Release Behaviors of Arsenic and Heavy Metals from Arsenic Sulfide Sludge during Simulated Storage
Abstract
:1. Introduction
2. Materials and Method
2.1. Materials
2.2. Experimental Procedure
2.3. Analysis
2.3.1. Determination of Elemental Composition
2.3.2. Determination of Phase Composition
2.3.3. Leaching Test and Sequential Extraction Scheme
2.3.4. Release Concentration and Release Ratio
2.3.5. Other Analyses
3. Results and Discussion
3.1. Characterization and Chemical Composition of the ASS
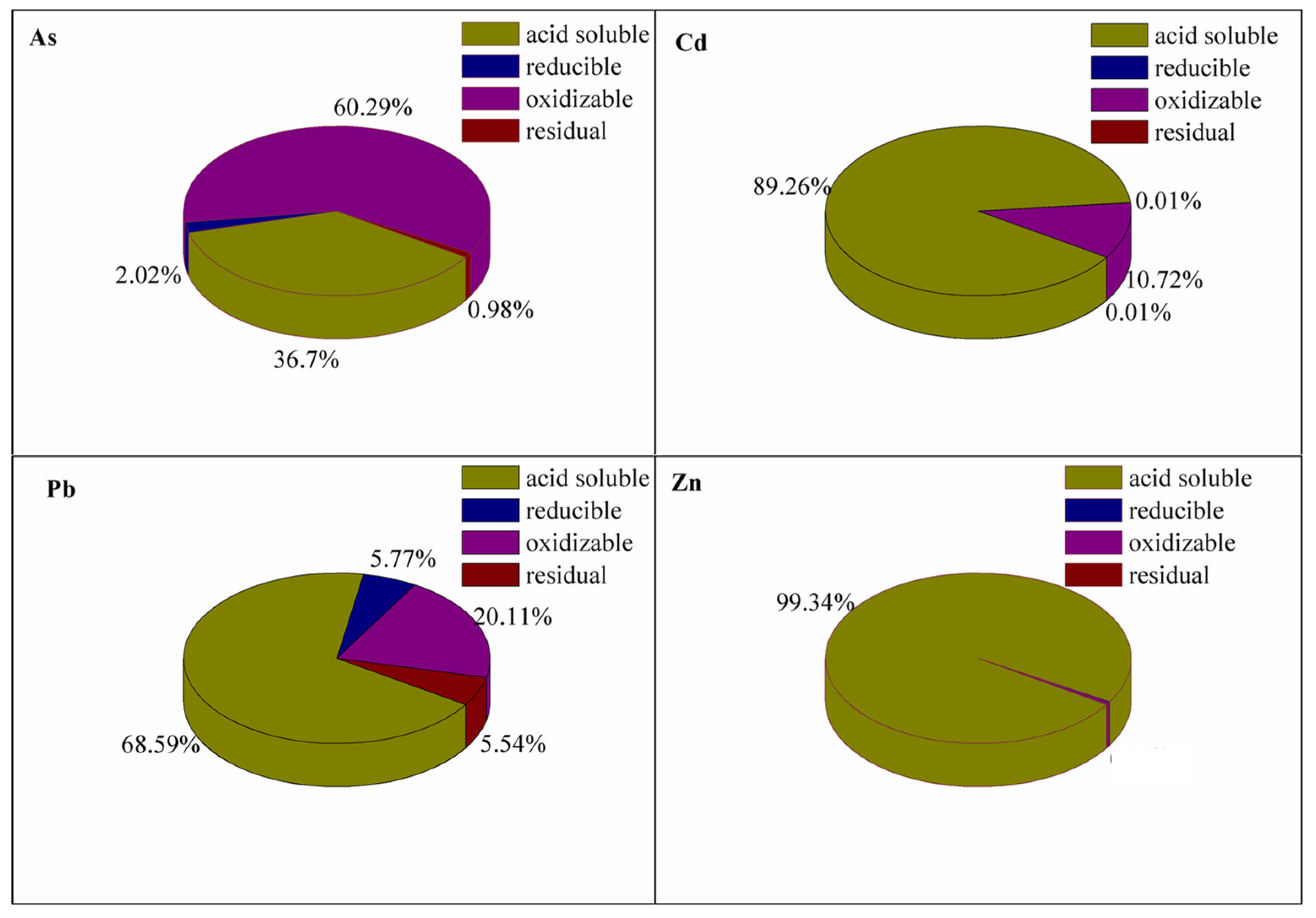
3.2. Chemical Speciation of As and Heavy Metals
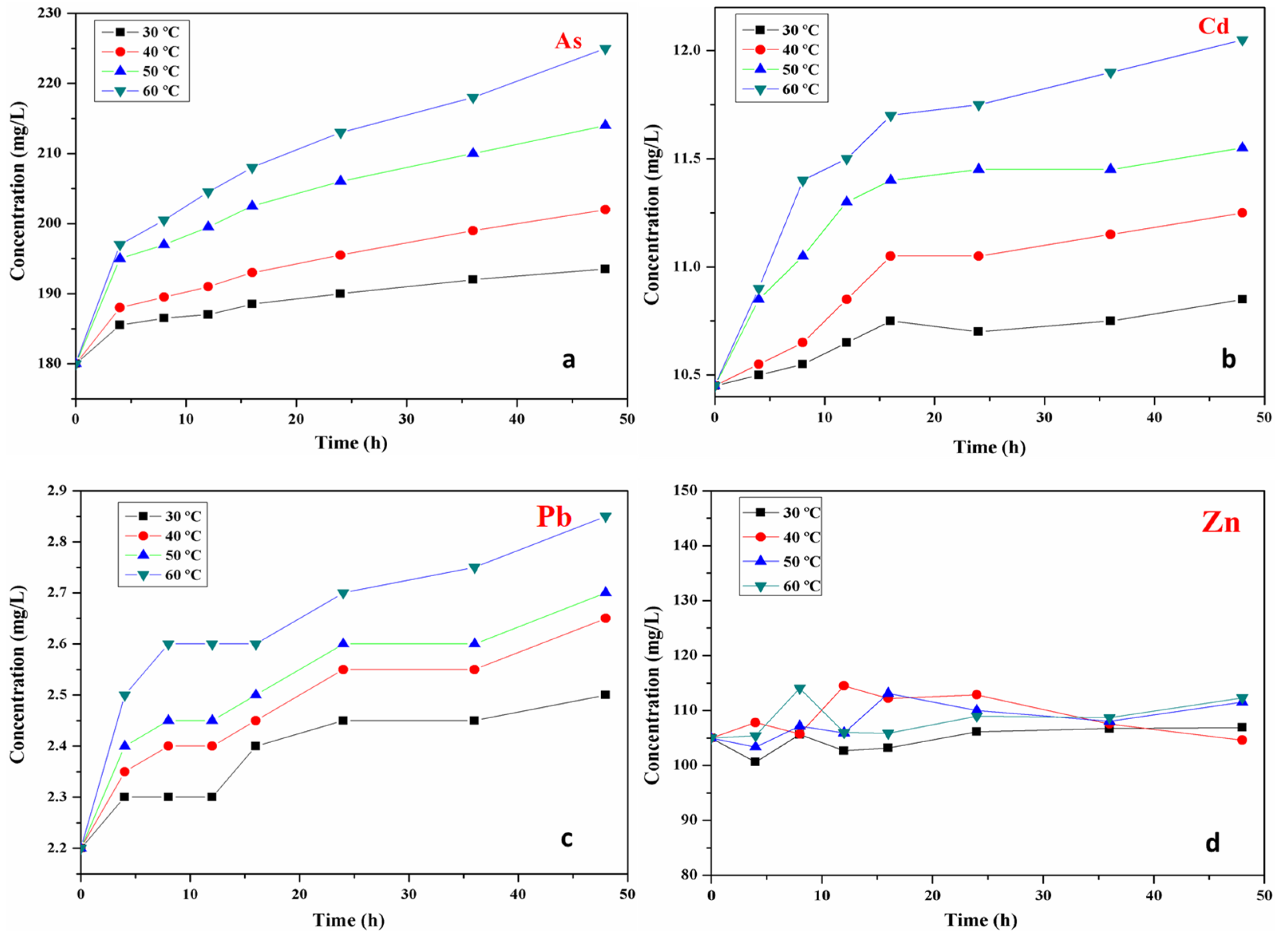
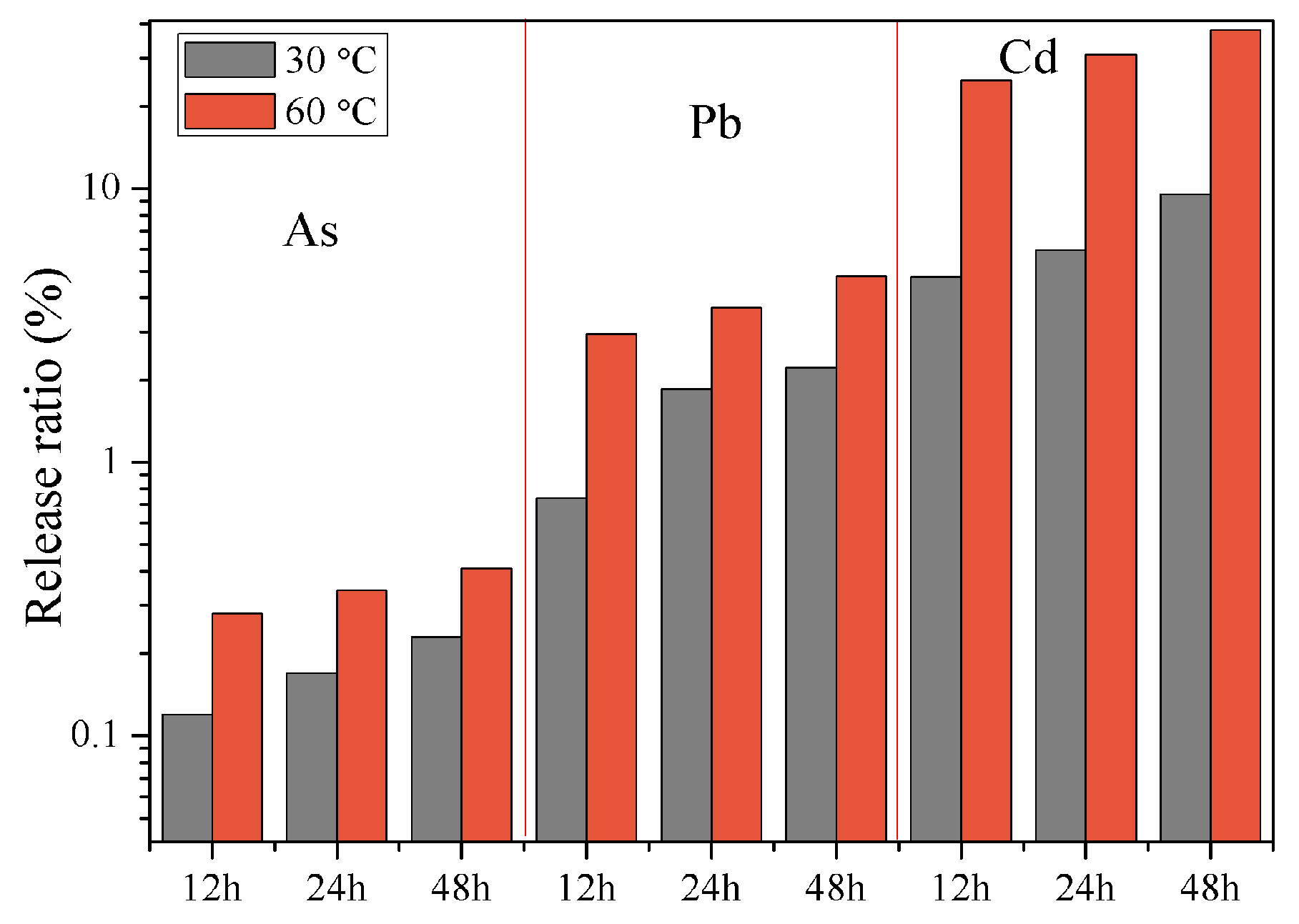
3.3. Release Characteristics of Heavy Metals
3.4. Influencing Factors
3.4.1. Effect of Atmosphere
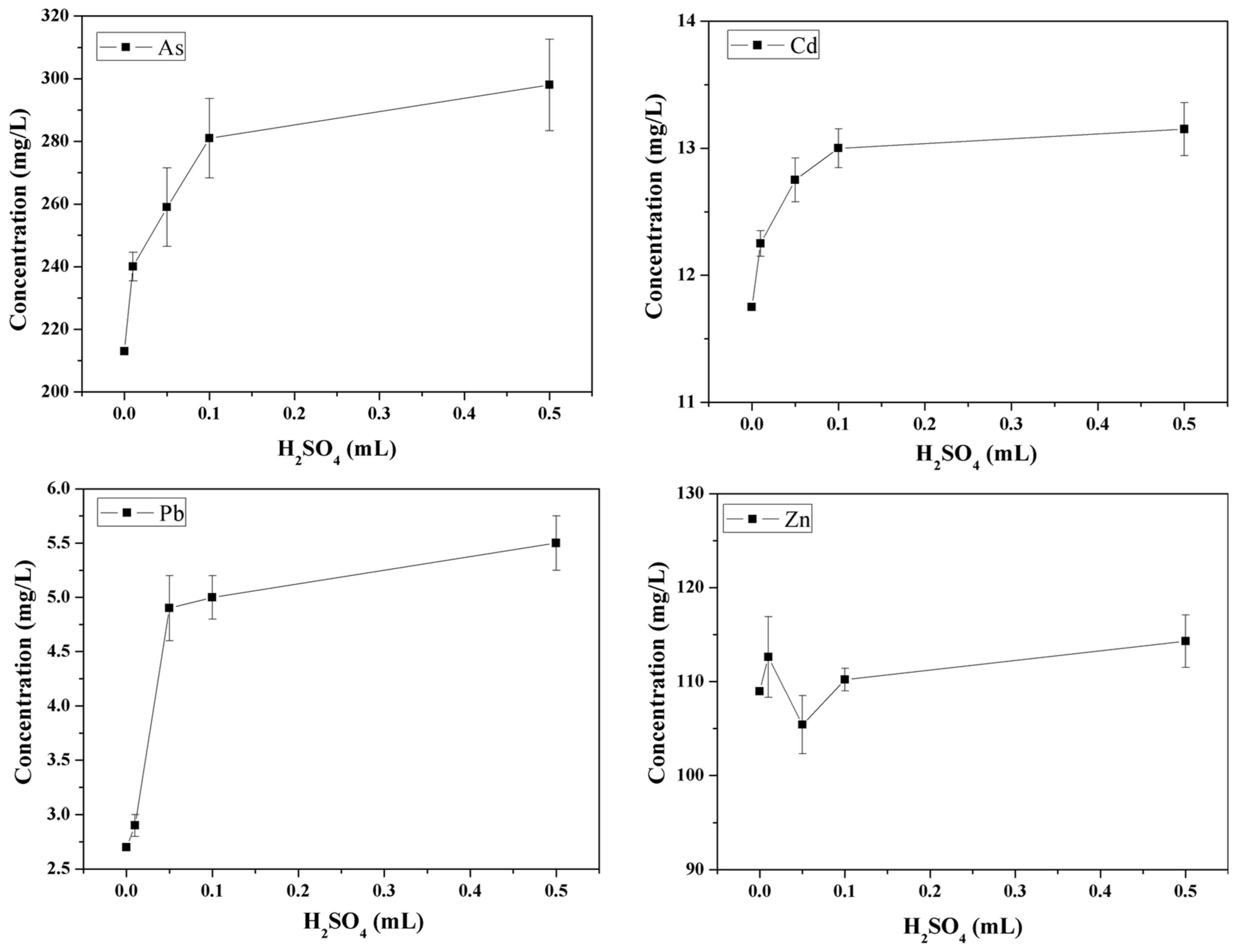
3.4.2. Effect of H2SO4
3.4.3. Effect of Ca(OH)2
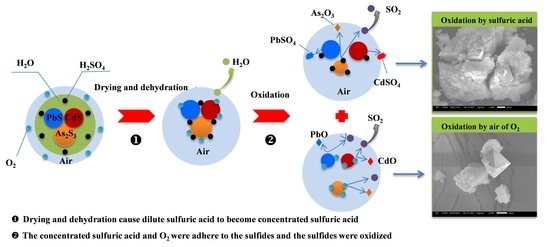
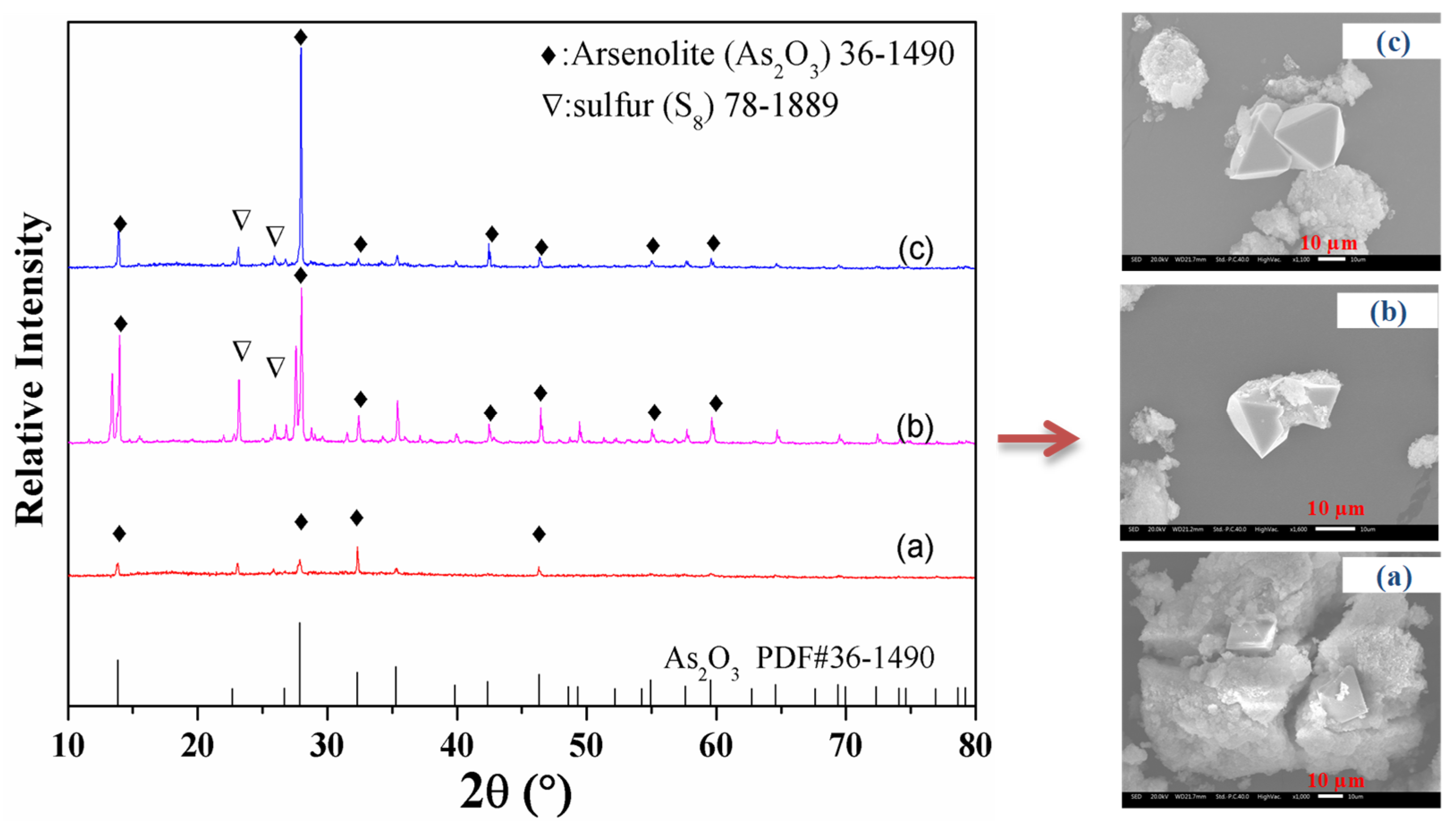
3.5. Morphology, Change and Phase Transformation
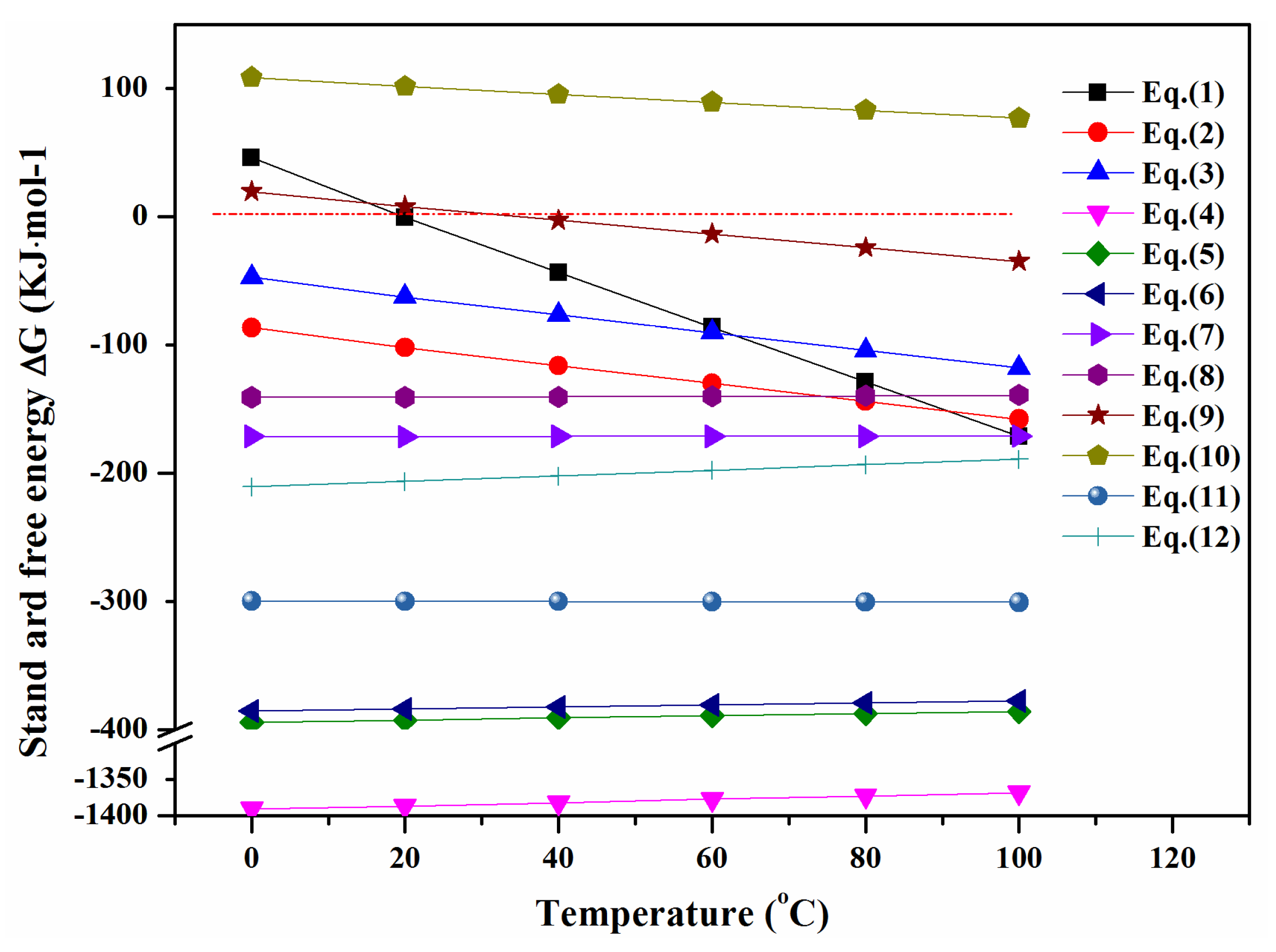
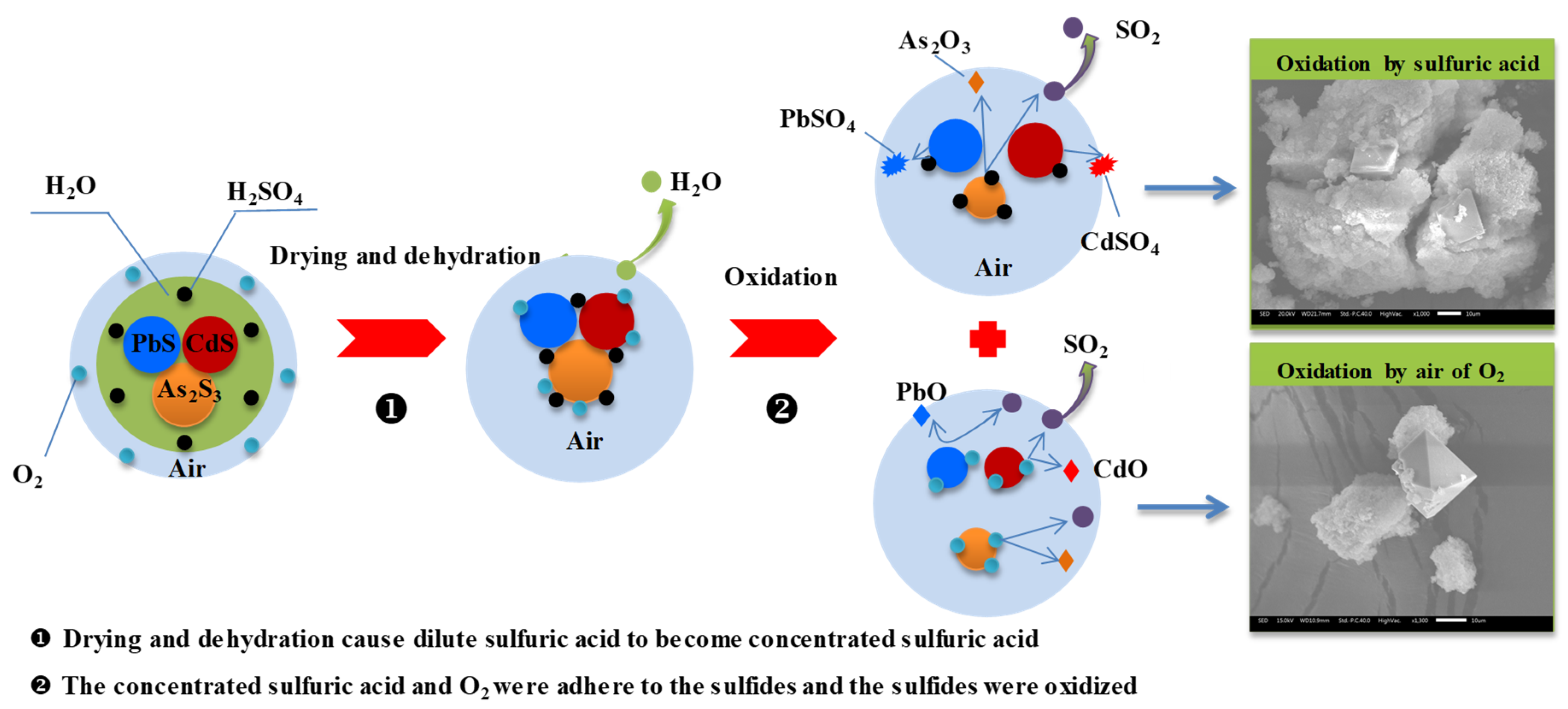
3.6. Release Mechanism
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Inam, M.A.; Khan, R.; Park, D.R.; Ali, B.A.; Uddin, A.; Yeom, I.T. Influence of pH and contaminant redox form on the competitive removal of arsenic and antimony from aqueous media by coagulation. Minerals 2018, 8, 574. [Google Scholar] [CrossRef]
