Interpretation of Hydrophobization Behavior of Dodecylamine on Muscovite and Talc Surface through Dynamic Wettability and AFM Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples and Chemicals
2.2. Methods
2.2.1. Dynamic Wettability Tests
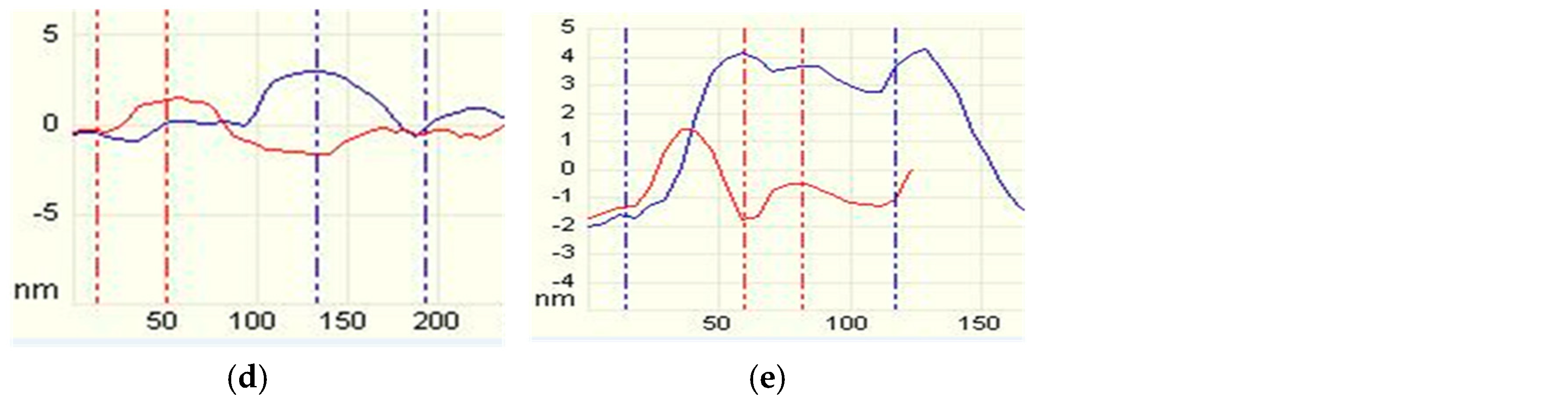
2.2.2. Tapping Mode AFM Imaging
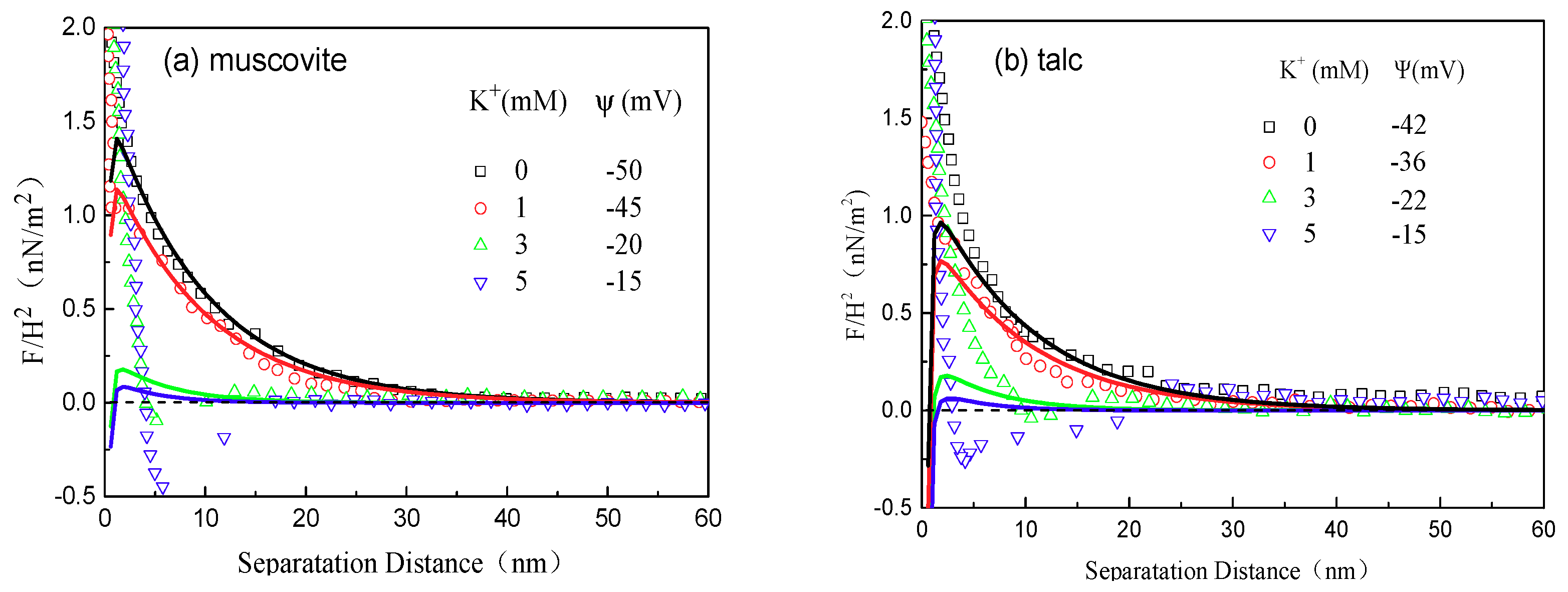
2.2.3. AFM Force Measurements
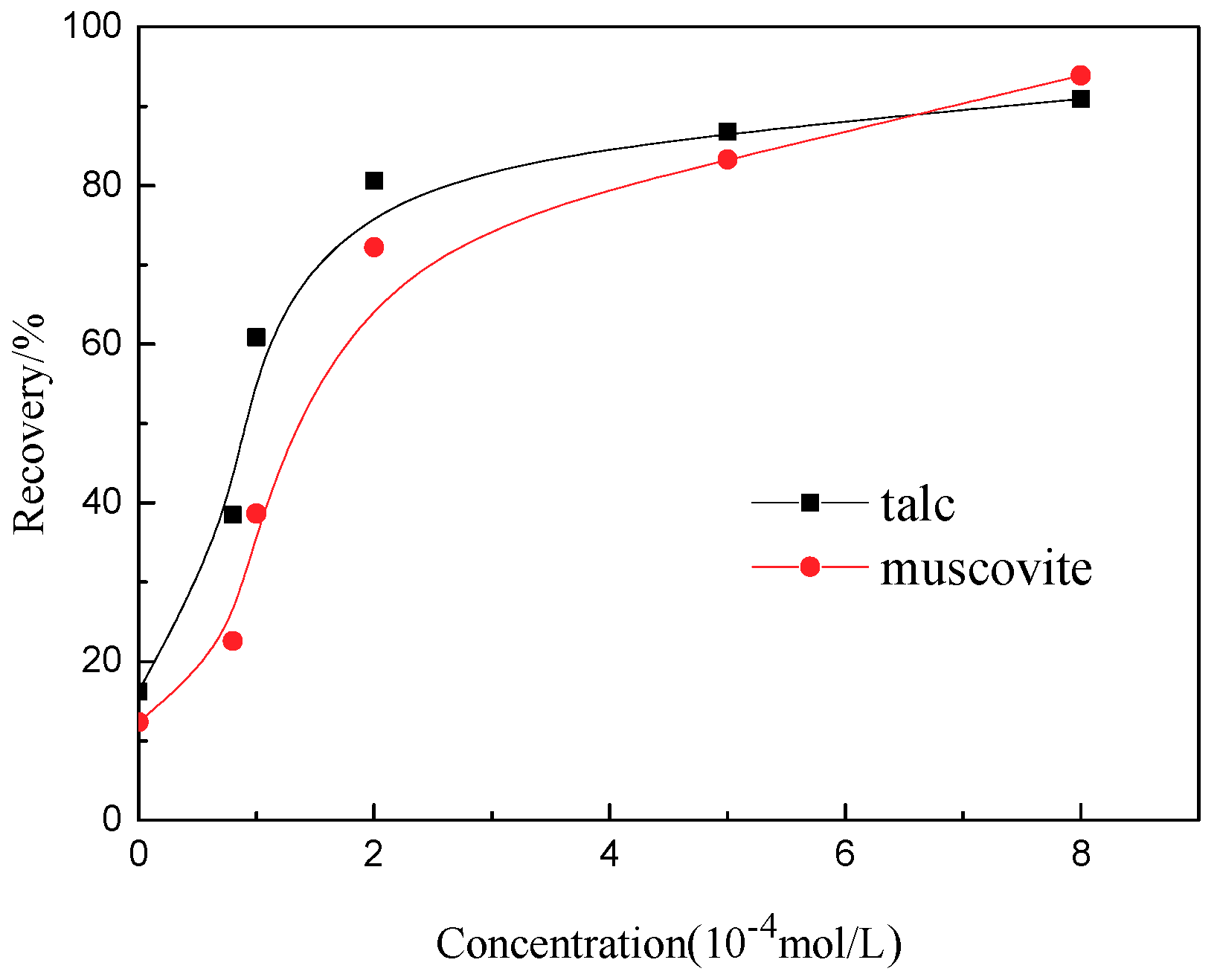
2.2.4. Microflotation Tests
3. Results and Discussion
3.1. Dynamic Wettability Analysis
3.2. AFM Imaging
3.3. AFM Force Analysis
3.4. Flotation Results
4. Conclusions
- (1)
- After treatments with DDA, the contact angles became much larger compared to initial angles, particularly for muscovite, and the attachment of bubbles on the talc surface was found to be much easier than muscovite due to its natural hydrophobicity.
- (2)
- From AFM imaging, both the muscovite and talc showed a similar tendency: the higher the DDA concentration, the more the adsorbed amount. However, the adsorbed amount of DDA on talc surface was obviously more than that on muscovite.
- (3)
- As for the interaction forces between the minerals, the maximum attractions occurred at different concentrations respectively for muscovite and talc and agreed well with the AFM imaging results.
- (4)
- Results obtained from flotation tests were promising and quite in agreement with the phenomenon of these techniques.
Author Contributions
Funding
Conflicts of Interest
References
- Morris, G.E.; Fornasiero, D.; Ralston, J. Polymer depressants at the talc–water interface: Adsorption isotherm, microflotation and electrokinetic studies. Int. J. Miner. Process. 2002, 67, 211–227. [Google Scholar] [CrossRef]
