Jurassic Non-Carbonate Microbialites from the Betic-Rifian Cordillera (Tethys Western End): Textures, Mineralogy, and Environmental Reconstruction
Abstract
:1. Introduction
1.1. Fe-Mn Crusts
1.2. Clay Microbialites
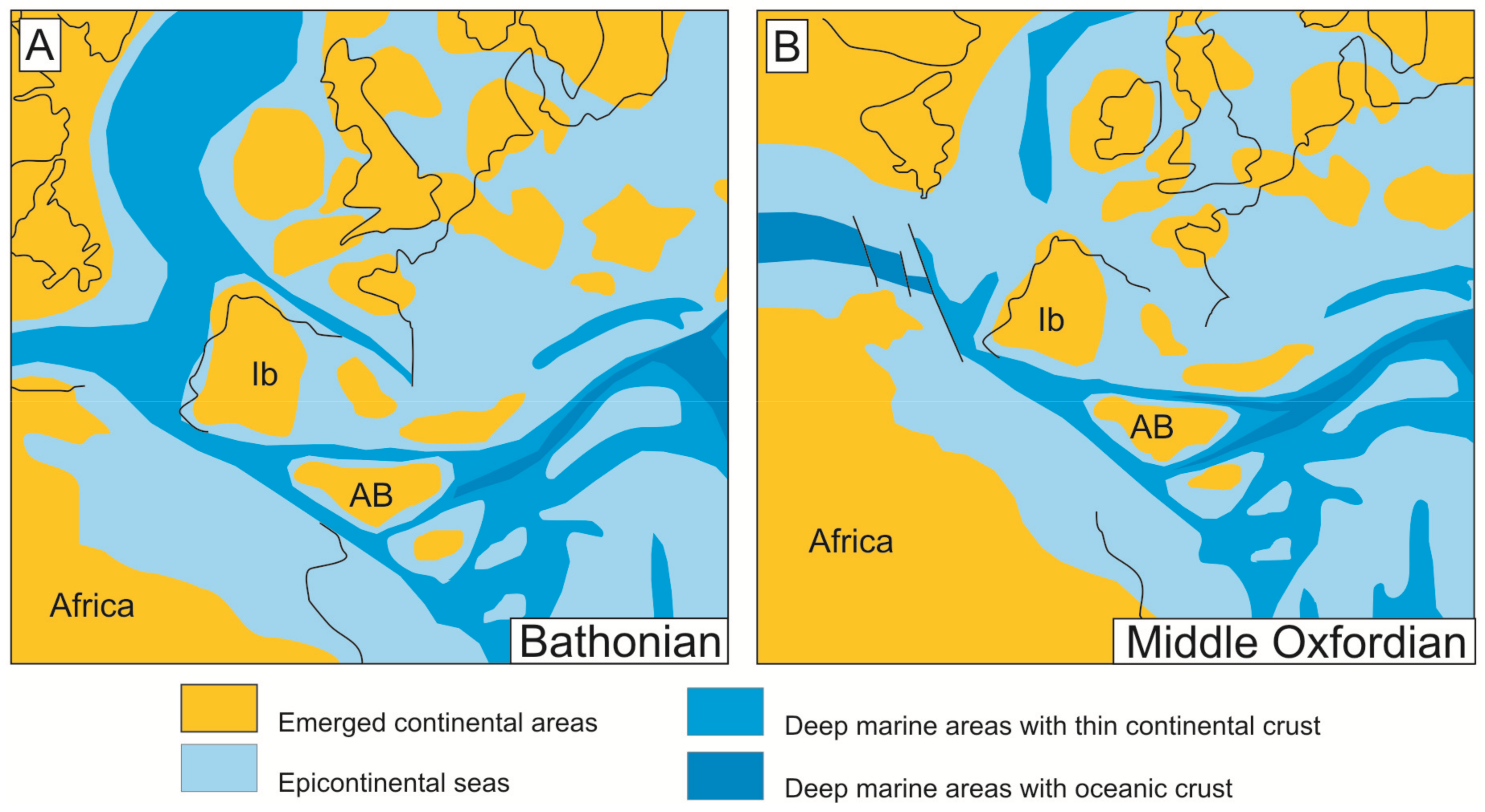
2. Geological Setting and Jurassic Evolution
3. Studied Outcrops
3.1. External Subbetic
3.2. Median Subbetic
3.3. Rifian Calcareous Chain
4. Methods
5. Results
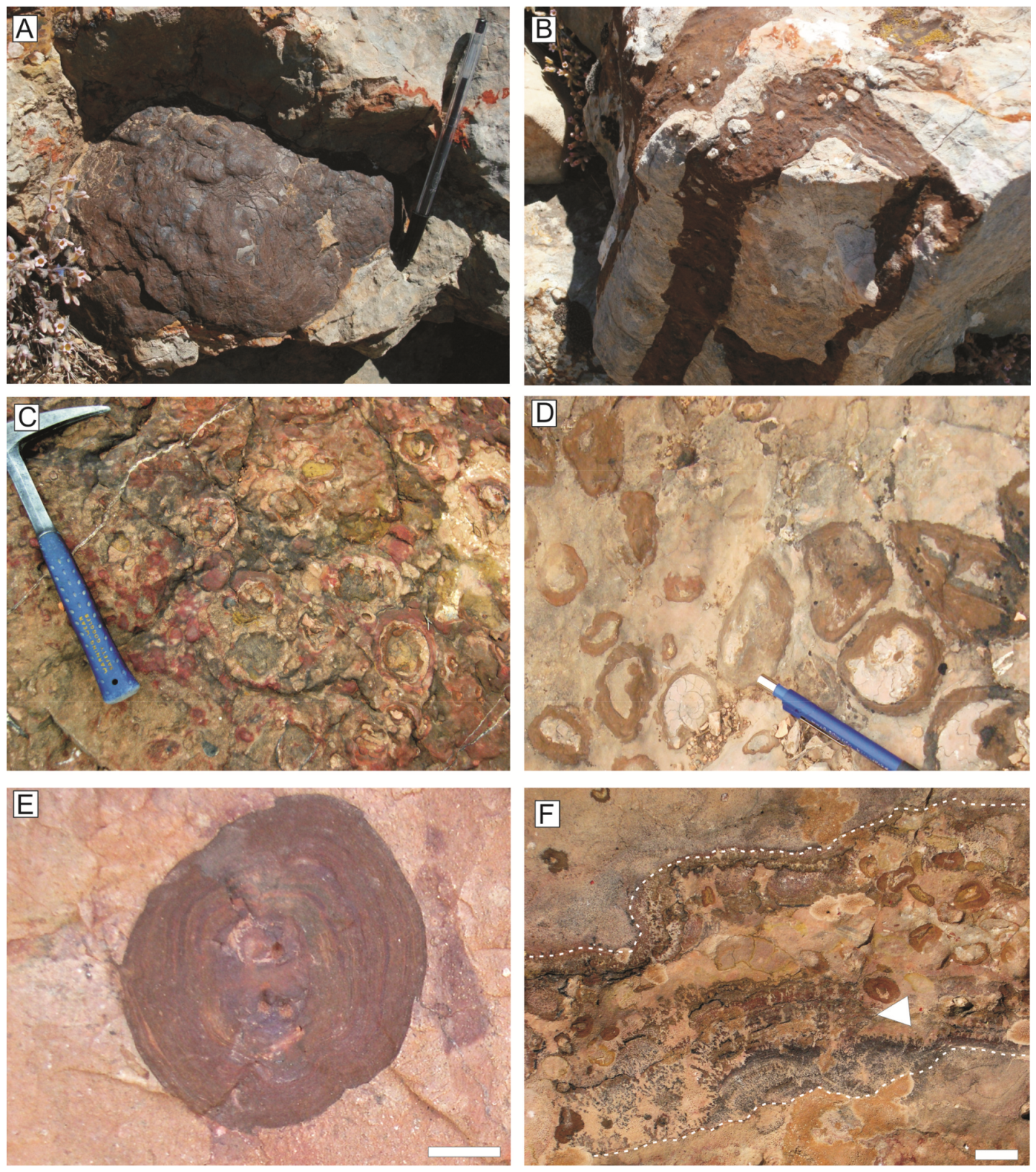
5.1. Iron Microbialite Crusts
5.1.1. Texture
5.1.2. Mineralogy and Geochemistry
5.1.3. Microbiota
5.2. Mn Microbial Crusts
5.2.1. Texture
5.2.2. Mineralogy and Geochemistry
5.2.3. Microbiota
5.3. Glauconitic Microbial Crusts
5.3.1. Texture
5.3.2. Mineralogy and Geochemistry
5.3.3. Microbiota
6. Interpretation
6.1. Iron Crusts and Macro-Oncoids on Hardgrounds
6.2. Cryptic Fe-Mn Frutexites Crusts
6.3. Hydrothermal Mn Microbial Crusts
6.4. Hydrothermal Glauconitic Microbial Crusts
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burne, R.V.; Moore, L.S. Microbialites: Organosedimentary deposits of benthic microbial communities. Palaios 1987, 2, 241–254. [Google Scholar] [CrossRef]
- Riding, R. Calcified Cyanobacteria. In Calcareous Algae and Stromatolites; Riding, R., Ed.; Springer: Berlin, Germany, 1991; pp. 21–51. [Google Scholar]
- Riding, R. Structure and composition of organic reefs and carbonate mud mounds: Concepts and categories. Earth-Sci. Rev. 2002, 58, 163–231. [Google Scholar] [CrossRef]
- Riding, R. Microbial carbonate abundance compared with fluctuations in metazoans diversity over geological time. Sed. Geol. 2006, 185, 229–238. [Google Scholar] [CrossRef]
- Mata, S.A.; Bottjer, D.J. Microbes and mass extinctions: Paleoenvironmental distribution of microbialites during times of biotic crisis. Geobiology 2012, 10, 3–24. [Google Scholar] [CrossRef] [PubMed]
- Lazar, I.; Gradinaru, M.; Petrescu, L. Ferruginous microstromatolites related to Middle Jurassic condensed sequences and hardgrounds (Bucegi Mountains, Southern Carpathians, Romania). Facies 2013, 59, 359–390. [Google Scholar] [CrossRef]
- Salama, W.; El Aref, M.M.; Gaupp, R. Mineral evolution and processes of ferruginous microbialite accretion—An example from the Middle Eocene stromatolitic and ooidal ironstones of the Bahariya depression, Western Desert, Egypt. Geobiology 2013, 11, 15–28. [Google Scholar] [CrossRef] [PubMed]
- Lozano, R.P.; Rossi, C. Exceptional preservation of Mn-oxidizing microbes in cave stromatolites (El Soplano, Spain). Sed. Geol. 2012, 255, 42–55. [Google Scholar] [CrossRef]
- Wang, X.; Gan, L.; Wiens, M.; Schlossmacher, U.; Schroeder, H.C.; Muller, W.E.G. Distribution of microfossils within polymetallic nodules: Biogenic clusters within manganese layers. Mar. Biotech. 2012, 14, 96–105. [Google Scholar] [CrossRef]
- Wu, Y.H.; Liao, L.; Wang, C.S.; Ma, W.L.; Meng, F.X.; Wu, M.; Xu, X.W. A comparison of microbial communitites in deep-sea polymetallic nodules and the surrounding sediments in the Pacific Ocean. Deep-Sea Res. Part I-Oceanogr. Res. Pap. 2013, 79, 40–49. [Google Scholar] [CrossRef]
- Martín-Algarra, A.; Sánchez-Navas, A. Phosphate stromatolites from condensed cephalopod limestones, Upper Jurassic, Southern Spain. Sedimentology 1995, 42, 893–919. [Google Scholar] [CrossRef]
- Soudry, D. Microbial phosphate sediment. In Microbial Sediments; Riding, R., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 127–136. [Google Scholar]
