Distribution of Rare Earth Elements plus Yttrium among Major Mineral Phases of Marine Fe–Mn Crusts from the South China Sea and Western Pacific Ocean: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geological Setting and Samples
2.2. Analytical Techniques
2.2.1. Mineralogical Analysis of Bulk Samples
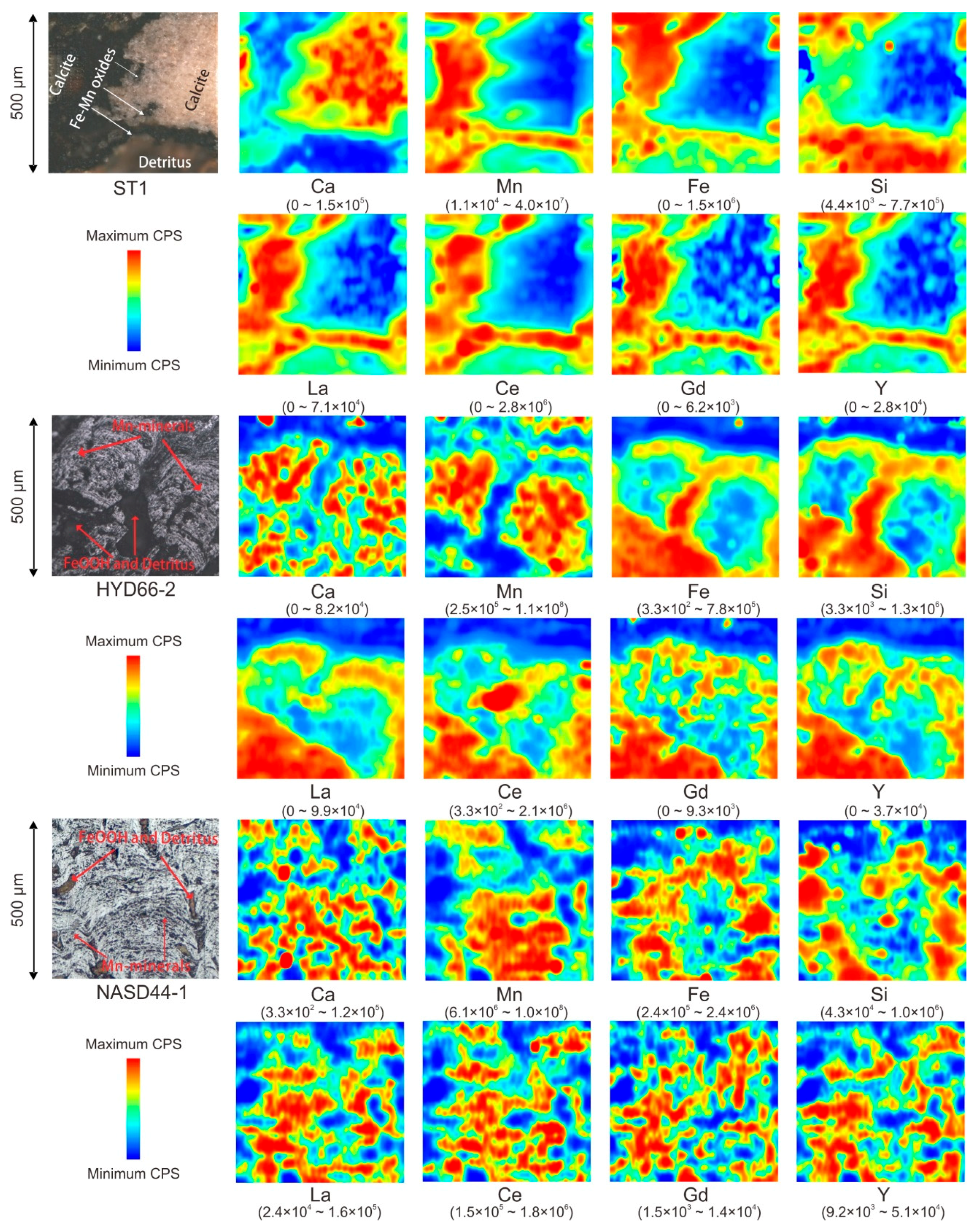
2.2.2. Qualitative Element Mapping by LA-ICP-MS
2.2.3. Geochemical Analysis of Bulk Samples
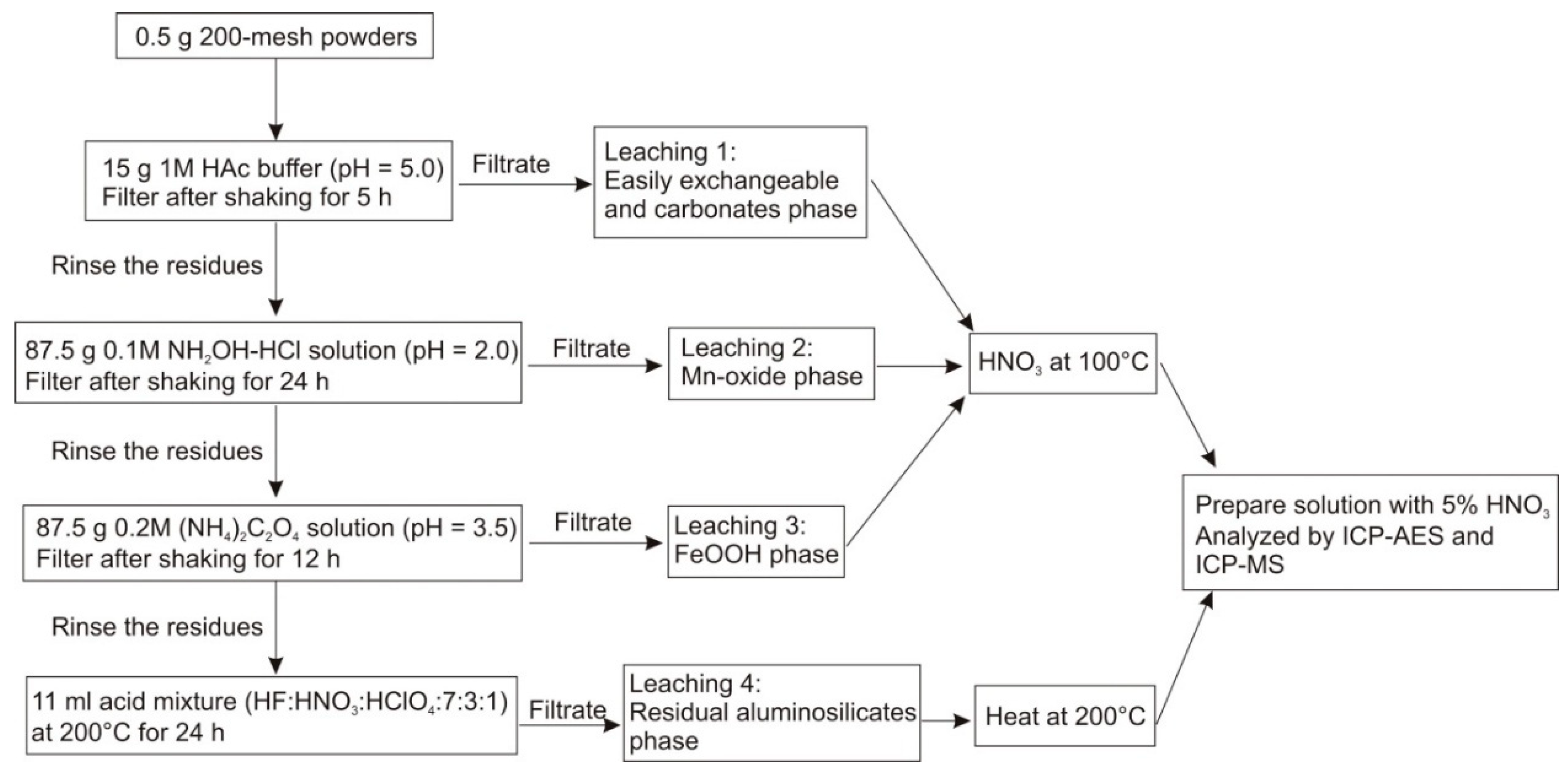
2.2.4. Sequential Leaching Experiment
3. Results
3.1. Mineralogy of the Fe–Mn Crusts
3.2. Distribution of REY in Fe–Mn Crusts
3.3. Geochemistry of Bulk Fe–Mn Crusts
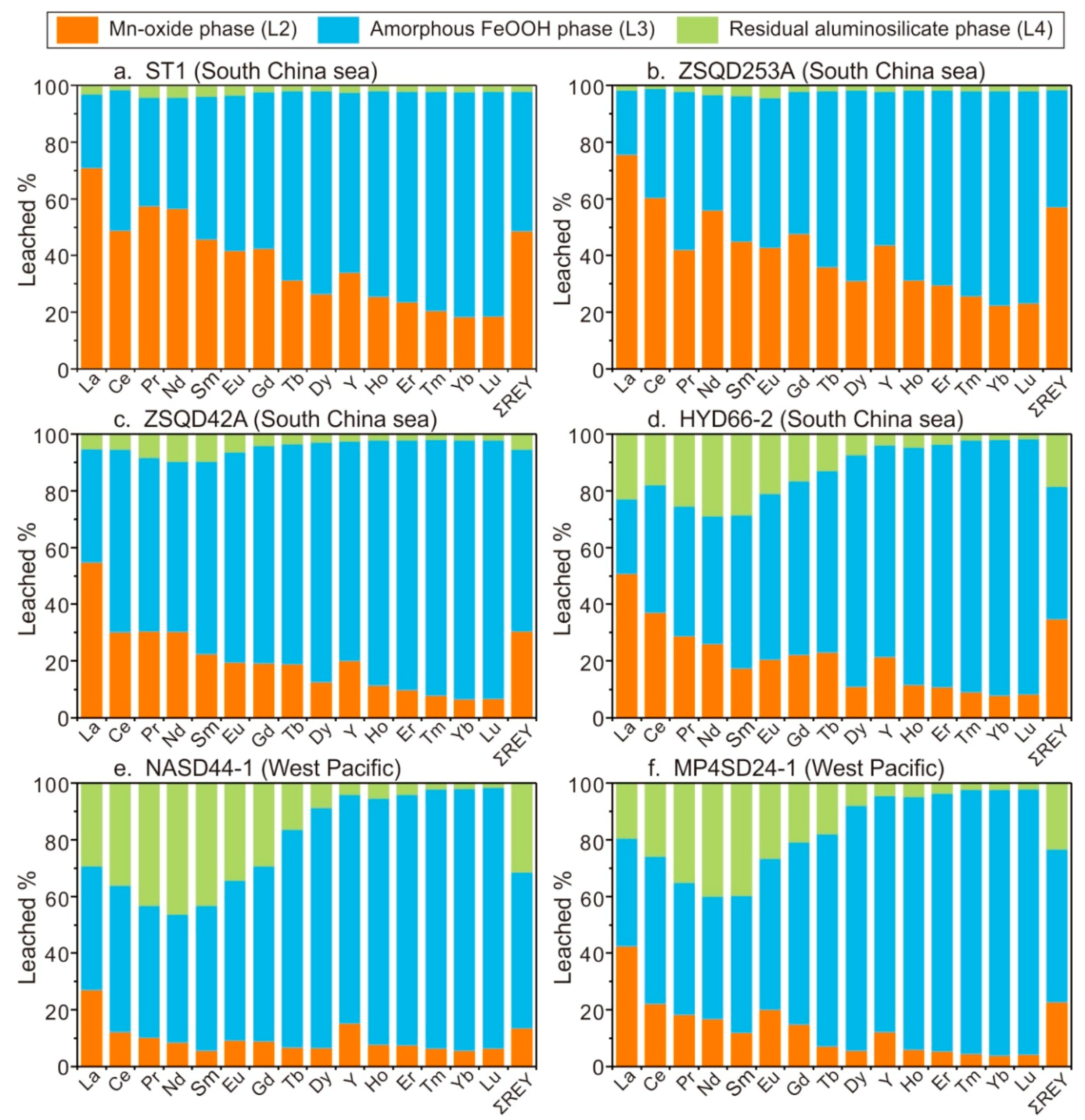
3.4. REY in Major Mineral Phases
4. Discussion