- Khraisheh, M.; Holland, C.; Creany, C.; Harris, P.; Parolis, L. Effect of molecular weight and concentration on the adsorption of CMC onto talc at different ionic strengths. Int. J. Miner. Process. 2005, 75, 197–206. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Beattie, D.A. The effect of impurities and cleavage characteristics on talc hydrophobicity and polymer adsorption. Int. J. Miner. Process. 2013, 118, 34–42. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Dong, F.; Gao, Z.; Wu, H.; Wang, Z. Anisotropic adsorption of oleate on diaspore and kaolinite crystals: Implications for their flotation separation. Appl. Surf. Sci. 2014, 321, 331–338. [Google Scholar] [CrossRef]
- Vidal, C.A.G.; Pawlik, M. Molecular weight effects in interactions of guar gum with talc. Int. J. Miner. Process. 2015, 138, 38–43. [Google Scholar] [CrossRef]
- Burdukova, E.; Van Leerdam, G.C.; Prins, F.E.; Smeink, R.G.; Bradshaw, D.J.; Laskowski, J.S. Effect of calcium ions on the adsorption of CMC onto the basal planes of New York talc–A ToF-SIMS study. Min. Eng. 2008, 21, 1020–1025. [Google Scholar] [CrossRef]
- Mishchuk, N.A. The model of hydrophobic attraction in the framework of classical DLVO forces. Adv. Colloid Interface Sci. 2011, 168, 149–166. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Miller, J.D. Surface force measurements at the basal planes of ordered kaolinite particles. J. Colloid Interface Sci. 2010, 344, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, J.W.G.; Attard, P. Atomic force microscope images of nanobubbles on a hydrophobic surface and corresponding force−Separation data. Langmuir 2002, 18, 160–167. [Google Scholar] [CrossRef]
- Hartley, P.G.; Larson, I.; Scales, P.J. Electrokinetic and direct force measurements between silica and mica surfaces in dilute electrolyte solutions. Langmuir 1997, 13, 2207–2214. [Google Scholar] [CrossRef]
- Kumar, N.; Andersson, M.P.; Van den Ende, D.; Mugele, F.; Siretanu, I. Probing the surface charge on the basal planes of kaolinite particles with high resolution atomic force microscopy. Langmuir 2017, 33, 14226–14237. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Masliyah, J.H.; Xu, Z. Interaction of divalent cations with basal planes and edge surfaces of phyllosilicate minerals: Muscovite and talc. J Colloid Interface Sci. 2013, 404, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Xie, Z.; Liu, G.-R.; Yu, Y.-W.; Zhang, D. Interaction forces between muscovite and silica surfaces in electrolyte solutions measured with AFM. Trans. Nonferrous Met. Soc. China. 2013, 23, 1783–1788. [Google Scholar] [CrossRef]
- Ozcan, O.; Gonul, B.B.; Bulutcu, A.N.; Manav, H. Correlations between the shear strength of mineral filter cakes and particle size and surface tension. Colloids Surf. A Phys. Eng. Asp. 2001, 187, 405–413. [Google Scholar] [CrossRef]
- Chang, Q.; Wei, B.; He, Y. Capillary pressure method for measuring lipophilic hydrophilic ratio of filter media. Chem. Eng. J. 2009, 150, 323–327. [Google Scholar]
- Stroh, G.; Stahl, W. Effect of surfactants on the filtration properties of fine particles. Filtr. Sep. 1990, 27, 197–199. [Google Scholar] [CrossRef]
- Chau, T.T. A review of techniques for measurement of contact angles and their applicability on mineral surfaces. Min. Eng. 2009, 22, 213–219. [Google Scholar] [CrossRef]
- Brossard, S.K.; Du, H.; Miller, J.D. Characteristics of dextrin adsorption by elemental sulfur. J. Colloid Interf. Sci. 2008, 317, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Miller, J.D. Adsorption states of amphipatic solutes at the surface of naturally hydrophobic minerals: A molecular dynamics simulation study. Langmuir 2007, 23, 11587–11596. [Google Scholar] [CrossRef] [PubMed]
- Leung, A.; Wiltshire, J.; Blencowe, A.; Fu, Q.; Solomon, D.H.; Qiao, G.G. The effect of acrylamide—vinylpyrrolidinone copolymer on the depression of talc in mixed nickel mineral flotation. Min. Eng. 2011, 24, 449–454. [Google Scholar] [CrossRef]
- Jin, S.; Shi, Q.; Li, Q.; Ou, L.; Ouyang, K. Effect of calcium ionic concentrations on the adsorption of carboxymethyl cellulose onto talc surface: Flotation, adsorption and AFM imaging study. Powder Technol. 2018, 331, 155–161. [Google Scholar] [CrossRef]