- Sallstedt, T.; Bengtson, S.; Broman, C.; Crill, P.M.; Canfield, D.E. Evidence of oxygenic phototrophy in ancient phosphatic stromatolites from the Paleoproterozoic Vindhyan and Aravalli Supergroups, India. Geobiology 2018, 16, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Rouchy, J.M.; Monty, C. Gypsum microbial sediments: Neogene and Modern examples. In Microbial Sediments; Riding, R., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 209–216. [Google Scholar]
- Taher, A.G. Formation and calcification of modern gypsum-dominated stromatolites, EMISAL, Fayium, Egypt. Facies 2014, 60, 721–735. [Google Scholar] [CrossRef]
- Stevens, E.W.N.; Bailey, J.V.; Flood, B.E.; Jones, D.S.; Gilhooly, W.P.; Joye, S.B.; Teske, A.; Mason, O.U. Barite encrustation of benthic sulfur-oxidizing bacteria at a marine cold seep. Geobiology 2015, 13, 588–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strohmenger, C.J.; Jameson, J. Gypsum stromatolites from Sawda nathil: Relicts from a southern coastline of Qatar. Carbonates Evaporites 2018, 33, 169–186. [Google Scholar] [CrossRef]
- Wacey, D.; Urosevic, L.; Saunders, M.; George, A.D. Mineralisation of filamentous cyanobacteria in Lake Thetis stromatolites, Western Australia. Geobiology 2018, 16, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, G.; Krumbein, W.E.; Nofke, N. Evaporite microbial sediments. In Microbial Sediments; Riding, R., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 196–208. [Google Scholar]
- Renaut, R.W.; Jones, B. Microbial precipitates around continental hot springs and geysers. In Microbial Sediments; Riding, R., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 186–195. [Google Scholar]
- Miller, S.L.; Bada, J.L. Submarine hot springs and the origin of life. Nature 1988, 334, 609–611. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B. Filamentous microfossils in a 3,235-million-year-old volcanogenic massive sulphide deposit. Nature 2000, 405, 676–679. [Google Scholar] [CrossRef] [PubMed]
- Martin, W.; Baross, J.; Kelley, D.; Russell, M.J. Hydrothermal vents and the origin of life. Nature Rev. Microbiol. 2008, 6, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Boston, P.J.; Ivanov, M.V.; McKay, C.P. On the possibility of chemosynthetic ecosystems in subsurface habitat on Mars. Icarus 1992, 95, 300–308. [Google Scholar] [CrossRef]
- Matsubara, T.; Fujishima, K.; Saltikov, C.W.; Nakamura, S.; Rothschild, L.J. Earth analogues for past and future life on Mars: Isolation of perchlorate resistant halophiles from Big Soda Lake. Int. J. Astrobiol. 2017, 16, 218–228. [Google Scholar] [CrossRef]
- Brasier, A.; Wacey, D.; Rogerson, M.; Guagliardo, P.; Saunders, M.; Kellner, S.; Mercedes-Martín, R.; Prior, T.; Taylor, C.; Matthews, A.; et al. A microbial role in the construction of Mono Lake carbonate chimneys? Geobiology 2018, 16, 540–555. [Google Scholar] [CrossRef] [PubMed]
- Krumbein, W.E. Microbial Geochemistry; Blackwell Scientific Publisher: London, UK, 1983. [Google Scholar]
- Stolz, J.F. Structure of microbial mats and biofilms. In Microbial Sediments; Riding, R., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 1–8. [Google Scholar]
- Tazaki, K. Microbial formation of a halloysite-like mineral. Clays Clay Miner. 2005, 53, 224–233. [Google Scholar] [CrossRef] [Green Version]
- Lusty, P.A.; Murton, B.J. Deep-ocean mineral deposits: Metal resources and windows into Earth processes. Elements 2018, 14, 301–306. [Google Scholar] [CrossRef]
- Perri, E.; Tucker, M.E.; Slowakiewicz, M.; Whitaker, F.; Bowen, L.; Perrotta, I.D. Carbonate and silicate biomineralization in a hypersaline microbial mat (Mesaieed sabkha, Qatar): Roles of bacteria, extracellular polymeric substances and viruses. Sedimentology 2018, 65, 1213–1245. [Google Scholar] [CrossRef]
