4.1. The Effect of Primary and Post-Depositional Changes, Evidence from Grain Texture, Bulk Chemical Compositions, and Mineral Chemistry
Detailed studies on separated mineral grains from sediments in hydrothermal environments can help enhance interpretation of the precipitation conditions of hydrothermal particles feeding the sediments. However geochemical investigations on isolated grains texture from metalliferous sediments are rare. Among the few studies are the work of Dias and Barriga [
3] who used the occurrence of micro-chimney particles from metalliferous sediment of Mount Saldanha hydrothermal field to suggest the intensity of hydrothermal activity. The observed Cu and Fe sulfide-rich micro-chimney-like structure (
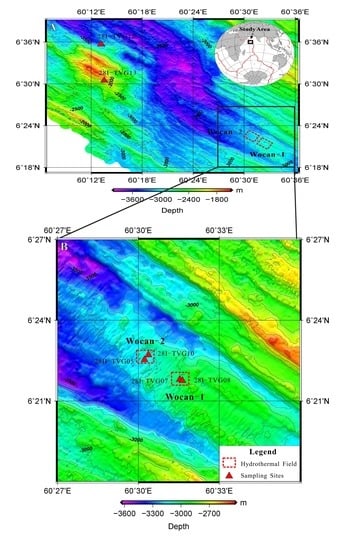
Figure 2G,H) at station TVG-08 can be related to pervasive hydrothermal contributions into the station relative to TVG-07, TVG-10 and TVG-05. In contrast, the typical etched features shown by the pyrite and chalcopyrite separates of TVG-10 from Wocan-2 sediment (
Figure 2C–F), can be related to dissolution arising from the effect of seafloor weathering and hydrothermal reworking process.
Concerning the bulk sediment geochemical compositions, previous studies had utilized elevated U/Fe ratios in metalliferous sediments to suggest secondary sulfide oxidation in hydrothermal environments [
8,
11,
12,
18,
19]. Moreover, high U contents (>10 ppm) in hydrothermal sediments are consistent with the uptake of seawater and sulfide oxidation [
8,
11,
18]. The high U concentrations (up to 19.6 ppm), and U/Fe enrichments (up to 6.24 × 10
−5) in the bulk sediment samples of station TVG-10 from Wocan-2 suggested a higher rate of sulfide oxidation. The dominant weathering features on sulfide grain separates of metalliferous sediment of station TVG-10 (
Figure 2C–F) is consistent with the high U/Fe ratio. Additionally, References [
8,
18,
20] reported a wide range of Fe/Mn ratio in the near vent metal-enriched sediments of Lucky Strike hydrothermal field (1000–3000), Trans-Atlantic Geotraverse (TAG) hydrothermal field (900–2000), and OBS vent field(900–2400), and attributed the high ratio (e.g., >1000) to near-vent hydrothermal settings. The narrow range (1373–1545) in the Fe/Mn ratio of the Wocan-1 sediment relative to the OBS, TAG and Lucky Strike sediment suggests a homogeneous chemical composition of Wocan-1 sediment. Moreover, the >1000 values of the Fe/Mn ratio of the sediment samples of TVG-07 and TVG-08 suggest the proximity of the sampling stations to high temperature venting sites.
Concerning the mineral chemistry, Se concentration in pyrite had been suggested as a new geothermometer for hydrothermal ore deposits, and varied according to changes in pH and redox conditions of ore-forming fluids [
21,
22,
23]. High concentration of Se and Co in pyrite are typical of high-temperature Cu-rich ores in the volcanogenic massive sulfide deposits (VMS) [
22]. Distinct enrichments of Se (up to 0.046 wt.%,
n = 6) and Co (up to 0.07 wt.%,
n = 26), in pyrite separates of the Wocan-1 sediment, and below detection limit (< 0.01 wt.%) in the pyrite separates of TVG-10 and TVG-05 of Wocan-2 station (
Table S2, n=11) can be related to higher temperature conditions and hydrothermal intensity in the Wocan-1 site, relative to Wocan-2.
Generally, the enriched bulk sediment Fe/Mn ratio in Wocan-1 (up to ~1545,
Table 3) is consistent with the enriched Se, and Co content in pyrite separates (
Table 4) and higher values of calculated sphalerite precipitation temperature (~278 °C) (
Table 5). Moreover, it is also consistent with the low S isotopes (3.0–3.6‰) values (
Table 6) and euhedral grain morphology (
Figure 2A,B). According to these signatures, we suggest that the Wocan-1 stations (TVG-08 and TVG-07) were near high-temperature hydrothermal discharge environments (
Figure 4).