4.1. Validity of LA-ICP-MS Mapping
4.2. Occurrence Phases of REY
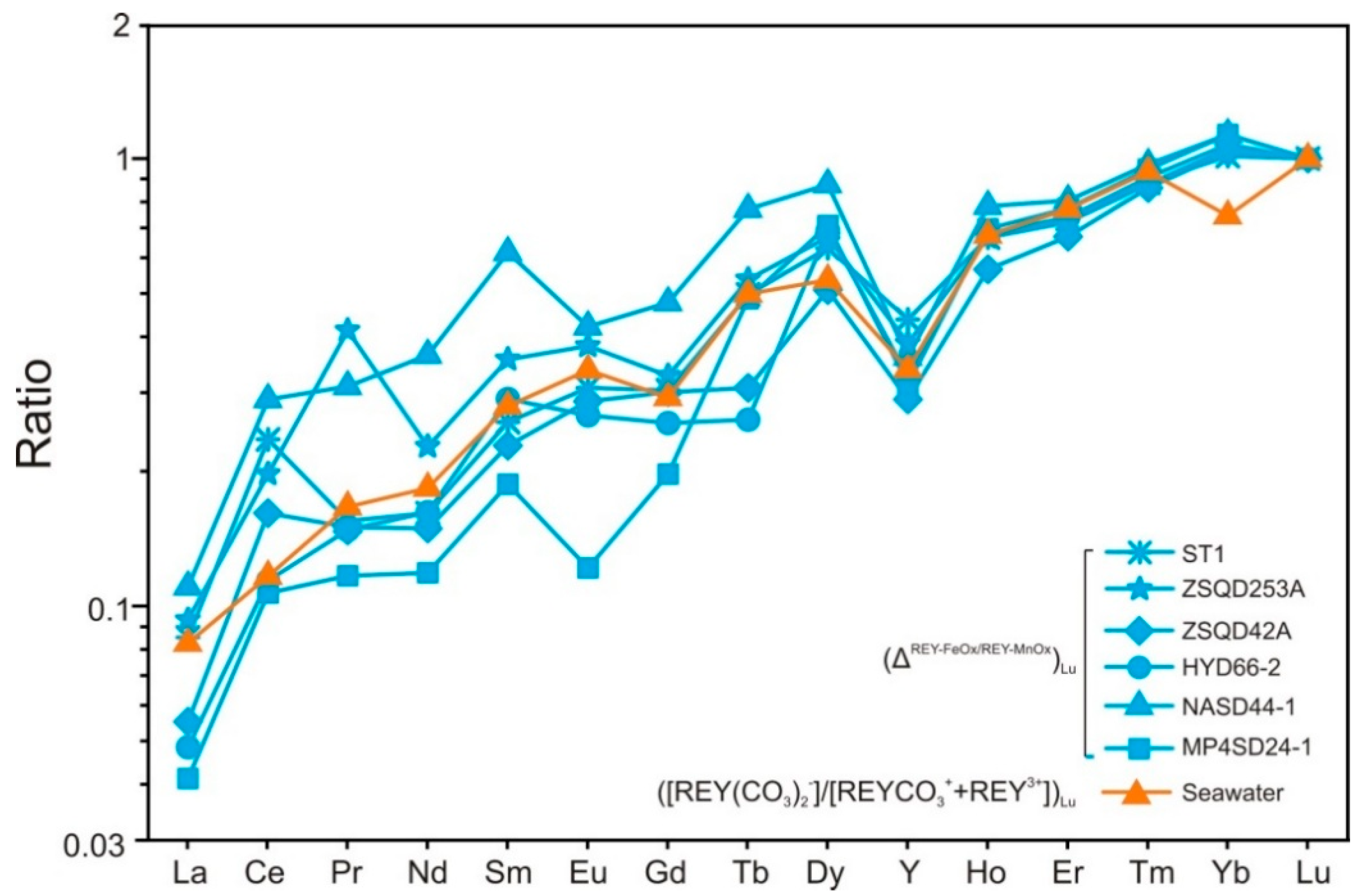
4.3. Enrichment Process of REY
4.4. Factors of the Difference in REY Enrichment in the SCS and WPO Crusts
4.4.1. Terrigenous Input
4.4.2. Growth Rate
5. Conclusions
- 1)
- Mineral composition is a considerable factor in the REY enrichment. Primary occurrence phases for REY in the SCS crusts are δ-MnO2 and amorphous FeOOH, both of which have advantageous crystal structures for REY enrichment. The WPO crusts have a large proportion of aluminosilicate-bound REY. We suggest that REY adsorption by clay minerals may play a role in the REY enrichment in the open-ocean Fe–Mn crusts.
- 2)
- In the hydrogenetic Fe–Mn crusts from the SCS and WPO, most of the ΣREY is bound to Mn-oxide and amorphous FeOOH phases, with LREY and HREY preferentially adsorbed onto δ-MnO2 and FeOOH, respectively. REY-carbonate complexes are the dominate REY species in seawater. Thus, the selective adsorption of major REY complex species from seawater by δ-MnO2 and FeOOH is the predominant mechanism for REY enrichment in the hydrogenetic Fe–Mn crusts.
- 3)
- The SCS crusts grow in the continental margin environment and they are significantly affected by terrigenous materials. The terrigenous aluminosilicate minerals in the SCS crusts dilute the abundance of REY. In contrast, Fe–Mn crusts in open oceans are rarely affected by the continent, and they contain a higher concentration of REY.