- Zubkov, M.V.; Plucinski, P.K.; Dartiguelongue, A.C.Y.; Lusty, P.A. Metal extraction from deep-ocean mineral deposits. Elements 2018, 14, 319–324. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Harauz, G.; Beveridge, T.J. Participation of a cyanobacterial S-layer in fine-grain mineral formation. J. Bacteriol. 1992, 174, 7971–7981. [Google Scholar] [CrossRef] [PubMed]
- Schultze-Lam, S.; Ferris, F.G.; Sherwood-Lollar, B.; Gerits, J.P. Ultrastructure and seasonal growth patterns of microbial mats in a temperature climate saline-alkaline lake: Goodenough Lake, British Columbia, Canada. Can. J. Microbiol. 1996, 42, 147–162. [Google Scholar] [CrossRef]
- Schultze-Lam, S.; Fortin, D.; Beveridge, T.J. Mineralization of bacterial surfaces. Chem. Geol. 1996, 132, 171–181. [Google Scholar] [CrossRef]
- Cuadros, J. Clay minerals interaction with microorganisms: A review. Clay Miner. 2017, 52, 235–261. [Google Scholar] [CrossRef]
- Krumbein, W.E.; Jens, K. Biogenic rock varnishes of the Negev Desert (Israel), an ecological study of iron and manganese transformation by cyanobacteria and fungi. Oecologia 1981, 50, 25–38. [Google Scholar] [CrossRef]
- Nealson, K.H. The microbial iron cycle. In Microbial Geochemistry; Krumbein, W.E., Ed.; Blackwell Scientific Publications: Oxford, UK, 1983; pp. 159–190. [Google Scholar]
- Dahanayake, K.; Krumbein, W.E. Microbial structures in oolitic iron formations. Mineral. Depos. 1986, 21, 85–94. [Google Scholar] [CrossRef]
- Frankel, R.B.; Bazylinski, D.A. Biologically induced mineralization by bacteria. Rev. Mineral. Geochem. 2003, 54, 95–114. [Google Scholar] [CrossRef]
- Bolton, B.R.; Both, R.; Exon, N.F.; Hamilton, T.F.; Ostwald, J.; Smith, J.D. Geochemistry and mineralogy of seafloor hydrothermal and hydrogenetic Mn oxide deposits from the Manus Basin and Bismarck Archipelago region of the southwest Pacific Ocean. Mar. Geol. 1988, 85, 65–87. [Google Scholar] [CrossRef]
- Hein, J.R.; Koschinsky, A.; Halbach, P.; Manheim, F.T.; Bau, M.; Kang, J.K.; Lubick, N. Iron and manganese oxide mineralization in the Pacific. Geol. Soc. Special Publ. 1997, 119, 123–138. [Google Scholar] [CrossRef]
- Glasby, G.P. Manganese: Predominant role of nodules and crusts. In Marine Geochemistry; Schultz, H.D., Zabel, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 350–372. [Google Scholar]
- Rojkovič, I.; Aubrecht, R.; Mišik, M. Mineral and chemical composition of manganese hardgrounds in Jurassic limestones of the Western Carpathians. Geol. Carpathica 2003, 54, 317–328. [Google Scholar]
- Jach, R.; Dudek, T. Origin of Toarcian manganese carbonate/silicate deposits from the Krížna unit, Tatra Mountains, Poland. Chem. Geol. 2005, 224, 136–152. [Google Scholar] [CrossRef]
- Préat, A.; Mamet, B.; Di Stefano, P.; Martire, L.; Kolo, K. Microbially-induced Fe and Mn oxides in condensed pelagic sediments (Middle-Upper Jurassic, Western Sicily). Sed. Geol. 2011, 237, 179–188. [Google Scholar] [CrossRef]
- Reolid, M.; El Kadiri, K.; Abad, I.; Olóriz, F.; Jiménez-Millán, J. Jurassic microbial communities in hydrothermal manganese crust from the Rifian Calcareous Chain, Northern Morocco. Sed. Geol. 2011, 233, 159–172. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, M.; Heim, C.; Simon, K.; Zilla, T.; Reitner, J. Tolypammina vagans Wendt 1969—Frutexites assemblage and ferromanganese crust: A coupled nutrient-metal interplay in the Carnian sedimentary condensed record of Hallstatt Facies (Austria). Lect. Notes Earth Sci. 2011, 31, 409–434. [Google Scholar]
