Variations of Major and Minor Elements in Pt–Fe Alloy Minerals: A Review and New Observations
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Variations in the Pt–Fe Alloys from Lode Deposits
3.2. Variations in the Compositions of the Pt–Fe Alloys from Placer Deposits
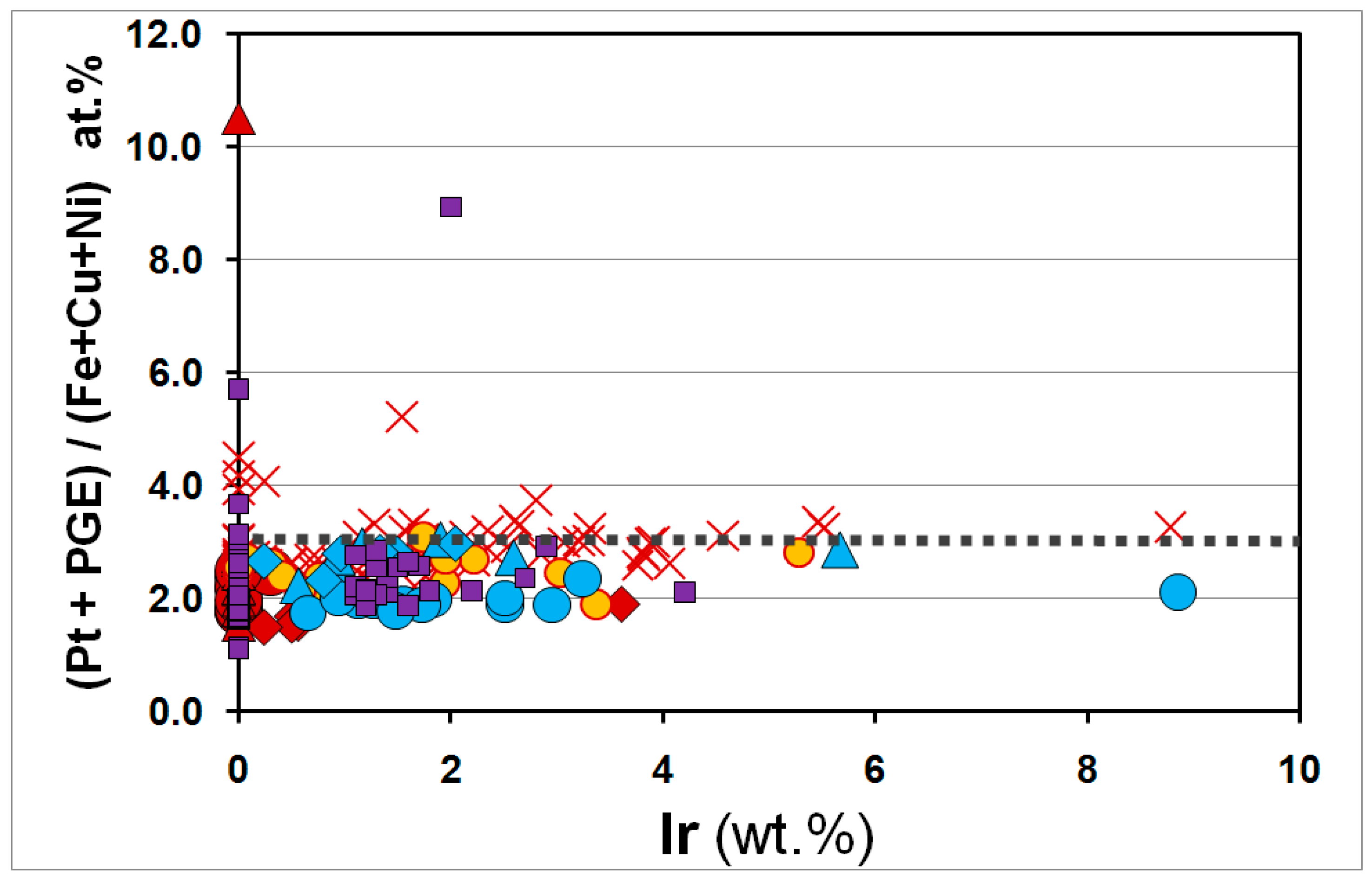
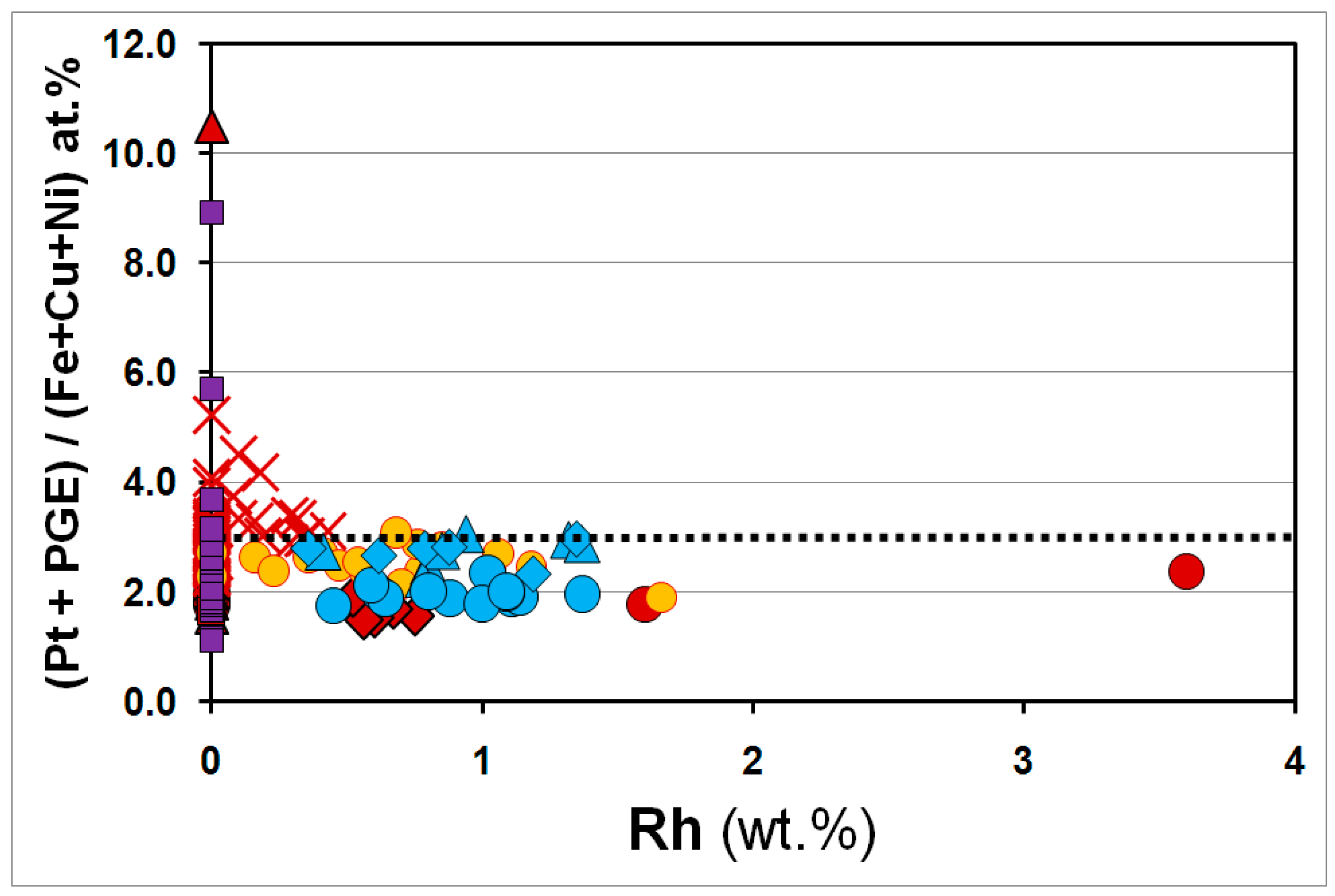
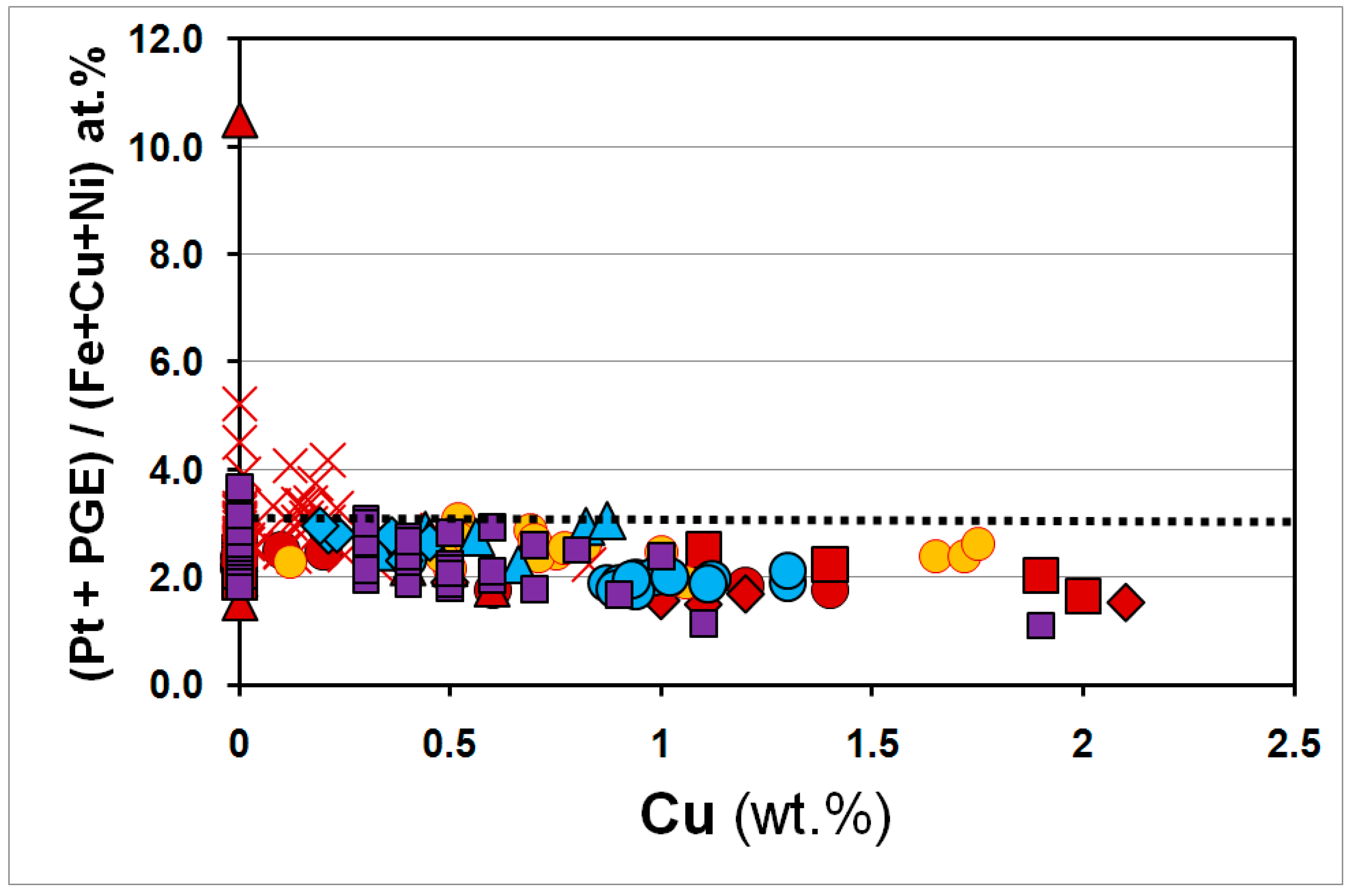
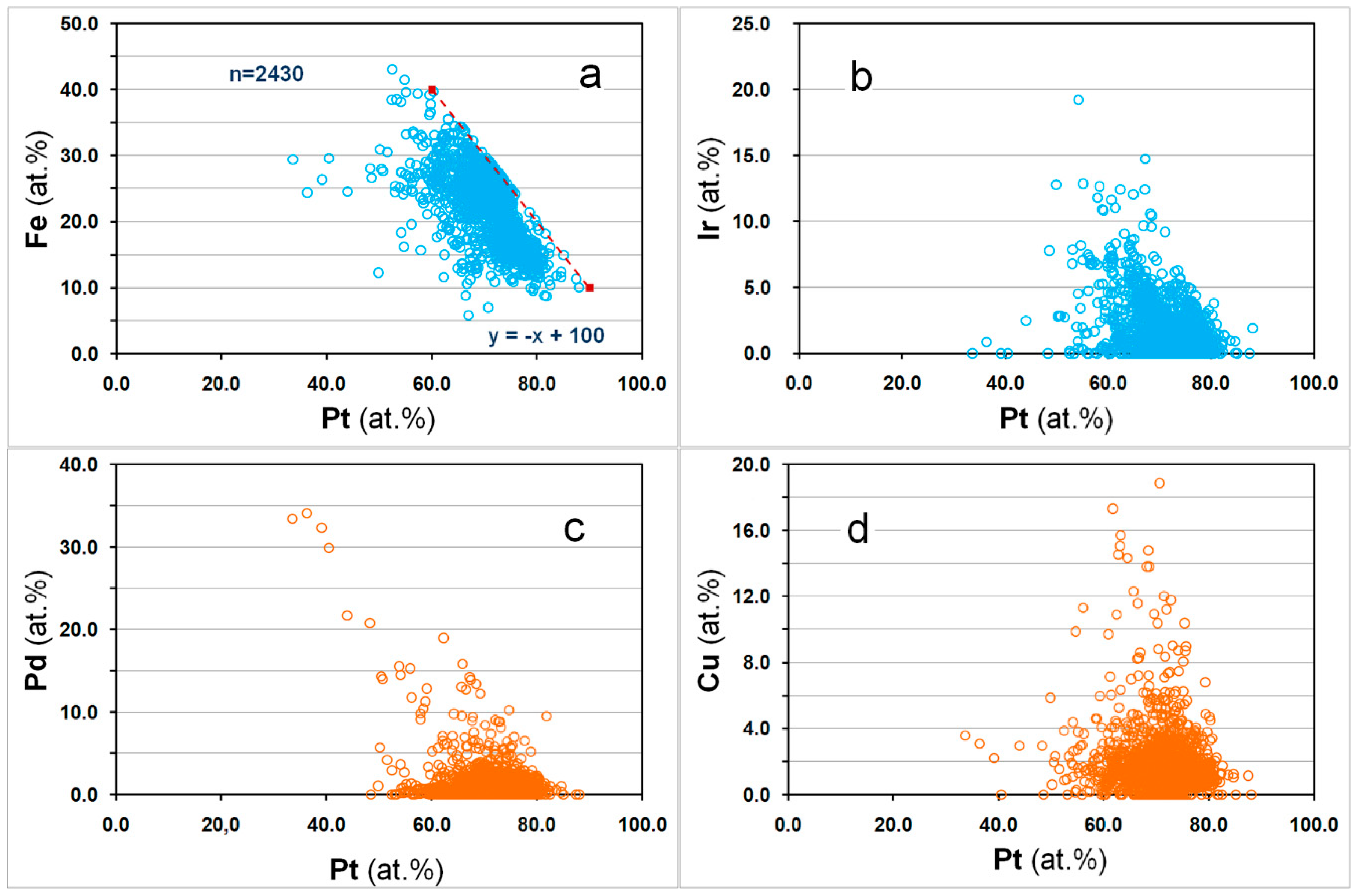
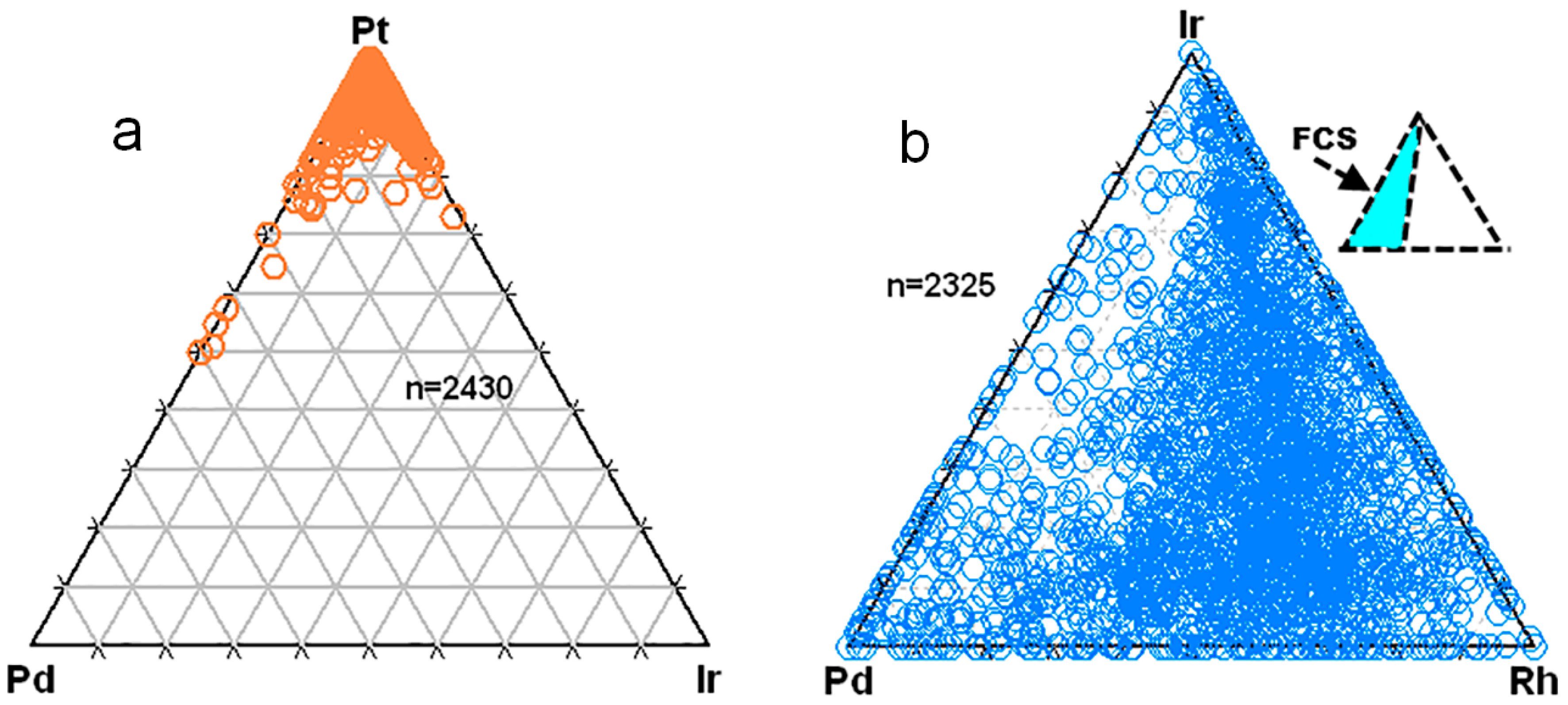
3.3. Overall Variations in the Compositions of the Pt–Fe Alloys on a Global Scale
4. Discussion and Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cabri, L.J.; Feather, C.E. Platinum-iron alloys: A nomenclature based on a study of natural and synthetic alloys. Can. Mineral. 1975, 13, 117–126. [Google Scholar]
- Cabri, L.J.; Rosenzweig, A.; Pinch, W.W. Platinum-group minerals from Onverwacht. I. Pt-Fe-Cu-Ni alloys. Can. Mineral. 1977, 15, 380–384. [Google Scholar]
- Cabri, L.J. (Ed.) The Geology, Geochemistry, Mineralogy, Mineral Beneficiation of the Platinum-Group Elements; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2002; Volume 54, p. 852. [Google Scholar]
- Cabri, L.J.; Harris, D.C.; Weiser, T.W. Mineralogy and distribution of platinum-group mineral (PGM) placer deposits of the world. Explor. Min. Geol. 1996, 5, 73–167. [Google Scholar]
- Bowles, J.F.W. Platinum-iron alloys, their structural and magnetic characteristics in relation to hydrothermal and low-temperature genesis. Mineral. Petrol. 1990, 43, 37–47. [Google Scholar] [CrossRef]
- Nosé, Y.; Kushida, A.; Ikeda, T.; Nakajima, H.; Tanaka, K.; Numakura, H. Re-examination of Phase Diagram of Fe–Pt System. Mater. Trans. 2003, 44, 2723–2731. [Google Scholar] [CrossRef]
- Bowles, J.F.W.; Suárez, S.; Prichard, H.M.; Fisher, P.C. Weathering of PGE sulfides and Pt–Fe alloys in the Freetown Layered Complex, Sierra Leone. Miner. Depos. 2017, 52, 1127–1144. [Google Scholar] [CrossRef] [Green Version]
- Evstigneeva, T.L. Phases in the Pt-Fe system. Vestnik Otdeleniya nauk o Zemle RAN 2009, 1, 1–2. [Google Scholar]
- Barkov, A.Y.; Fleet, M.E.; Nixon, G.T.; Levson, V.M. Platinum-group minerals from five placer deposits in British Columbia, Canada. Can. Mineral. 2005, 43, 1687–1710. [Google Scholar] [CrossRef]