- Wang, Z.B.; Zhao, Z.W.; Zhang, L.F.; Liu, F.S.; Peng, B.; Chai, L.Y.; Liu, D.C.; Liu, D.G.; Wang, T.Y.; Liu, H.; et al. Formation mechanism of zinc-doped fayalite (Fe2−xZnxSiO4) slag during copper smelting. J. Hazard. Mater. 2019, 364, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Min, X.B.; Ke, Y.; Liu, D.G.; Tang, C.J. Preparation of red mud-based geopolymer materials from MSWI fly ash and red mud by mechanical activation. Waste Manag. 2019, 83, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Fei, J.C.; Wang, T.; Zhou, Y.Y.; Wang, Z.X.; Min, X.B.; Ke, Y.; Hu, W.Y.; Chai, L.Y. Aromatic organoarsenic compounds (ADCs) occurrence and remediation methods. Chemosphere 2018, 207, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Min, X.B.; Liu, D.G.; Chai, L.Y.; Ke, Y.; Liang, Y.J.; Shi, M.Q.; Li, Y.C.; Tang, C.J.; Wang, Y.Y.; Wang, Z.B. Comparison of arsenic immobilization properties among calcium silicate hydrate, ettringite, and friedel’s salt in a slag-based binder. Environ. Prog. Sustain. 2018. [Google Scholar] [CrossRef]
- Liu, D.G.; Min, X.B.; Ke, Y.; Chai, L.Y.; Liang, Y.J.; Li, Y.C.; Yao, L.W.; Wang, Z.B. Co-treatment of flotation waste, neutralization sludge, and arsenic-containing gypsum sludge from copper smelting: Solidification/stabilization of arsenic and heavy metals with minimal cement clinker. Environ. Sci. Pollut. Res. 2018, 25, 7600–7607. [Google Scholar] [CrossRef] [PubMed]