- Mandernack, K.W.; Tebo, B.M. Manganese scavenging and oxidation at hydrothermal vents and in vent plumes. Geochim. Cosmochim. Acta 1993, 57, 3907–3923. [Google Scholar] [CrossRef]
- Templeton, A.S.; Knowles, E.J.; Eldridge, D.L.; Arey, B.W.; Dohnalkova, A.C.; Webb, S.M.; Bailey, B.E.; Tebo, B.M.; Staudigel, H. A seafloor microbial biome hosted within incipient ferromanganese crusts. Nat. Geosci. 2009, 2, 872–876. [Google Scholar] [CrossRef]
- Reolid, M.; Nieto, L.M. Jurassic Fe-Mn macro-oncoids from pelagic swells of the External Subbetic (Spain): Evidences of microbial origin. Geol. Acta 2010, 8, 151–168. [Google Scholar]
- Reolid, M.; Abad, I. The Middle-Upper Jurassic unconformity in the South Iberian palaeomargin (Western Tethys): A history of carbonate platform fragmentation, emersion and subsequent drowning. Jour. Iberian Geol. 2018. [Google Scholar] [CrossRef]
- Tashiro, Y.; Tazaki, K. The primitive stage of microbial mats comprising iron hydroxides. Earth Sci. 1999, 53, 27–35. [Google Scholar]
- Douglas, S.; Beveridge, T. Mineral formation by bacteria in natural microbial communities. FEMS Microbiol. Ecol. 1998, 26, 79–88. [Google Scholar] [CrossRef] [Green Version]
- Fiore, S.; Dumontet, S.; Huertas, F.J.; Pasquale, V. Bacteria-induced crystallization of kaolinite. Appl. Clay Sci. 2011, 53, 566–571. [Google Scholar] [CrossRef]
- Konhauser, K.O.; Fyfe, W.S.; Ferris, F.G.; Beveridge, T.J. Metal sorption and mineral precipitation by bacteria in tow Amazonian river systems: Rio Solimoes and Rio Negro, Brazil. Geology 1993, 21, 1103–1106. [Google Scholar] [CrossRef]
- Konhauser, K.; Urrutia, M. Bacterial clay authigenesis: A common biogeochemical process. Chem. Geol. 1999, 161, 399–413. [Google Scholar] [CrossRef]
- Kawano, M.; Tomita, K. Microbial biomineralization in weathered volcanic ash deposit and formation of biogenic minerals by experimental incubation. Am. Mineral. 2001, 86, 400–410. [Google Scholar] [CrossRef]
- Ueshima, M.; Tazaki, K. Possible role of microbial polysaccharides in nontronite formation. Clays Clay Miner. 2001, 49, 292–299. [Google Scholar] [CrossRef]
- Sánchez-Navas, A.; Martín-Algarra, A.; Nieto, F. Bacterially-mediated authigenesus of clays in phosphate stromatolites. Sedimentology 1998, 45, 519–533. [Google Scholar] [CrossRef]
- Guerrera, F.; Martín-Algarra, A.; Perrone, V. Late Oligocene-Miocene syn-/-late-orogenic successions in Western and Central Mediterranean Chains from the Betic Cordillera to the Southern Apennines. Terra Nova 1993, 5, 525–544. [Google Scholar] [CrossRef]
- Vera, J.A. Evolution of the Iberian Continental Margin. Mémoires Muséum Natl. d’Histoire Nat. Paris 2001, 186, 109–143. [Google Scholar]
- Durand-Delga, M.; Villiaumey, M. Sur la stratigraphie et la tectonique du Groupe du Jbel Musa (Rif septentrional, Maroc). Bull. Soc. Géol. France 1969, 5, 70–79. [Google Scholar]
- Durand-Delga, M. La courbure de Gibraltar, extrémité occidentale des chaînes alpines, unit l’Europe et l’Afrique. Eclog. Geol. Helv. 1972, 65, 267–278. [Google Scholar]
- El Kadiri, K.; Linares, A.; Olóriz, F. Les éléments du Groupe du Jbel Moussa (Chaîne Calcaire, Rif, Maroc): Évolutions stratigraphique el géodynamique au cours du Jurassique-Crétacé. Comunic. Serv. Geol. Portugal 1990, 76, 141–161. [Google Scholar]
- El Hatimi, N. Rifting mésozoïque sur la Bordure Occidentale du Rif Interne (Maroc). Evolution Géodynamique d’un Secteur de la Marge Oust-Téthysienne. Exemples du Haouz et du Jbel Moussa. Ph.D. Thesis, Université Pau, Pau, France, 1991. [Google Scholar]