- Cabri, L.J. Mineralogical Study of Five Samples from a PGE Regolith, Kapalagulu Intrusion, Tanzania; Unpublished Report; Cabri Consulting Inc.: Ottawa, ON, Canada, 2004. [Google Scholar]
- Mostert, A.B.; Hofmeyr, P.K.; Potgieter, G.A. The platinum-group mineralogy of the Merensky Reef at the Impala platinum mines, Bophuthatswana. Econ. Geol. 1982, 77, 1385–1394. [Google Scholar] [CrossRef]
- Rudashevsky, N.S.; Avdontsev, S.N.; Dneprovskaya, M.B. Evolution of PGE mineralization in hortonolitic dunites of the Mooihoek and Onverwacht pipes, Bushveld Complex. Mineral. Petrol. 1992, 47, 37–54. [Google Scholar] [CrossRef]
- Wilhelmij, H.R.; Cabri, L.J. Platinum mineralization in the Kapalagulu Intrusion, western Tanzania. Miner. Depos. 2016, 51, 343–367. [Google Scholar] [CrossRef]
- Cabri, L.J.; Wilhelmij, H.R.; Eksteen, J.J. Contrasting mineralogical and processing potential of two mineralization types in the platinum group element and nickel-bearing Kapalagulu Intrusion, western Tanzania. Ore Geol. Rev. 2017, 90, 772–789. [Google Scholar] [CrossRef]
- Nixon, G.T.; Cabri, L.J.; Laflamme, J.H.G. Platinum-group element mineralization in lode and placer deposits associated with the Tulameen Alaskan type complex, British Columbia. Can. Mineral. 1990, 28, 503–535. [Google Scholar]
- Good, D.J.; Cabri, L.J.; Ames, D.E. PGM Facies variations for Cu–PGE deposits in the Coldwell Alkaline Complex, Ontario, Canada. Ore Geol. Rev. 2017, 90, 748–771. [Google Scholar] [CrossRef]
- Sidorov, E.G.; Kozlov, A.P.; Tolstykh, N.D. The Gal’moenan Basic-Ultrabasic Massif and Its Platinum Potential; Nauchnyi Mir Publisher: Moscow, Russia, 2012; p. 288. (In Russian) [Google Scholar]
- Nekrasov, I.Y.; Lennikov, A.M.; Zalishchak, B.L.; Oktyabrsky, R.A.; Ivanov, V.V.; Sapin, V.I.; Taskaev, V.I. Compositional variations in platinum-group minerals and gold, Konder alkaline-ultrabasic massif, Aldan Shield, Russia. Can. Mineral. 2005, 43, 637–654. [Google Scholar] [CrossRef]
- Augé, T.; Genna, A.; Legendre, O.; Ivanov, K.S.; Volchenko, Y.A. Primary Platinum Mineralization in the Nizhny Tagil and Kachkanar Ultramafic Complexes, Urals, Russia: A Genetic Model for PGE Concentration in Chromite-Rich Zones. Econ. Geol. 2005, 100, 707–732. [Google Scholar] [CrossRef]