- He, X.Y.; Deng, F.; Shen, T.T.; Yang, L.M.; Chen, D.Z.; Luo, J.F.; Luo, X.B.; Min, X.Y.; Wang, F. Exceptional adsorption of arsenic by zirconium metal-organic frameworks: Engineering exploration and mechanism insight. J. Colloid Interface Sci. 2019, 539, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.J.; Min, X.B.; Chai, L.Y.; Wang, M.; Liyang, W.J.; Pan, Q.L.; Okido, M. Stabilization of arsenic sludge with mechanochemically modified zero valent iron. Chemosphere 2017, 168, 1142–1151. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.L.; Zheng, Y.J.; Chen, W.M. The oxidation of arsenic from As(III) to As(V) during copper electrorefining. Hydrometallurgy 2012, 129, 156–160. [Google Scholar] [CrossRef]
- Zheng, Y.J.; Peng, Y.L.; Ke, L.; Chen, W.M. Separation and recovery of Cu and As from copper electrolyte through electrowinning and SO2 reduction. Trans. Nonferr. Met. Soc. China 2013, 23, 2166–2173. [Google Scholar] [CrossRef]
- Bai, M.; Zheng, Y.J.; Liu, W.Y.; Zhang, C.F. Alkaline leaching and leaching kinetics of arsenic sulfide residue. J. Cent. South Univ. 2008, 39, 268–272. (In Chinese) [Google Scholar]
- Jong, T.; Parry, D.L. Evaluation of the stability of arsenic immobilized by microbial sulfate reduction using TCLP extractions and long-term leaching techniques. Chemosphere 2005, 60, 254–265. [Google Scholar] [CrossRef] [PubMed]
- Nazari, A.M.; Radzinski, R.; Ghahreman, A. Review of arsenic metallurgy: Treatment of arsenical minerals and the immobilization of arsenic. Hydrometallurgy 2016, 174, 258–281. [Google Scholar] [CrossRef]
- Hu, H.J.; Qiu, K.Q. Three-step vacuum separation for treating arsenic sulfide residue. Vacuum 2015, 111, 170–175. [Google Scholar] [CrossRef]