- García-Hernández, M.; López-Garrido, A.C.; Martín-Algarra, A.; Molina, J.M.; Ruiz-Ortiz, P.A.; Vera, J.A. Las discontinuidades mayores del Jurásico de las Zonas Externas de las Cordilleras Béticas: Análisis e interpretación de los ciclos sedimentarios. Cuad. Geol. Ibérica 1989, 13, 35–52. [Google Scholar]
- Comas, M.C.; Puga, E.; Bargossi, G.M.; Morten, L.; Rossi, P.L. Paleogeography, sedimentation and volcanism of the Central Subbetic Zone, Betic Cordilleras, Southeastern Spain. Neues Jahrbuch Geol. Paläontol. Monatsh. 1986, 7, 385–404. [Google Scholar]
- Vera, J.A.; Molina, J.M.; Montero, P.; Bea, F. Jurassic guyots in the Southern Iberian Continental Margin: A model of isolated carbonate platforms on volcanic submarine edifices. Terra Nova 1997, 9, 163–166. [Google Scholar] [CrossRef]
- Soussi, M.; Ben Ismail, M.H. Platform collapse and pelagic seamount facies: Jurassic development of vcentral Tunisia. Sed. Geol. 2000, 133, 93–113. [Google Scholar] [CrossRef]
- Bouaziz, S.; Barrier, E.; Soussi, M.; Turki, M.M.; Zouari, H. Tectonic evolution of the northern African margin in Tunisia from paleostress data and sedimentary record. Tectonophysics 2002, 357, 227–253. [Google Scholar] [CrossRef]
- Tanfous Amri, D.; Bédir, M.; Soussi, M.; Azaiez, H.; Zitouni, L.; Inoubli, M.H.; Boubaker, K.M. Halocinèse précoce associée au rifting jurassique dans l’Atlas central de Tunisie (region de Majoura-El Hfay). C. R. Géosci. 2005, 337, 703–711. [Google Scholar] [CrossRef]
- Marok, A.; Reolid, M. Lower Jurassic sediments from the Rhar Roubane Mountains (Western Algeria): Stratigraphic precisions and synsedimentary block-faulting. J. Afr. Earth Sci. 2012, 76, 50–65. [Google Scholar] [CrossRef]
- Soussi, M.; M’rabet, A. Las facies à oolithes ferrugineuses (‘oolitic ironstones’) du Jurassique moyen de l’Axe Nord-Sud (Tunisie centrale): Caractéristiques et significations. Notes Serv. Géol. Tunisie 1991, 57, 71–85. [Google Scholar]
- El Kadiri, K. Jurassic ferruginous hardgrounds of the “Dorsale Calcaire” and the Jbel Moussa Group (Internal Rif, Morocco): Stratigraphical context and paleoceanographic consequences of mineralization processes. Geol. Romana 2002, 36, 33–69. [Google Scholar]
- Aurell, M.; Fernández-López, S.; Meléndez, G. The Middle-Upper Jurassic oolitic ironstone level in the Iberian Range (Spain). Eustatic implications. Geobios 1994, 17, 549–561. [Google Scholar] [CrossRef]
- Aurell, M.; Badenas, B.; Bello, J.; Delvene, G.; Meléndez, G.; Pérez-Urresti, I.; Ramajo, J. El Calloviense y el Jurasico Superior en la Cordillera Ibérica Nororiental y la zona de enlace con la Cordillera Costero Catalana, en los sectores de Sierra de Arcos, Calanda y Xerta-Paüls. Cuad. Geol. Ibérica 1999, 25, 73–110. [Google Scholar]
- Ramajo, J.; Aurell, M. Análisis sedimentológico de las discontinuidades y depósitos del Calloviense superior-Oxfordiense medio en la Cordillera Ibérica Noroccidental. Cuad. Geol. Ibérica 1997, 22, 213–236. [Google Scholar]
- Ramajo, J.; Aurell, M.; Cepría, J. Análisis de facies de la Capa de oolitos ferruginosos de Arroyofrío en la Sierra de Arcos (Jurásico, Cordillera Ibérica septentrional). J. Iberian Geol. 2002, 28, 45–64. [Google Scholar]
- Meléndez, G.; Ramajo, J.; Martínez-Cotanda, S. El desarrollo de la Capa de Arroyofrío (límite Calloviense-Oxfordiense) al Sur de Zaragoza, entre Ricla y Aguilón: Bioestratigrafía y facies. Geogaceta 2005, 38, 3–6. [Google Scholar]