- Zaccarini, F.; Garuti, G.; Pushkarev, E.; Thalhammer, O. Origin of Platinum-Group Minerals (PGM) Inclusions in Chromite Deposits of the Urals. Minerals 2018, 8, 379. [Google Scholar] [CrossRef]
- Cabri, L.J. Electro-Hydraulic (EH) Crushing and Hydroseparation (HS) Concentration of Three Samples from Yubdo, Ethiopia; Unpublished CNT-MC report 2007-3; CNT-MC Inc.: Ottawa, ON, Canada, 2007; 40p. [Google Scholar]
- Barkov, A.Y.; Martin, R.F.; LeBarge, W.; Fedortchouk, Y. Grains of Pt-Fe alloy and inclusions in a Pt-Fe alloy from Florence creek, Yukon, Canada: Evidence for mobility of Os in a Na-H2O-Cl-rich fluid. Can. Mineral. 2008, 46, 343–360. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Martin, R.F.; Fleet, M.E.; Nixon, G.T.; Levson, V.M. New data on associations of platinum-group minerals in placer deposits of British Columbia, Canada. Mineral. Petrol. 2007, 92, 9–29. [Google Scholar] [CrossRef]
- Laflamme, J.H.G. Mineralogical Study of Platinum-Group Minerals from Au-Pt-Bearing Placer Samples from British Columbia; Report MMSL 02-038(CR): Appendix “Electron Microprobe Data”; CANMET Mining and Mineral Sciences Laboratories: Ottawa, ON, Canada, 2002; pp. A-1–A-19. [Google Scholar]
- Fedortchouk, Y.; LeBarge, W.; Barkov, A.Y.; Fedele, L.; Bodnar, R.J.; Martin, R.F. Platinum-group minerals from a placer deposit in Burwash creek, Kluane area, Yukon Territory, Canada. Can. Mineral. 2010, 48, 583–596. [Google Scholar] [CrossRef]
- Tolstykh, N.D.; Foley, J.Y.; Sidorov, E.G.; Laajoki, K.V.O. Composition of the platinum-group minerals in the Salmon River placer deposit, Goodnews bay, Alaska. Can. Mineral. 2002, 40, 463–471. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Martin, R.F.; Shi, L.; Feinglos, M.N. New data on PGE alloy minerals from a very old collection (probably 1890s), California. Am. Miner. 2008, 93, 1574–1580. [Google Scholar] [CrossRef]
- Gornostayev, S.S.; Crocket, J.H.; Mochalov, A.G.; Laajoki, K.V.O. The Platinum-Group Minerals of the Baimka Placer Deposits, Aluchin Horst, Russian Far East. Can. Mineral. 1999, 37, 1117–1129. [Google Scholar]