- 4)
- The terrigenous inputs and the development of OMZ determine the growth rate of the hydrogenetic Fe–Mn crusts. A fast growth rate is unfavorable for the adsorption of REY from the surrounding seawater into the FeOOH and aluminosilicates. Therefore, the SCS crusts with a faster growth rate contain lower REY enrichment.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hein, J.R.; Koschinsky, A.; Bau, M.; Manheim, F.T.; Kang, J.K.; Roberts, L. Cobalt-rich ferromanganese crusts in the Pacific. In Handbook of Marine Mineral Deposits; CRC Press: Boca Raton, FL, USA, 2000; pp. 239–279. [Google Scholar]
- Hein, J.R.; Reid, J.A.; Conrad, T.A.; Dunham, R.E.; Clague, D.A.; Schulz, M.S.; Davis, A.S. Seamounts and Ferromanganese Crusts Within and Near the U.S. EEZ off California—Data for RV Farnella Cruise F7-87-SC; U.S. Geological Survey: Reston, VA, USA, 2010. [CrossRef]
- Halbach, P.E.; Jahn, A.; Cherkashov, G. Marine Co-rich ferromangannese crust deposits: Description and formation, occurrences and distribution, estmated world-wide resources. In Deep-Sea Minerals: Distribution Characteristics and Their Resource Potential; Sharma, R., Ed.; Springer Nature: Basingstoke, UK, 2017; pp. 65–140. [Google Scholar]
- Bau, M.; Schmidt, K.; Koschinsky, A.; Hein, J.; Kuhn, T.; Usui, A. Discriminating between different genetic types of marine ferro-manganese crusts and nodules based on rare earth elements and yttrium. Chem. Geol. 2014, 381, 1–9. [Google Scholar] [CrossRef]
- Josso, P.; Pelleter, E.; Pourret, O.; Fouquet, Y.; Etoubleau, J.; Cheron, S.; Bollinger, C. A new discrimination scheme for oceanic ferromanganese deposits using high field strength and rare earth elements. Ore Geol. Rev. 2017, 87, 3–15. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A. Deep-ocean ferromanganese crusts and nodules. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 273–291. [Google Scholar]
- Hein, J.R.; Mizell, K.; Koschinsky, A.; Conrad, T.A. Deep-ocean mineral deposits as a source of critical metals for high- and green-technology applications: Comparison with land-based resources. Ore Geol. Rev. 2013, 51, 1–14. [Google Scholar] [CrossRef]
- Marino, E.; González, F.J.; Lunar, R.; Reyes, J.; Medialdea, T.; Castillo-Carrión, M.; Bellido, E.; Somoza, L. High-resolution analysis of critical minerals and elements in Fe–Mn crusts from the Canary Island Seamount Province (Atlantic Ocean). Minerals 2018, 8, 285. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Mikesell, M.; Mizell, K.; Glenn, C.; Wood, R. Marine phosphorites as potential resources for heavy rare earth elements and yttrium. Minerals 2016, 6, 88. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halbach, P.; Manheim, F.T.; Bau, M. Iron and manganese oxide mineralization in the Pacific. Geol. Soc. Lond. Spec. Publ. 1997, 119, 123–138. [Google Scholar] [CrossRef]
- Koschinsky, A.; Gerven, M.V.; Halbach, P. First investigations of massive ferromanganese crusts in the NE Atlantic in comparison with hydrogenetic Pacific occurrences. Mar. Georesour. Geotechnol. 1995, 13, 375–391. [Google Scholar] [CrossRef]
- Hein, J.R.; Conrad, T.; Mizell, K.; Banakar, V.K.; Frey, F.A.; Sager, W.W. Controls on ferromanganese crust composition and reconnaissance resource potential, Ninetyeast Ridge, Indian Ocean. Deep Sea Res. Part I Oceanogr. Res. Pap. 2016, 110, 1–19. [Google Scholar] [CrossRef]
- Prakash, L.S.; Ray, D.; Paropkari, A.L.; Mudholkar, A.V.; Satyanarayanan, M.; Sreenivas, B.; Chandrasekharam, D.; Kota, D.; Raju, K.A.K.; Kaisary, S.; et al. Distribution of REEs and yttrium among major geochemical phases of marine Fe–Mn-oxides: Comparative study between hydrogenous and hydrothermal deposits. Chem. Geol. 2012, 312–313, 127–137. [Google Scholar] [CrossRef]
- Konstantinova, N.; Hein, J.R.; Gartman, A.; Mizell, K.; Barrulas, P.; Cherkashov, G.; Mikhailik, P.; Khanchuk, A. Mineral Phase-Element Associations Based on Sequential Leaching of Ferromanganese Crusts, Amerasia Basin Arctic Ocean. Minerals 2018, 8, 460. [Google Scholar] [CrossRef]
- Grigoriev, A.G.; Zhamoida, V.A.; Gruzdov, K.A.; Krymsky, R.S. Age and growth rates of ferromanganese concretions from the gulf of Finland derived from 210Pb measurements. Oceanology 2013, 53, 345–351. [Google Scholar] [CrossRef]
- Hein, J.R. Mercury- and silver-rich ferromanganese oxides, southern California borderland: Deposit model and environmental implications. Econ. Geol. 2005, 100, 1151–1168. [Google Scholar] [CrossRef]