- Scouflaire, Q.; Marchand, D.; Bonnot, A.; Courville, P.; Raffray, M.; Huault, V. Le contact Callovien-Oxfordien dans les environs de Chaignay: Nouvelles données stratigraphiques et paléontologiques. Bull. Sci. Bour. 1997, 49, 45–63. [Google Scholar]
- Courville, P.; Collin, P.Y. La série du Callovien et de l’Oxfordien de Veuxhaulles (Châtillonnais, Côte d’Or): Problèmes de datation, de géométrie et de paléoenvironnements dans une série “condensée”. Bull. Sci. Bourg. 1997, 49, 29–43. [Google Scholar]
- Lorin, S.; Courville, P.; Collin, P.Y.; Thierry, J.; Tort, A. Modalités de réinstallation d’une plate-forme carbonatée après une crise sédimentaire: Exemple de la limite Oxfordien moyen—Oxfordien supérieur dans le Sud-Est du Bassin de Paris. Bull. Soc. Géol. Fr. 2004, 175, 289–302. [Google Scholar] [CrossRef]
- Collin, P.Y.; Loreau, J.P.; Courville, P. Depositional environments and iron ooids formation in condensed sections (Callovian-Oxfordian, south-eastern Paris basin, France). Sedimentology 2005, 52, 969–985. [Google Scholar] [CrossRef]
- Gygi, R.A. Oolitic iron formations: Marine or not marine? Eclog. Geol. Helv. 1981, 74, 233–254. [Google Scholar]
- Huber, B.; Müller, B.; Luterbacher, H. Mikropaläontologische Untersuchungen an der Callovien/Oxfordien-Grenze im Schweizer Jura und auf der Schwäbischen Alb (vorläufige Mitteilung). Eclog. Geol. Helv. 1987, 80, 449–459. [Google Scholar]
- Gygi, R.A.; Persoz, F. The epicontinental sea of Swabia (southern Germany) in the Late Jurassic: Factors controlling sedimentation. N. J. Geol. Paläont. Abh. 1987, 176, 49–65. [Google Scholar]
- Sequeiros, L. Paleobiogeografía del Calloviense y el Oxfordiense en el Sector Central de la Zona Subbética. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1974. [Google Scholar]
- Sequeiros, L. Caracterización cuali-cuantitativa del Calloviense de Cabra (Cordillera Bética, España). Bol. Real Soc. Española Hist. Nat. 1987, 83, 25–46. [Google Scholar]
- Seyfried, H. Der Subbetische Jura von Murcia (Sudost-Spanien). Geol. J. 1978, 29, 3–204. [Google Scholar]
- García-Hernández, M.; López-Garrido, A.C.; Rivas, P.; Sanz de Galdeano, C.; Vera, J.A. Mesozoic paleogeographic evolution of the External Zones of the Betic Cordillera. Geol. Minjbow 1980, 59, 155–168. [Google Scholar]
- Sandoval, J. Bioestratigrafía y Paleontología (Stephanocerataceae y Perisphinctaceae) del Bajocense y Bathonense en las Cordilleras Béticas. Ph.D. Thesis, Universidad Granada, Granada, Spain, 1983. [Google Scholar]
- Marques, B.; Olóriz, F.; Rodríguez-Tovar, F.J. Interactions between tectonics and eustasy during the Upper Jurassic and lowermost Cretaceous. Examples from the south of Iberia. Bull. Soc. Géol. Fr. 1991, 162, 1109–1124. [Google Scholar]
- Martín-Algarra, A.; Vera, J.A. Mesozoic pelagic phosphate stromatolites from the Penibetic (Betic Cordillera Southern Spain). In Phanerozoic Stromatolites II; Bertrand-Sarfati, J., Monty, C., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1994; pp. 345–391. [Google Scholar]
- O’Dogherty, L.; Sandoval, J.; Vera, J.A. Ammonite faunal turnover tracing sea level changes during the Jurassic (Betic Cordillera, southern Spain). Jour. Geol. Soc. Lond. 2000, 157, 723–736. [Google Scholar] [CrossRef]
- Reolid, M.; Abad, I.; Martín-García, J.M. Palaeoenvironmental implications of ferruginous deposits related to a Middle-Upper Jurassic discontinuity (Prebetic Zone, Betic Cordillera, Southern Spain). Sed. Geol. 2008, 203, 1–16. [Google Scholar] [CrossRef]