- Airiyants, E.V.; Zhmodik, S.M.; Ivanov, P.O.; Belyanin, D.K.; Agafonov, L.V. Mineral inclusions in Fe-Pt solid solution from the alluvial ore occurrences of the Anabar basin (northeastern Siberian Platform). Russ. Geol. Geophys. 2014, 55, 945–958. [Google Scholar] [CrossRef]
- Sidorov, E.G.; Tolstykh, N.D.; Podlipsky, M.Yu.; Pakhomov, I.O. Placer PGE minerals from the Filippa clinopyroxenite-dunite massif (Kamchatka). Russ. Geol. Geophys. 2004, 45, 1080–1097. [Google Scholar]
- Barkov, A.Y.; Shvedov, G.I.; Silyanov, S.A.; Martin, R.F. Mineralogy of Platinum-Group Elements and Gold in the Ophiolite-Related Placer of the River Bolshoy Khailyk, Western Sayans, Russia. Minerals 2018, 8, 247. [Google Scholar] [CrossRef]
- Cabri, L.J.; Laflamme, J.H.G. Platinum-group minerals from the Konder massif, Russian Far East. Mineral. Rec. 1997, 28, 97–106. [Google Scholar]
- Malitch, K.N.; Thalhammer, O.A.R. Pt-Fe nuggets derived from clinopyroxenite-dunite massifs, Russia: A structural, compositional and osmium-isotope study. Can. Mineral. 2002, 40, 395–418. [Google Scholar] [CrossRef]
- Tolstykh, N.D.; Sidorov, E.G.; Laajoki, K.V.O.; Krivenko, A.P.; Podlipskiy, M. The association of platinum-group minerals in placers of the Pustaya River, Kamchatka, Russia. Can. Mineral. 2000, 38, 1251–1264. [Google Scholar] [CrossRef]
- Barkov, A.Y.; Shvedov, G.I.; Martin, R.F. PGE–(REE–Ti)-rich micrometer-sized inclusions, mineral associations, compositional variations, and a potential lode source of platinum-group minerals in the Sisim Placer Zone, Eastern Sayans, Russia. Minerals 2018, 8, 181. [Google Scholar] [CrossRef]
- Krivenko, A.P.; Tolstykh, N.D.; Nesterenko, G.V.; Lazareva, E.V. Types of mineral assemblages of platinum metals in auriferous placers of the Altai-Sayan region. Russ. Geol. Geophys. 1994, 35, 58–65. [Google Scholar]
- Zhmodik, S.M.; Nesterenko, G.V.; Airiyants, E.V.; Belyanin, D.K.; Kolpakov, V.V.; Podlipsky, M.Y.; Karmanov, N.S. Alluvial platinum—Group minerals as indicators of primary PGE mineralization (placers of southern Siberia). Russ. Geol. Geophys. 2016, 57, 1437–1464. [Google Scholar] [CrossRef]
- Zaccarini, F.; Pushkarev, E.; Garuti, G.; Krause, J.; Dvornik, G.P.; Stanley, C.; Bindi, L. Platinum–group minerals (PGM) nuggets from alluvial—Eluvial placer deposits in the concentrically zoned mafic-ultramafic Uktus complex (Central Urals, Russia). Eur. J. Miner. 2013, 25, 519–531. [Google Scholar] [CrossRef]