- Ke, Y.; Shen, C.; Min, X.B.; Shi, M.Q.; Chai, L.Y. Separation of Cu and As in Cu-As-containing filter cakes by Cu2+-assisted acid leaching. Hydrometallurgy 2017, 172, 45–50. [Google Scholar] [CrossRef]
- Chen, W.P.; Li, Z.Y.; Bian, K.J.; Yang, X.; Chen, F.C. Application of wet-method for extracting arsenic in treating industrial wastewater and residues. China Environ. Sci. 1999, 19, 310–312. (In Chinese) [Google Scholar]
- Dong, S.L. Study on wet treatment of arsenic sulfide. Sulfuric Acid Ind. 1994, 5, 3–5. (In Chinese) [Google Scholar]
- Liu, S.L.; Li, J.H.; Meng, W.J.; Feng, S.B.; Tang, D.Y. Current situation of resource recovery treatment technology of arsenic sulfide residues. Phosphate Compd. Fertil. 2009, 24, 60–63. (In Chinese) [Google Scholar]
- Perez-Lopez, R.; Alvarez-Valero, A.M.; Nieto, J.M. Changes in mobility of toxic elements during the production of phosphoric acid in the fertilizer industry of Huelva (SW Spain) and environmental impact of phosphogypsum wastes. J. Hazard. Mater. 2007, 148, 745–750. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y.S.; Bae, M.J.; Park, Y.S. Changes of heavy metals in pollutant release and transfer registers (PRTRs) in Korea. Int. J. Environ. Res. Public Health 2014, 11, 2381–2394. [Google Scholar] [CrossRef] [PubMed]
- Peterson, G.S.; Ankley, G.T.; Leonard, E.N. Effect of bioturbation on metal-sulfide oxidation in surficial freshwater sediments. Environ. Toxicol. Chem. 2010, 15, 2147–2155. [Google Scholar] [CrossRef]