- Reolid, M.; Nieto, L.M.; Rey, J. Taphonomy of cephalopod assemblages from Middle Jurassic hardgrounds of pelagic swells (South-Iberian Palaeomargin, Western Tethys). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2010, 292, 257–271. [Google Scholar] [CrossRef]
- Vera, J.A.; Martín-Algarra, A.; Sánchez-Gómez, M.; Fornós, J.J.; Gelabert, B. Cordillera Bética y Baleares. In Geología de España; Vera, J.A., Ed.; SGE-IGME: Madrid, Spain, 2004; pp. 347–464. [Google Scholar]
- Molina, J.M. Análisis de facies del Mesozoico en el Subbético Externo (Provincia de Córdoba y Sur de Jaén). Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1987. [Google Scholar]
- Rey, J. Análisis de la Cuenca Subbética durante el Jurásico y el Cretácico en la transversal Caravaca-Vélez Rubio. Ph.D. Thesis, Universidad de Granada, Granada, Spain, 1993. [Google Scholar]
- Nieto, L.M. La Cuenca Subbética Mesozoica en el Sector Oriental de las Cordilleras Béticas. Ph.D. Thesis, Universidad de Jaén, Jaén, Spain, 1997. [Google Scholar]
- Molina, J.M.; Ruiz-Ortiz, P.A.; Vera, J.A. Neptunian dykes and associated features in southern Spain: Mechanisms of formation and tectonic implications. Discussion. Sedimentology 1995, 42, 957–960. [Google Scholar] [CrossRef]
- Molina, J.M.; Ruiz-Ortiz, P.A.; Vera, J.A. A review of polyphase karstification in extensional tectonic regimes: Jurassic and Cretaceous examples, Betic Cordillera, southern Spain. Sed. Geol. 1999, 129, 71–84. [Google Scholar] [CrossRef]
- Reolid, M.; Molina, J.M. Serpulid-Frutexites assemblage from shadow-cryptic environments in Jurassic marine caves (Betic Cordillera, South Spain). Palaios 2010, 25, 468–474. [Google Scholar] [CrossRef]
- Puga, E.; van de Fliert, J.R.; Torres-Roldán, R.L.; Sanz de Galdeano, C. Attempts of the whole-rock K/Ar dating of Mesozoic volcanic and hypabissal igneous rocks from the central Subbetic (Southern Spain): A case of differential argon loss related to very low-grade metamorphism. Estud. Geol. 1988, 44, 47–59. [Google Scholar] [CrossRef]
- Puga, E.; Portugal, M.; Díaz de Federico, A.; Bargossi, G.M.; Morten, L. The evolution of the magmatism in the external zones of the Betic Cordilleras during the Mesozoic. Geodinamica Acta 1989, 3, 253–266. [Google Scholar] [CrossRef]
- Morata, D.A.; Puga, E.; Demant, A.; Aguirre, L. Evolución petrogenética del magmatismo básico mesozoico en las Zonas Externas de las Cordilleras Béticas (S. España). Geogaceta 1986, 20, 576–578. [Google Scholar]
- Abad, I.; Jiménez-Millán, J.; Molina, J.M.; Nieto, F.; Vera, J.A. Anomalous reverse zoning of saponite and corrensite caused by contact metamorphism and hydrothermal alteration of marly rocks associated with subvolcanic bodies. Clays Clay Miner. 2003, 51, 543–554. [Google Scholar] [CrossRef]
- Jiménez-Millán, J.; Abad, I.; Nieto, F. Contrasting alteration processes in hydrothermally altered dolerites from the Betic Cordillera, Spain. Clay Miner. 2008, 43, 267–280. [Google Scholar] [CrossRef]
- El Kadiri, K. La Dorsale Calcaire (Rif Interne, Maroc): Stratigraphie, Sédimentologie Etévolution Géodynamique d’une Marge Alpine Durant le Mésozoïque. Mise en Evidence d’un Modèle. Ph.D. Thesis, Université Tétouan, Tétouan, Morocco, 1991. [Google Scholar]
- Reolid, M.; Abad, I. Glauconitic laminated crusts from hydrothermal alteration of Jurassic pillow-lavas (Betic Cordillera, S Spain): A microbial influence case. J. Iberian Geol. 2014, 40, 389–408. [Google Scholar] [CrossRef]