- Johan, Z.; Ohnenstetter, M.; Fischer, M.; Amossé, J. Platinum-Group Minerals from the Durance River Alluvium, France. Mineral. Petrol. 1990, 42, 287–306. [Google Scholar] [CrossRef]
- Augé, T.; Legendre, O. Pt-Fe nuggets from alluvial deposits in eastern Madagascar. Can. Mineral. 1992, 30, 983–1004. [Google Scholar]
- Oberthür, T.; Weiser, T.W.; Melcher, F.; Gast, L.; Wöhrl, C. Detrital platinum-group minerals in rivers draining the Great Dyke, Zimbabwe. Can. Mineral. 2013, 51, 197–222. [Google Scholar] [CrossRef]
- Oberthür, T.; Melcher, F.; Gast, L.; Wöhrl, C.; Lodziak, J. Detrital platinum-group minerals in rivers draining the eastern Bushveld complex, South Africa. Can. Mineral. 2004, 42, 563–582. [Google Scholar] [CrossRef]
- Melcher, F.; Oberthür, T.; Lodziak, J. Modification of detrital platinum-group minerals from the eastern Bushveld complex, South Africa. Can. Mineral. 2005, 43, 1711–1734. [Google Scholar] [CrossRef]
- Yu, Z. Chengdeite—An ordered natural iron-iridium alloy. Dizhi Xuebao 1995, 69, 215–220. (In Chinese) [Google Scholar]
- Bose, S.K.; Kudrnovsky, J.; van Schilfgaarde, M.; Blöchl, P.; Jepsen, O.; Methfessel, M.; Paxton, A.T.; Andersen, O.K. Electronic structure of ordered and disordered Pd3Fe. J. Magn. Magn. Mater. 1990, 87, 97–105. [Google Scholar] [CrossRef]
- Zhernovsky, I.V.; Mochalov, A.G.; Rudashevsky, N.S. Phase inhomogeneity of isoferroplatinum enriched in iron. Dokl. Akad. Nauk SSSR 1985, 283, 196–200. (In Russian) [Google Scholar]
- Barkov, A.Y.; Martin, R.F.; Halkoaho, T.A.A.; Criddle, A.J. Laflammeite, Pd3Pb2S2, a new platinum-group mineral species from the Penikat layered complex, Finland. Can. Mineral. 2002, 40, 671–678. [Google Scholar] [CrossRef]
- Tarkian, M.; Krstić, S.; Klaska, K.-H.; Ließmann, W. Rhodarsenide, (Rh, Pd)2As, a new mineral. Eur. J. Mineral. 1997, 9, 1321–1326. [Google Scholar] [CrossRef]
- Britvin, S.N.; Rudashevskiy, N.S.; Bogdanova, A.N.; Shcherbachev, D.K. Palladodymite (Pd,Rh)2As—A new mineral from placer in Miass River, Urals. Zap. Vseross. Mineral. Obshch. 1999, 128, 39–42. (In Russian) [Google Scholar]