- Xu, L.; Hu, Y.; Tian, J.; Wu, H.; Wang, L.; Yang, Y.; Wang, Z. Synergistic effect of mixed cationic/anionic collectors on flotation and adsorption of muscovite. Colloids Surf. A Phys. Eng. Asp. 2016, 492, 181–189. [Google Scholar] [CrossRef]
- Liu, J.; Xu, Z.; Masliyah, J. Colloidal forces between bitumen surfaces in aqueous solutions measured with atomic force microscope. Colloids Surf. A Phys. Eng. Asp. 2005, 260, 217–228. [Google Scholar] [CrossRef]
- Ducker, W.A.; Senden, T.J.; Pashley, R.M. Measurement of forces in liquids using a force microscope. Langmuir 1992, 8, 1831–1836. [Google Scholar] [CrossRef]
- Ducker, W.A.; Xu, Z.; Israelachvili, J.N. Measurements of hydrophobic and DLVO forces in bubble-surface interactions in aqueous solutions. Langmuir 1994, 10, 3279–3289. [Google Scholar] [CrossRef]
- Rabinovich, Y.I.; Yoon, R.H. Use of atomic force microscope for the measurements of hydrophobic forces. Colloids Surf. A Phys. Eng. Asp. 1994, 93, 263–273. [Google Scholar] [CrossRef]
- Cappella, B.; Dietler, G. Force-distance curves by atomic force microscopy. Surf. Sci. Rep. 1999, 34, 1–3. [Google Scholar] [CrossRef]
- Douillard, J.M.; Zajac, J.; Malandrini, H.; Clauss, F. Contact angle and film pressure: Study of a talc surface. J. Colloid Interface Sci. 2002, 255, 341–351. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Sun, W.; Hu, Y. New insights into the dodecylamine adsorption on scheelite and calcite: An adsorption model. Min. Eng. 2015, 79, 54–61. [Google Scholar] [CrossRef]
- Bastidas, J.M.; Polo, J.L.; Cano, E. Substitutional inhibition mechanism of mild steel hydrochloric acid corrosion by hexylamine and dodecylamine. J. Appl. Electrochem. 2000, 30, 1173–1177. [Google Scholar] [CrossRef] [Green Version]
- Qu, G.Z.; Hu, Y.H. The theory and application of coarse particle effect in flotation of fine particles. J. Central-South Inst. Min. Metall. 1993, 24, 743–748. [Google Scholar]