- Ghosh, D.; Sarkar, S.; Sengupta, A.K.; Gupta, A. Investigation on the long-term storage and fate of arsenic obtained as a treatment residual: A case study. J. Hazard. Mater. 2014, 271, 302–310. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Delapp, R.C.; Kosson, D.S.; van der Sloot, H.A.; Liu, J.G. Release of heavy metals during long-term land application of sewage sludge compost: Percolation leaching tests with repeated additions of compost. Chemosphere 2017, 169, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Chai, L.Y.; Min, X.B.; Tang, C.J.; Chen, J.; Wang, Y.; Liang, Y.J. Sulfidation of heavy-metal-containing neutralization sludge using zinc leaching residue as the sulfur source for metal recovery and stabilization. Min. Eng. 2014, 61, 105–112. [Google Scholar] [CrossRef]
- Feng, S.P. Analytical Chemistry of Arsenic; Environmental Science Press: Beijing, China, 1986. (In Chinese) [Google Scholar]
- Zhang, H.B. Chemical Phase Analysis of Ore and Industrial Product; Metallurgical Industry Press: Beijing, China, 1992. (In Chinese) [Google Scholar]
- Min, X.B.; Xie, X.D.; Chai, L.Y.; Liang, Y.J.; Mi, L.I.; Yong, K.E. Environmental availability and ecological risk assessment of heavy metals in zinc leaching residue. Trans. Nonferrr. Met. Soc. China 2013, 23, 208–218. [Google Scholar] [CrossRef]
- Kong, L.; Peng, X.; Hu, X. Mechanisms of UV-Light promoted removal of As(V) by sulfide from strongly acidic wastewater. Environ. Sci. Technol. 2017, 51, 12583–12591. [Google Scholar] [CrossRef] [PubMed]