- Rahm, M.; Hoffmann, R.; Ashcroft, N.W. Atomic and Ionic Radii of Elements 1–96. Chem. Eur. J. 2016, 22, 14625–14632. [Google Scholar] [CrossRef]
- Oberthür, T. The Fate of Platinum-Group Minerals in the Exogenic Environment—From Sulfide Ores via Oxidized Ores into Placers: Case Studies Bushveld Complex, South Africa, and Great Dyke, Zimbabwe. Minerals 2018, 8, 581. [Google Scholar] [CrossRef]
- Weiser, T.W. Platinum-Group Minerals (PGM) from placer deposits in the mineral collection of the Museum of Natural History, Vienna, Austria. Ann. Naturhist. Mus. Wien 2004, 105A, 1–28. [Google Scholar]
- Barkov, A.Y.; Shvedov, G.I.; Polonyankin, A.A.; Martin, R.F. New and unusual Pd-Tl-bearing mineralization in the Anomal’nyi deposit, Kondyor concentrically zoned complex, northern Khabarovskiy kray, Russia. Mineral. Mag. 2017, 81, 679–688. [Google Scholar] [CrossRef]
- Mertie, J.B., Jr. The Goodnews platinum deposits Alaska. U.S. Geol. Survey 1940, 918, 97. [Google Scholar]
- Mertie, J.B., Jr. Platinum deposits of the Goodnews Bay District Alaska. U.S. Geol. Survey 1976, 938, 42. [Google Scholar]
- O’Driscoll, B.; González-Jiménez, J.M. Petrogenesis of the Platinum-Group Minerals. Rev. Mineral. Geochem. 2015, 81, 489–578. [Google Scholar] [CrossRef] [Green Version]
- Garuti, G.; Pushkarev, E.V.; Zaccarini, F. Composition and paragenesis of Pt alloys from chromitites of the Uralian Alaskan-type Kytlym and Uktus complexes, northern and central Urals, Russia. Can. Mineral. 2002, 40, 357–376. [Google Scholar] [CrossRef]
- Ohnenstetter, M. Platinum group element enrichment in the upper mantle peridotites of the Monte Maggiore ophiolitic massif (Corsica, France): Mineralogical evidence for ore-fluid metasomatism. Mineral. Petrol. 1992, 46, 85–107. [Google Scholar] [CrossRef]
- Tolstykh, N.; Kozlov, A.; Telegin, Yu. Platinum mineralization of the Svetly Bor and Nizhny Tagil intrusions, Ural Platinum Belt. Ore Geol. Rev. 2015, 67, 234–243. [Google Scholar] [CrossRef]
- Stepanov, S.Y.; Malitch, K.N.; Kozlov, A.V.; Badanina, I.Y.; Antonov, A.V. Platinum group element mineralization of the Svetly Bor and Veresovy Bor clinopyroxenite–dunite massifs, Middle Urals, Russia. Geol. Ore Depos. 2017, 59, 244–255. [Google Scholar] [CrossRef]