- Conrad, T.; Hein, J.R.; Paytan, A.; Clague, D.A. Formation of Fe–Mn crusts within a continental margin environment. Ore Geol. Rev. 2017, 87, 25–40. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, X.; Jiang, X.; Sa, R.; Zhou, L.; Huang, Y.; Liu, Y.; Li, X.; Lu, R.; Wang, C. The effect of Fe–Mn minerals and seawater interface and enrichment mechanism of ore-forming elements of polymetallic crusts and nodules from the South China Sea. Acta Oceanol. Sin. 2017, 36, 34–46. [Google Scholar] [CrossRef]
- Guan, Y.; Sun, X.; Ren, Y.; Jiang, X. Mineralogy, geochemistry and genesis of the polymetallic crusts and nodules from the South China Sea. Ore Geol. Rev. 2017, 89, 206–227. [Google Scholar] [CrossRef]
- Zhong, Y.; Chen, Z.; González, F.J.; Hein, J.R.; Zheng, X.; Li, G.; Luo, Y.; Mo, A.; Tian, Y.; Wang, S. Composition and genesis of ferromanganese deposits from the northern South China Sea. J. Asian Earth Sci. 2017, 138, 110–128. [Google Scholar] [CrossRef] [Green Version]
- Guan, Y.; Ren, Y.; Sun, X.; Xiao, Z.; Guo, Z. Helium and argon isotopes in the Fe–Mn polymetallic crusts and nodules from the South China Sea: Constraints on their genetic sources and origins. Minerals 2018, 8, 471. [Google Scholar] [CrossRef]
- Zhang, Z.; Gao, L.; Shen, P.; Wang, Y.; Feng, G. Polimetallic nodules at the northwest continental margin of the South China Sea. Mar. Geol. Lett. 2010, 26, 32. [Google Scholar]
- Guan, Y.; Sun, X.; Shi, G.; Jiang, X.; Lu, H. Rare earth elements composition and constraint on the genesis of the polymetallic crusts and nodules in the South China Sea. Acta Geol. Sin. 2017, 91, 1751–1766. [Google Scholar] [CrossRef]
- Bau, M.; Koschinsky, A. Oxidative scavenging of cerium on hydrous Fe oxide: Evidence from the distribution of rare earth elements and yttrium between Fe oxides and Mn oxides in hydrogenetic ferromanganese crusts. Geochem. J. 2009, 43, 37–47. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Manceau, A.; Geoffroy, N.; Marcus, M.A.; Usui, A. Chemical and structural control of the partitioning of Co, Ce, and Pb in marine ferromanganese oxides. Geochim. Cosmochim. Acta 2007, 71, 984–1008. [Google Scholar] [CrossRef]
- Koschinsky, A.; Hein, J.R. Uptake of elements from seawater by ferromanganese crusts: Solid-phase associations and seawater speciation. Mar. Geol. 2003, 198, 331–351. [Google Scholar] [CrossRef]
- Koschinsky, A.; Halbach, P. Sequential leaching of marine ferromanganese precipitates: Genetic implications. Geochim. Cosmochim. Acta 1995, 59, 5113–5132. [Google Scholar] [CrossRef]
- Wang, Z.L.; Yamada, M. Geochemistry of dissolved rare earth elements in the Equatorial Pacific Ocean. Environ. Geol. 2006, 52, 779–787. [Google Scholar] [CrossRef]
- Zhou, D.; Yao, B. Tectonics and sedimentary basins of the South China Sea: Challenges and progresses. J. Earth Sci. 2009, 20, 1–12. [Google Scholar] [CrossRef]
- Yang, S.X.; Qiu, Y.; Zhu, B.Y. Atlas of Geology and Geophysics of the South China Sea; China Navigation Publications: Tianjing, China, 2015. [Google Scholar]
- Li, D.; Fu, Y.; Sun, X.; Hollings, P.; Liao, J.; Liu, Q.; Feng, Y.; Liu, Y.; Lai, C. LA-ICP-MS trace element mapping: Element mobility of hydrothermal magnetite from the giant Beiya Fe-Au skarn deposit, SW China. Ore Geol. Rev. 2018, 92, 463–474. [Google Scholar] [CrossRef]
- Zhou, H.; Sun, X.; Wu, Z.; Liao, J.; Fu, Y.; Li, D.; Hollings, P.; Liu, Y.; Lin, H.; Lin, Z. Hematite U-Pb geochronometer: Insights from monazite and hematite integrated chronology of the Yaoan gold deposit, southwest China. Econ. Geol. 2017, 112, 2023–2039. [Google Scholar] [CrossRef]
- McLennan, S.M. Rare earth elements in sedimentary rocks; Influence of provenance and sedimentary processes. Rev. Mineral. Geochem. 1989, 21, 169–200. [Google Scholar]
- Manheim, F.T.; Lane-Bostwick, C.M. Cobalt in ferromanganese crusts as a monitor of hydrothermal discharge on the Pacific sea floor. Nature 1988, 335, 59–62. [Google Scholar] [CrossRef]
- Hirata, J.; Takahashi, K.; Sahoo, Y.V.; Tanaka, M. Laser ablation inductively coupled plasma mass spectrometry for quantitative imaging of elements in ferromanganese nodule. Chem. Geol. 2016, 427, 65–72. [Google Scholar] [CrossRef]
- Raimondo, T.; Payne, J.; Wade, B.; Lanari, P.; Clark, C.; Hand, M. Trace element mapping by LA-ICP-MS: Assessing geochemical mobility in garnet. Contrib. Mineral. Petrol. 2017, 172, 17. [Google Scholar] [CrossRef]