The enriched bulk sediment U/Fe (up to 6.24 × 10
−5) ratio, U contents (up to 19.6 ppm), and low Fe/Mn (288–523) ratio in the Wocan-2 sediments are consistent with the low Se and Co concentrations in pyrite separate. Moreover, the lower values of calculated sphalerite precipitation temperature (mean value, ~233 °C,
n = 8) are also consistent with the heavy S isotopes (4.1–8.7‰) values and the weathered grain morphology (
Figure 2C–F). The lower Fe/Mn ratio, high U values and U/Fe ratio, the relatively low Sph.PT, and depleted Se, Co concentration suggest lower temperature conditions in the sampling stations of TVG-10 and TVG-05 in Wocan-2 relative to Wocan-1 (
Figure 4). Based on these signatures, we suggest that the Wocan-2 samples had undergone secondary oxidation in relation to seafloor weathering.
4.2. The Contributions of Hydrothermal Fluid, Evidence from S Isotope Values
Sulfur isotopes may be applied in hydrothermal systems to understand the isotope exchange process, the relative source of sulfur from magmatic rocks, biogenic pyrite re-mobilization and seawater sulfate (SO
42−) reductions [
24].
The S sources in sediment-starved fields were mainly seawater-derived sulfate and magmatic sulfur [
25]. Based on two-component mixing model (δ
34S
-mix = X × δ
34S
seawater + (1 − X) × δ
34S
basalt [
25,
26,
27]). The δ
34S
seawater, δ
34S
basalt, and δ
34S
-mix represent 21‰, 0‰ and the isotope values of the Wocan-1 and Wocan-2 samples, while, the unknown value (X) represents the amount of seawater component. We quantify the relative contributions of seawater-derived sulfur and magmatic sulfur to the Wocan-1 and Wocan-2 samples (
Table 6). The sulfur source in the metalliferous sediments of Wocan-1 was dominated by magmatic sulfur and lower (<20%) seawater-derived components relative to Wocan-2.
The higher δ
34S values of Wocan-2 sediment samples (e.g., 6.4–8.7‰) at station TVG-05 suggests a reduction of admixed seawater-sulfate (SO
42−)-derived sulfur (H
2S). This assumption is supported by the S isotope end-member modelling according to References [
28,
29,
30] who attributed δ
34S values of 1.5–7.0‰, 0.1 ± 0.5‰, and 21 ± 0.2‰ to vent-fluid/sulfide chimney, Mid Ocean Ridge Basalt (MORB), and seawater, respectively. Furthermore, References [
9,
31] suggested that the mixture of hydrothermal fluid with seawater is not liable to generate δ
34S values that are heavier than 4.5‰. Also, References [
32,
33] suggested that a δ
34S value more than 4.5‰ is an indication of excessive seawater sulfate derived sulfur.
The overlap in S-isotope values (e.g., <4.5‰) between the Wocan-1 (3.0–3.6‰) and Wocan-2 sediments at station TVG-10 (4.1–4.3‰) suggest that station TVG-10 may not be entirely extinct. This assumption is supported by the dominant and minor abundance of chalcopyrite and secondary Cu-mineral assemblages (
Table 2), and higher Cu (5.19 wt.%) contents in bulk sediment (
Table 3) at TVG-10. A previous study [
34] has suggested Fe-dominated mineral assemblages in extinct sulfide mounds in the TAG hydrothermal field. Also, the calculated sphalerite precipitation temperature (~233 °C,
n = 5) further suggests the occurrence of a low-intermediate temperature hydrothermal venting, rather than extinction at TVG-10.
Similar heavier isotope values (6.4–7.4‰) at station TVG-05 (Wocan-2) were reported for sulfide-rich samples from the inactive sulfide chimney at a Yamanaka site in the back-arc spreading center of the southern Mariana Trough. The higher δ
34S values were attributed to the incorporation of seawater sulfate derived sulfur without isotopic fractionation during the reducing process in a seafloor environment [
9]. Therefore, the heavier isotope (6.4–8.7%) values and seawater-derived sulfur components of 37.6–41.4% (
Table 6) further support pervasive post-depositional process via seafloor weathering on Wocan-2 sediments at station TVG-05 relative to TVG-10. However, the low δ
34S values (3.0‰), the magmatic sulfur derived components of > 85%, and the seawater-derived sulfur of <15% (
Table 6) support pervasive hydrothermal processes at station TVG-08 relative to TVG-07. Moreover, the observed micro-chimney structures (
Figure 2G,H) further supports pervasive hydrothermal process at station TVG-08.
In summary, the grain texture, bulk geochemical analysis, mineral chemistry, calculated sphalerite precipitation temperature, and S isotope values show two distinct signatures (type-1 and type-2). Stations TVG-07 and TVG-08 from Wocan-1 shows the characteristics of the type-1 signature, while the type-2 signatures were mainly related to the station TVG-05 and TVG-10 of Wocan-2.