| Number of Data-Points (N) | Reference | ||
|---|---|---|---|
| Lode Deposits | |||
| 1 | Dunite pipes and Merensky Reef; Bushveld Layered Complex; South Africa | 9 | [2,11,12] |
| 2 | Kapalagulu Layered Intrusion; western Tanzania | 9 | [10,13,14] |
| 3 | Tulameen Alaskan-type Complex; British Columbia; Canada | 6 | [15] |
| 4 | Coldwell Alkaline Complex; Ontario; Canada | 10 | [16] |
| 5 | Gal’moenan Mafic-Ultramafic Complex; Koryak Upland; Kamchatka krai; Russia | 70 | [17] |
| 6 | Kondyor concentrically zoned Alkaline Ultramafic Complex, northern Khabarovskiy krai; Aldan Shield; Russia | 19 | [18] |
| 7 | Kachkanar Alaskan -Uralian-type Ultramafic Complex; Urals; Russia | 7 | [19] |
| 8 | Nizhniy Tagil Alaskan -Uralian-type Ultramafic Complex; Urals; Russia | 13 | [19] |
| 9 | Ophiolitic Chromitites; Uktus and Kytlym areas; central Urals; Russia | 6 | [20] |
| 10 | Saprolite after mineralized dunite weathered; Yubdo; Ethiopia | 83 | [21] |
| Placer Deposits | |||
| 11 | Placers of North Saskatchewan River; Alberta; Canada | 295 | [4] |
| 12 | Similkameen-Tulameen River System; British Columbia; Canada | 18 | [4,15] |
| 13 | Placers of Liard River; Northwest Territories; Canada | 196 | [4] |
| 14 | Placers of Saskatchewan River; Saskatchewan; Canada | 18 | [4] |
| 15 | Florence Creek; Yukon; Canada | 35 | [4,22] |
| 16 | Au-PGE placer deposits; British Columbia; Canada | 77 | [9,23] |
| 17 | Detrital grains from McConnell Stream; Dease Stream; Birch Stream; British Columbia; Canada | 99 | [24] |
| 18 | Detrital grains; Burwash Creek; Kluane area; Yukon; Canada | 15 | [25] |
| 19 | Fox Gulch; Alaska; USA | 2 | [4] |
| 20 | Salmon River Placer; Goodnews bay; Alaska; USA | 29 | [26] |
| 21 | Detrital grains from Trinity County; California; USA | 4 | [4,27] |
| 22 | Syssert Placer Zone; Omutnaya River; Urals; Russia | 6 | [4] |
| 23 | Placers from Nizhniy Tagil; Kushvinskiy and Nevyansk areas; Urals; Russia | 137 | [4] |
| 24 | Placers; western Chukotka; Russia | 47 | [4,28] |
| 25 | Placers; Anabar basin; northeastern Siberian Platform; Russia | 3 (mean of 105) | [29] |
| 26 | Placers derived from Filippa clinopyroxenite-dunite complex; Kamchatka; Russia | 43 | [30] |