- Yao, L.W.; Min, X.B.; Xu, H.; Ke, Y.; Liang, Y.J.; Yang, K. hydrothermal treatment of arsenic sulfide residues from arsenic-bearing acid wastewater. Int. J. Environ. Res. Public Health 2018, 15, 1863. [Google Scholar] [CrossRef] [PubMed]
- Rochette, E.A.; Bostick, B.C.; Li, G.C.; Fendorf, S. Kinetics of arsenate reduction by dissolved sulfide. Environ. Sci. Technol. 2000, 34, 4714–4720. [Google Scholar] [CrossRef]
- Kong, L.H.; Peng, X.J.; Hu, X.Y.; Chen, J.Y.; Xia, Z.L. UV-light-induced aggregation of arsenic and metal sulfide particles in acidic wastewater: The role of free radicals. Environ. Sci. Technol. 2018, 52, 10719–10727. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Du, Y.; Liu, Y.; Cao, L.; Du, D. Property reserach of industrial arsenic sulfide residue and environmental risk analysis. Sulfuric Acid Ind. 2013, 2, 41–46. (In Chinese) [Google Scholar]
- Margui, E.; Queralt, I.; Carvalho, M.L.; Hidalgo, M. Assessment of metal availability to vegetation (Betula pendula) in Pb-Zn ore concentrate residues with different features. Environ. Pollut. 2007, 145, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Michailov, M.D.; Mamedov, S.B.; Tsventarnyi, S.V. Dissolution kinetics of glassy arsenic sulfide in alkali and amine solutions. J. Non-Cryst. Solids 1994, 176, 258–262. [Google Scholar] [CrossRef]
- Mamedov, S.B.; Mikhailov, M.D. Dissolution kinetics of glassy and crystalline As2S3 in aqueous sodium sulfide and hydroxide. J. Non-Cryst. Solids 1997, 221, 181–186. [Google Scholar] [CrossRef]