- Yan, L.; Englert, A.H.; Masliyah, J.H.; Xu, Z. Determination of anisotropic surface characteristics of different phyllosilicates by direct force measurements. Langmuir 2011, 27, 12996–13007. [Google Scholar] [CrossRef] [PubMed]
- Van Oss, C.J.; Omenyi, S.N.; Neumann, A.W. Negative Hamaker coefficients. Colloid Polym. Sci. 1979, 257, 737–744. [Google Scholar] [CrossRef]
- Van Oss, C.J.; Absolom, D.R.; Neumann, A.W. The “hydrophobic effect”: Essentially a van der Waals interaction. Colloid Polym. Sci. 1980, 258, 424–427. [Google Scholar] [CrossRef]
- Rajagopalan, R.; Hiemenz, P.C. Principles of Colloid and Surface Chemistry; Marcel Dekker: Newwork, NY, USA, 1986. [Google Scholar]
- Jiang, H.; Ji, W.; Yang, Q.; Xu, L.; Zhao, C.; Hu, Y. Synergistic adsorption and flotation of new mixed cationic/nonionic collectors on muscovite. Minerals 2017, 7, 74. [Google Scholar] [CrossRef]











| Sample | Al2O3 | SiO2 | Fe2O3 | TiO2 | CaO | MgO | K2O | Na2O | H2O | LOI 1 |
|---|---|---|---|---|---|---|---|---|---|---|
| Muscovite | 37.46 | 44.55 | 2.30 | 0.74 | 0.19 | 0.97 | 6.53 | 2.24 | 2.33 | 2.69 |
| Talc | 0.84 | 60.61 | 0.71 | - | - | 29.07 | - | - | 6.65 | 2.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, H.; Gao, Y.; Khoso, S.A.; Ji, W.; Hu, Y. Interpretation of Hydrophobization Behavior of Dodecylamine on Muscovite and Talc Surface through Dynamic Wettability and AFM Analysis. Minerals 2018, 8, 391. https://doi.org/10.3390/min8090391
Jiang H, Gao Y, Khoso SA, Ji W, Hu Y. Interpretation of Hydrophobization Behavior of Dodecylamine on Muscovite and Talc Surface through Dynamic Wettability and AFM Analysis. Minerals. 2018; 8(9):391. https://doi.org/10.3390/min8090391
Chicago/Turabian StyleJiang, Hao, Ya Gao, Sultan Ahmed Khoso, Wanying Ji, and Yuehua Hu. 2018. "Interpretation of Hydrophobization Behavior of Dodecylamine on Muscovite and Talc Surface through Dynamic Wettability and AFM Analysis" Minerals 8, no. 9: 391. https://doi.org/10.3390/min8090391