| 27 | Placer of River Bolshoy Khailyk; western Sayans; Russia | 10 | [31] |
| 28 | Placer derived from Kondyor Alkaline Ultramafic Complex; northern Khabarovskiy krai; Russia | 14 | [32] |
| 29 | Placers associated with Kondyor, Inagli and Guli concentrically zoned Complexes; northeastern Russia | 13 | [33] |
| 30 | Placer at Pustaya River; Kamchatka; Russia | 15 | [34] |
| 31 | Sisim Placer Zone; eastern Sayans; Russia | 19 | [35] |
| 32 | Placer at River Ko; eastern Sayans; Russia | 17 | [36] |
| 33 | Placers of southern Siberia; Russia | 20 | [37] |
| 34 | Placers associated with concentrically zoned Uktus complex; central Urals; Russia | 4 | [38] |
| 35 | Placers from Rio Condoto area; Chocó; Colombia | 461 | [4] |
| 36 | Placers from Santiago River area; Esmeraldas Province; Ecuador | 104 | [4] |
| 37 | Placers at Yubdo; Ethiopia | 5 | [4] |
| 38 | Placers at Riam Kanan; South Kalimantan; Indonesia | 51 | [4] |
| 39 | Placers of Borneo; Sabah Province; Malaysia | 20 | [4] |
| 40 | Placers from Papua New Guinea | 6 | [4] |
| 41 | Placer of Ortakale River; Kars Province; Turkey | 3 | [4] |
| 42 | Placers of Transvaal and Orange Free State; South Africa | 24 | [4] |
| 43 | Placers of Tasmania; Australia | 9 | [4] |
| 44 | Placers from Itabira; Brazil | 5 | [4] |
| 45 | Placers of Chindwin River; Burma | 315 | [4] |
| 46 | Durance River; France | 5 | [39] |
| 47 | Placers of eastern Madagascar | 18 | [40] |
| 48 | Placers of rivers draining Great Dyke; Zimbabwe | 3 | [41] |
| 49 | Placers of rivers draining eastern Bushveld complex; South Africa | 7 | [42,43] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barkov, A.Y.; Cabri, L.J. Variations of Major and Minor Elements in Pt–Fe Alloy Minerals: A Review and New Observations. Minerals 2019, 9, 25. https://doi.org/10.3390/min9010025
Barkov AY, Cabri LJ. Variations of Major and Minor Elements in Pt–Fe Alloy Minerals: A Review and New Observations. Minerals. 2019; 9(1):25. https://doi.org/10.3390/min9010025
Chicago/Turabian StyleBarkov, Andrei Y., and Louis J. Cabri. 2019. "Variations of Major and Minor Elements in Pt–Fe Alloy Minerals: A Review and New Observations" Minerals 9, no. 1: 25. https://doi.org/10.3390/min9010025