- Zhu, Y.N.; Zhang, X.H.; Xie, Q.L.; Wang, D.Q.; Cheng, G.W. Solubility and stability of calcium arsenates at 25 °C. Water Air Soil Pollut. 2006, 169, 221–238. [Google Scholar] [CrossRef]
- Bothe, J.V.; Brown, P.W. Arsenic immobilization by calcium arsenate formation. Environ. Sci. Technol. 1999, 33, 3806–3811. [Google Scholar] [CrossRef]





| Extraction Step | Species | Reagent | Program |
|---|---|---|---|
| 1 | Oxidation states | Distilled water | Stir and boil for 1.5 h |
| 2 | Arsenate/arsenite | 20% H2SO4 | Stir for 0.5 h at room temperature |
| 3 | Arsenic and sulfur compounds | NaOH (2 mol/L) | Stir for 0.5 h at 80 °C |
| 4 | Residual | Aqua regia | Boil for 0.5 h |
| Classification | As | S | Fe | Cu | Zn | Pb | Cd | Mg | Na | Ca |
|---|---|---|---|---|---|---|---|---|---|---|
| Elemental composition (wt %) | 39.2 | 22.9 | 0.81 | 0.53 | 0.6 | 0.10 | 0.093 | 0.016 | 0.19 | 0.055 |
| CSLT (mg/L) | 1474 | a | a | 0.2 | 469.1 | 3.35 | 61 | a | a | a |
| GB18598-2001 * | ≤2.5 | b | b | ≤75 | ≤75 | ≤0.5 | ≤5 | b | b | b |
| GB5085.3-2007 * | ≤5 | b | b | ≤100 | ≤100 | ≤1.0 | ≤5 | b | b | b |
| Arsenic Species (%) | Oxidation States | Arsenate and Arsenite | Sulfide | Residual | ||||||
| 49.56 | 0.02 | 48.78 | 1.64 | |||||||
| Other ion concentration (wet mass) | SO42− | H+ | ||||||||
| 72 mg/g | 8.125 mg/g | |||||||||
| Equation | ΔG (kcal·mol−1) | ΔH (kcal·mol−1) | Number |
|---|---|---|---|
| As2S3 + 9H2SO4 = As2O3 + 12SO2(g) + 9H2O | −20.634 | 149.383 | (1) |