- Ubide, T.; McKenna, C.A.; Chew, D.M.; Kamber, B.S. High-resolution LA-ICP-MS trace element mapping of igneous minerals: In search of magma histories. Chem. Geol. 2015, 409, 157–168. [Google Scholar] [CrossRef]
- Kuhn, T.; Wegorzewski, A.; Rühlemann, C.; Vink, A. Composition, Formation, and Occurrence of Polymetallic Nodules. In Deep-Sea Minerals: Distribution Characteristics and Their Resource Potential; Sharma, R., Ed.; Springer Nature: Basingstoke, UK, 2017; pp. 23–63. [Google Scholar]
- Lee, S.; Xu, H. Size-dependent phase map and phase transformation kinetics for nanometric Iron (III) Oxides (γ→ϵ→α pathway). J. Phys. Chem. C 2016, 120, 13316–13322. [Google Scholar] [CrossRef]
- Post, J.E. Manganese oxide minerals: Crystal structures and economic and environmental significance. Proc. Natl. Acad. Sci. USA 1999, 96, 3447–3454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcus, M.A.; Manceau, A.; Kersten, M. Mn, Fe, Zn and As speciation in a fast-growing ferromanganese marine nodule. Geochim. Cosmochim. Acta 2004, 68, 3125–3136. [Google Scholar] [CrossRef]
- Sahai, N.; Lee, Y.J.; Xu, H.; Ciardelli, M.; Gaillard, J.F. Role of Fe (II) and phosphate in arsenic uptake by coprecipitation. Geochim. Cosmochim. Acta 2007, 71, 3193–3210. [Google Scholar] [CrossRef]
- Coppin, F.; Berger, G.; Bauer, A.; Castet, S.; Loubet, M. Sorption of lanthanides on smectite and kaolinite. Chem. Geol. 2002, 182, 57–68. [Google Scholar] [CrossRef]
- De Carlo, E.H.; McMurtry, G.M. Rare-earth element geochemistry of ferromanganese crusts from the Hawaiian Archipelago, Central Pacific. Chem. Geol. 1992, 95, 235–250. [Google Scholar] [CrossRef]
- Sholkovitz, E.R.; Landing, W.M.; Lewis, B.L. Ocean particle chemistry: The fractionation of rare earth elements between suspended particles and seawater. Geochim. Cosmochim. Acta 1994, 58, 1567–1579. [Google Scholar] [CrossRef]
- Ohta, A.; Kawabe, I. Rare earth element partitioning between Fe oxyhydroxide precipitates and aqueous NaCl solutions doped with NaHCO3: Determinations of rare earth element complexation constants with carbonatc ions. Geochem. J. 2000, 34, 439–454. [Google Scholar] [CrossRef]
- Schijf, J.; Marshall, K.S. YREE sorption on hydrous ferric oxide in 0.5 M NaCl solutions: A model extension. Mar. Chem. 2011, 123, 32–43. [Google Scholar] [CrossRef]
- Patten, J.T.; Byrne, R.H. Assessment of Fe(III) and Eu(III) complexation by silicate in aqueous solutions. Geochim. Cosmochim. Acta 2017, 202, 361–373. [Google Scholar] [CrossRef]
- Byrne, R.H. Inorganic speciation of dissolved elements in seawater: The influence of pH on concentration ratios. Geochem. Trans. 2002, 2, 11. [Google Scholar] [CrossRef]
- Liu, X.; Byrne, R.H. Comprehensive investigation of yttrium and rare earth element complexation by carbonate ions using ICP-mass spectrometry. J. Solut. Chem. 1998, 27, 803–815. [Google Scholar] [CrossRef]
- Erel, Y.; Stolper, E.M. Modeling of rare-earth element partitioning between particles and solution in aquatic environments. Geochim. Cosmochim. Acta 1992, 57, 513–518. [Google Scholar] [CrossRef]
- Schijf, J.; Christenson, E.A.; Byrne, R.H. YREE scavenging in seawater: A new look at an old model. Mar. Chem. 2015, 177, 460–471. [Google Scholar] [CrossRef] [Green Version]
- Akagi, T. Rare earth element (REE)-silicic acid complexes in seawater to explain the incorporation of REEs in opal and the “leftover” REEs in surface water: New interpretation of dissolved REE distribution profiles. Geochim. Cosmochim. Acta 2013, 113, 174–192. [Google Scholar] [CrossRef]
- Luo, Y.R.; Byrne, R.H. Carbonate complexation of yttrium and the rare earth elements in natural waters. Geochim. Cosmochim. Acta 2004, 68, 691–699. [Google Scholar] [CrossRef]
- Catts, J.G.; Langmuir, D. Adsorption of Cu, Pb and Zn by δ-MnO2: Applicability of the site binding-surface complexation model. Appl. Geochem. 1986, 1, 255–264. [Google Scholar] [CrossRef]
- Yao, W.S.; Millero, F.J. Adsorption of phosphate on manganese dioxide in seawater. Environ. Sci. Technol. 1996, 30, 536–541. [Google Scholar] [CrossRef]
- Sverjensky, D.A.; Sahai, N. Theoretical prediction of single-site surface-protonation equilibrium constants for oxides and silicates in water. Geochim. Cosmochim. Acta 1996, 60, 3773–3797. [Google Scholar] [CrossRef]
- Ohta, A.; Kawabe, I. REE(III) adsorption onto Mn dioxide (δ-MnO2) and Fe oxyhydroxide: Ce(III) oxidation by δ-MnO2. Geochim. Cosmochim. Acta 2001, 65, 695–703. [Google Scholar] [CrossRef]
