesophilic and Thermophilic Cultures" Minerals 8, no. 9: 382. https://doi.org/10.3390/min8090382