| PbS + 4H2SO4 = PbSO4 * + 4SO2(g) + 4H2O | −31.115 | 24.368 | (2) |
| CdS + 4H2SO4 = CdSO4 + 4SO2(g) + 4H2O | −21.608 | 33.356 | (3) |
| As2S3 + 4.5O2(g) = As2O3 + 3SO2(g) | −329.242 | −347.395 | (4) |
| PbS + 1.5O2(g) = PbO + SO2(g) | −93.030 | −99.624 | (5) |
| CdS +1.5O2(g) = CdO + SO2(g) | −91.008 | −97.186 | (6) |
| PbO + H2SO4 = PbSO4 * + H2O | −40.953 | −41.552 | (7) |
| CdO + H2SO4 = CdSO4 + H2O | −33.469 | −35.113 | (8) |
| S + 2H2SO4 = 3SO2(g)+2H2O | −3.231 | 127.830 | (9) |
| As2O3 + 2H2SO4 = As2O5 + 2SO2(g) + 2H2O | 21.293 | 73.503 | (10) |
| S + O2(g) = SO2(g) | −71.810 | −71.039 | (11) |
| As2O3 + O2(g) = As2O5 | −47.287 | −64.614 | (12) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yao, L.; Min, X.; Ke, Y.; Wang, Y.; Liang, Y.; Yan, X.; Xu, H.; Fei, J.; Li, Y.; Liu, D.; et al. Release Behaviors of Arsenic and Heavy Metals from Arsenic Sulfide Sludge during Simulated Storage. Minerals 2019, 9, 130. https://doi.org/10.3390/min9020130
Yao L, Min X, Ke Y, Wang Y, Liang Y, Yan X, Xu H, Fei J, Li Y, Liu D, et al. Release Behaviors of Arsenic and Heavy Metals from Arsenic Sulfide Sludge during Simulated Storage. Minerals. 2019; 9(2):130. https://doi.org/10.3390/min9020130
Chicago/Turabian StyleYao, Liwei, Xiaobo Min, Yong Ke, Yunyan Wang, Yanjie Liang, Xu Yan, Hui Xu, Jiangchi Fei, Yuancheng Li, Degang Liu, and et al. 2019. "Release Behaviors of Arsenic and Heavy Metals from Arsenic Sulfide Sludge during Simulated Storage" Minerals 9, no. 2: 130. https://doi.org/10.3390/min9020130