- Bau, M. Scavenging of dissolved yttrium and rare earths by precipitating iron oxyhydroxide: Experimental evidence for Ce oxidation, Y-Ho fractionation, and lanthanide tetrad effect. Geochim. Cosmochim. Acta 1999, 63, 67–77. [Google Scholar] [CrossRef]
- Liu, Z.; Zhao, Y.; Colin, C.; Stattegger, K.; Wiesner, M.G.; Huh, C.A.; Zhang, Y.; Li, X.; Sompongchaiyakul, P.; You, C.F.; et al. Source-to-sink transport processes of fluvial sediments in the South China Sea. Earth Sci. Rev. 2016, 153, 238–273. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Colin, C.; Liu, Z.; Douville, E.; Dubois-Dauphin, Q.; Frank, N. New insights into hydrological exchange between the South China Sea and the Western Pacific Ocean based on the Nd isotopic composition of seawater. Deep Sea Res. Part II Top. Stud. Oceanogr. 2015, 122, 25–40. [Google Scholar] [CrossRef]
- Liu, J.; Xiang, R.; Chen, Z.; Chen, M.; Yan, W.; Zhang, L.; Chen, H. Sources, transport and deposition of surface sediments from the South China Sea. Deep Sea Res. Part I Oceanogr. Res. Pap. 2013, 71, 92–102. [Google Scholar] [CrossRef]
- Möller, P.; Muecke, G.K. Significance of europium anomalies in silicate melt and crystal-melt equilibria: A re-evaluation. Contrib. Mineral. Petrol. 1984, 87, 242–250. [Google Scholar] [CrossRef]
- Rudnick, R.L.; Gao, S. Composition of the continental crust. In The Crust; Holland, H.D., Turekian, K.K., Eds.; Elsevier-Pergamon: Oxford, UK, 2003; pp. 1–64. [Google Scholar]
- Akagi, T.; Fu, F.; Hongo, Y.; Takahashi, K. Composition of rare earth elements in settling particles collected in the highly productive North Pacific Ocean and Bering Sea: Implications for siliceous-matter dissolution kinetics and formation of two REE-enriched phases. Geochim. Cosmochim. Acta 2011, 75, 4857–4876. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, N.; Chen, H.; Li, L.; Yan, W. The surface sediment types and their rare earth element characteristics from the continental shelf of the northern South China Sea. Cont. Shelf Res. 2014, 88, 185–202. [Google Scholar] [CrossRef]
- Wei, G.; Liu, Y.; Ma, J.; Xie, L.; Chen, J.; Deng, W.; Tang, S. Nd, Sr isotopes and elemental geochemistry of surface sediments from the South China Sea: Implications for provenance tracing. Mar. Geol. 2012, 319–322, 21–34. [Google Scholar] [CrossRef]
- Kato, Y.; Fujinaga, K.; Nakamura, K.; Takaya, Y.; Kitamura, K.; Ohta, J.; Toda, R.; Nakashima, T.; Iwamori, H. Deep-sea mud in the Pacific Ocean as a potential resource for rare-earth elements. Nat. Geosci. 2011, 4, 535–539. [Google Scholar] [CrossRef]
- Dubinin, A.V. Geochemistry of rare earth elements in oceanic phillipsites. Lithol. Miner. Resour. 2000, 35, 101–108. [Google Scholar] [CrossRef]
- Sa, R.; Sun, X.; He, G.; Xu, L.; Pan, Q.; Liao, J.; Zhu, K.; Deng, X. Enrichment of rare earth elements in siliceous sediments under slow deposition: A case study of the central North Pacifi. Ore Geol. Rev. 2018, 94, 12–23. [Google Scholar] [CrossRef]
- Khanchuk, A.I.; Mikhailik, P.E.; Mikhailika, E.V.; Zarubina, N.V.; Blokhina, M.G. Peculiarities of the distribution of rare earth elements and yttrium in mineral phases of the ferromanganese crusts from the Detroit Guyot (Pacific Ocean). Dokl. Earth Sci. 2015, 465, 479–483. [Google Scholar] [CrossRef]
- Hein, J.R.; Schwab, W.C.; Davis, A. Cobalt- and platinum-rich ferromanganese crusts and associated substrate rocks from the Marshall Islands. Mar. Geol. 1988, 78, 255–283. [Google Scholar] [CrossRef]
- Halbach, P.; Hebisch, U.; Scherhag, C. Geochemical variations of ferromanganese nodules and crusts from different provinces of the Pacific Ocean and their genetic control. Chem. Geol. 1981, 34, 3–17. [Google Scholar] [CrossRef]
- Li, L.; Qu, T. Thermohaline circulation in the deep South China Sea basin inferred from oxygen distributions. J. Geophys. Res. 2006, 111, C05017. [Google Scholar] [CrossRef]
- Alibo, D.S.; Nozaki, Y. Dissolved rare earth elements in the South China Sea: Geochemical characterization of the water masses. J. Geophys. Res. Oceans 2000, 105, 28771–28783. [Google Scholar] [CrossRef] [Green Version]
- He, G.W.; Sun, X.M.; Xue, T. Marine environment and chemistry in working areas. In A Comparative Study of the Geology, Geochemistry and Metallogenetic Mechanism of Polymetallic Nodules and Cobalt-Rich Crusts from the Pacific Ocean; Zhao, J., Cai, W., Eds.; Geological Publishing House: Beijing, China, 2011; pp. 41–45. [Google Scholar]
- Nozaki, Y.; Alibo, D.S.; Amakawa, H.; Gamo, T.; Hasumoto, H. Dissolved rare earth elements and hydrography in the Sulu Sea. Geochim. Cosmochim. Acta 1999, 63, 2171–2181. [Google Scholar] [CrossRef]
- Marino, E.; González, F.J.; Somoza, L.; Lunar, R.; Ortega, L.; Vázquez, J.T.; Reyes, J.; Bellido, E. Strategic and rare elements in Cretaceous-Cenozoic cobalt-rich ferromanganese crusts from seamounts in the Canary Island Seamount Province (northeastern tropical Atlantic). Ore Geol. Rev. 2017, 87, 41–61. [Google Scholar] [CrossRef]

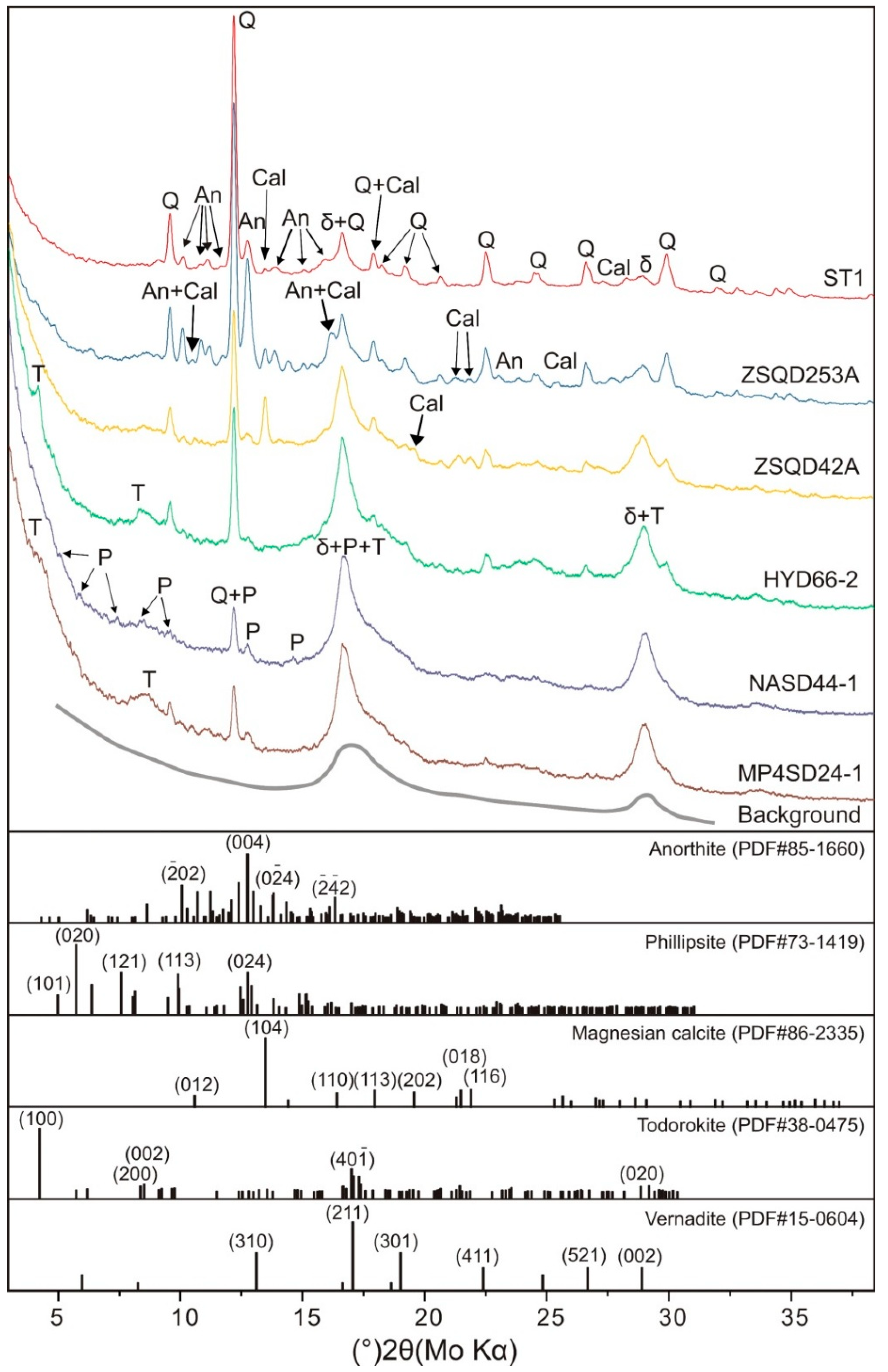



| Sample | Location | Depth (m) | Substrate | XRD Mineral Compositions | |
|---|---|---|---|---|---|
| SCS | ST1 | 117°54.6′ E, 20°28.4′ N | 1600 | Altered basalt covered by carbonate | δ-MnO2, quartz, anorthite, and calcite |
| ZSQD253A | 118°36.4′ E, 16°46′ N | 1150 | Bioclastic limestone | δ-MnO2, quartz, anorthite, and calcite | |
| ZSQD42A | 114°49′ E, 16°51′ N | 1230 | Reef limestone | δ-MnO2, quartz, anorthite, and calcite | |
| HYD66-2 | 115°16.4′ E, 13°40.7′ N | 1378 | Altered basalt | Todorokite, δ-MnO2, quartz, and anorthite | |
| WPO | NASD44-1 | 115°35.4′ E, 19°54.2′ N | 1747 | Breccia | δ-MnO2, quartz, and phillipsite |
| MP4SD24A-1 | 162°18.8′ E, 19°36′ N | 1191 | Breccia | Todorokite, δ-MnO2, quartz, and phillipsite | |
| Elements | South China Sea | Western Pacific Ocean | ||||
|---|---|---|---|---|---|---|
| ST1 | ZSQD253A | ZSQD42A | HYD66-2 | NASD44-1 | MP4SD24A-1 | |
| Mn (%) | 8.98 | 8.78 | 14.84 | 19.21 | 21.71 | 15.83 |
| Fe | 14.67 | 10.98 | 14.39 | 16.41 | 13.93 | 10.71 |
| Mn/Fe | 0.61 | 0.80 | 1.03 | 1.17 | 1.56 | 1.48 |
| Si a | 18.66 | 12.69 | 7.20 | 6.11 | 2.09 | 1.90 |
| Al | 2.88 | 2.02 | 0.88 | 0.99 | 0.88 | 0.44 |
| Ca | 1.85 | 2.87 | 3.34 | 1.90 | 2.14 | 2.06 |
| Mg | 0.96 | 1.16 | 1.23 | 1.07 | 1.04 | 0.99 |
| Ti | 0.31 | 0.42 | 0.35 | 0.35 | 0.92 | 0.35 |
| P | 0.28 | 0.22 | 0.32 | 0.37 | 0.31 | 0.41 |
| As (ppm) | 173 | 105 | 207 | 258 | 204 | 172 |
| Ba | 721 | 551 | 715 | 1527 | 1398 | 3035 |
| Co | 873 | 1101 | 2320 | 3256 | 5788 | 2667 |
| Cu | 424 | 418 | 366 | 571 | 1346 | 816 |
| Mo | 200 | 125 | 303 | 545 | 498 | 625 |
| Ni | 1540 | 2835 | 4016 | 3243 | 5762 | 3448 |
| Pb | 1709 | 1034 | 2179 | 2797 | 1612 | 1205 |
| Zn | 432 | 516 | 590 | 645 | 731 | 864 |
| La | 125.0 | 101.5 | 228.5 | 222.9 | 237.4 | 181.8 |
| Ce | 598.3 | 744.2 | 959.0 | 1552 | 924.7 | 883.1 |
| Pr | 29.53 | 20.64 | 39.80 | 49.33 | 45.01 | 30.37 |
| Nd | 126.3 | 90.0 | 168.8 | 200.6 | 187.0 | 130.4 |
| Sm | 27.74 | 21.02 | 38.29 | 45.12 | 38.27 | 25.34 |
| Eu | 6.94 | 5.49 | 9.46 | 10.91 | 9.95 | 7.21 |
| Gd | 32.63 | 28.34 | 46.11 | 54.07 | 49.78 | 33.75 |
| Tb | 6.26 | 3.85 | 6.34 | 7.10 | 6.95 | 3.51 |
| Dy | 32.99 | 21.60 | 33.85 | 36.48 | 40.35 | 23.53 |
| Y | 105.3 | 87.5 | 148.3 | 98.0 | 146.5 | 123.0 |
| Ho | 6.13 | 4.38 | 8.42 | 6.82 | 8.36 | 6.19 |
| Er | 17.24 | 12.93 | 23.83 | 19.14 | 24.76 | 20.13 |
| Tm | 2.37 | 1.83 | 3.27 | 2.67 | 3.61 | 2.86 |
| Yb | 15.20 | 11.97 | 20.63 | 16.89 | 23.40 | 18.89 |
| Lu | 2.35 | 1.90 | 3.14 | 2.54 | 3.65 | 3.22 |
| ΣREY | 1134 | 1157 | 1738 | 2324 | 1750 | 1493 |
| Ce/Ce* | 2.25 | 3.72 | 2.28 | 3.39 | 2.04 | 2.68 |
| Eu/Eu* | 2.25 | 1.01 | 1.03 | 1.01 | 1.03 | 1.11 |
| Yn/Hon | 0.63 | 0.73 | 0.65 | 0.53 | 0.64 | 0.73 |
| Lan/Ybn | 0.61 | 0.62 | 0.82 | 0.97 | 0.75 | 0.71 |
| Growth rate (mm/Myr) ** | 11.43 | 5.75 | 3.18 | 2.51 | 0.96 | 2.15 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, Y.; Sun, X.; Guan, Y.; Xiao, Z.; Liu, Y.; Liao, J.; Guo, Z. Distribution of Rare Earth Elements plus Yttrium among Major Mineral Phases of Marine Fe–Mn Crusts from the South China Sea and Western Pacific Ocean: A Comparative Study. Minerals 2019, 9, 8. https://doi.org/10.3390/min9010008
Ren Y, Sun X, Guan Y, Xiao Z, Liu Y, Liao J, Guo Z. Distribution of Rare Earth Elements plus Yttrium among Major Mineral Phases of Marine Fe–Mn Crusts from the South China Sea and Western Pacific Ocean: A Comparative Study. Minerals. 2019; 9(1):8. https://doi.org/10.3390/min9010008
Chicago/Turabian StyleRen, Yingzhi, Xiaoming Sun, Yao Guan, Zhenglian Xiao, Ying Liu, Jianlin Liao, and Zhengxing Guo. 2019. "Distribution of Rare Earth Elements plus Yttrium among Major Mineral Phases of Marine Fe–Mn Crusts from the South China Sea and Western Pacific Ocean: A Comparative Study" Minerals 9, no. 1: 8. https://doi.org/10.3390/min9010008