- Taylor, S.R.; McLennan, S.M. The Continental Crust: Its Composition and Evolution; Blackwell Scientific: Oxford, UK, 1985. [Google Scholar]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Monty, C. Cavity and fissure dwelling stromatolites (endostromatolites) from Belgian Devonian mud mounds. Ann. Soc. Géol. Belgique 1984, 105, 343–344. [Google Scholar]
- Burkhalter, R.M. Ooidal ironstones and ferruginous microbialites: Origin and relation to sequence stratigraphy (Aalenian and Bajocian, Swiss Jura mountains). Sedimentology 1995, 42, 57–74. [Google Scholar] [CrossRef]
- Lozano, R.P.; Rossi, C.; La Iglesia, A.; Matesanz, E. Zaccagnaite-3R, a new Zn-Al hydrotalcite polytype from El Soplao cave (Cantabria, Spain). Am. Mineral. 2012, 97, 513–523. [Google Scholar] [CrossRef]
- Rossi, C.; Villalain, J.J.; Lozano, R.P.; Hellstrom, J. Paleo-watertable definition using cave ferromanganese stromatolites and associated cave-wall notches (Sierra de Arnero, Spain). Geomorphology 2016, 261, 57–75. [Google Scholar] [CrossRef] [Green Version]
- Nieto, L.M.; Reolid, M.; Molina, J.M.; Ruiz-Ortiz, P.A.; Jiménez-Millán, J.; Rey, J. Evolution of pelagic swells from hardground analysis (Bathonian-Oxfordian, Eastern External Subbetic, southern Spain). Facies 2012, 58, 389–414. [Google Scholar] [CrossRef]
- Nieto, L.M.; Rodríguez-Tovar, F.J.; Molina, J.M.; Reolid, M.; Ruiz-Ortiz, P.A. Unconformity surfaces in pelagic carbonate environments: A case from the Middle Bathonian of the Betic Cordillera, SE Spain. Ann. Soc. Geol. Pol. 2014, 84, 281–295. [Google Scholar]
- Jiménez-Millán, J.; Nieto, L.M. Geochemical and mineralogical evidence of tectonic and sedimentary factors controlling the origin of ferromanganese crusts associates to stratigraphic discontinuities (Betic Cordilleras, SE of Spain). Chem. Erde 2008, 68, 323–336. [Google Scholar] [CrossRef]
- Han, X.; Jin, X.; Yang, S.; Fietzke, J.; Eisenhauer, A. Rhythmic growth of Pacific ferromanganese nodules and their Milankovitch climatic origin. Earth Planet. Sci. Lett. 2003, 211, 143–157. [Google Scholar] [CrossRef]
- Fleet, A.J. Hydrotermal and hydrogenous ferromanganese deposits: Do they from a continuum? The rare evidence. In Hydrothernal Processes at Seafloor Spreading Centers; Plenum Press: New York, NY, USA, 1983; pp. 535–555. [Google Scholar]
- Fromm, R.; Hachicha, T.; Smykatz-Kloss, W. Aridic crusts and vein mineralization in the playa Areg el Makrezene, South Tunisia. Chem. Erde Geochem. 2005, 65, 357–373. [Google Scholar] [CrossRef]
- Reolid, M.; Gaillard, C.; Olóriz, F.; Rodríguez-Tovar, F.J. Microbial encrustation from the Middle Oxfordian-earliest Kimmeridgian lithofacies in the Prebetic Zone (Betic Cordillera, southern Spain): Characterization, distribution and controlling factors. Facies 2005, 50, 529–543. [Google Scholar] [CrossRef]
- Nose, M.; Schmid, D.U.; Leinfelder, R.R. Significance of microbialites, calcimicrobes, and calcareous algae in reefal framework formation from the Silurian of Gotland, Sweden. Sed. Geol. 2006, 192, 243–265. [Google Scholar] [CrossRef]
- Perri, E.; Tucker, M. Bacterial fossils and microbial dolomite in Triassic stromatolites. Geology 2007, 35, 207–210. [Google Scholar] [CrossRef]
- Gerdes, G.; Klenke, T.; Nofke, N. Microbial signatures in peritidal siliciclastic sediments: A catalogue. Sedimentology 2000, 47, 279–308. [Google Scholar] [CrossRef]
- Cavalazzi, B.; Barbieri, R.; Ori, G.G. Chemosynthetic microbialites in the Devonian carbonate mounds of Hamar Laghdad (Anti-Atlas, Morocco). Sed. Geol. 2007, 200, 73–88. [Google Scholar] [CrossRef]
- Playford, P.E.; Cockbain, A.E.; Druce, E.C.; Wray, J.L. Devonian stromatolites from the Canning Basin, Western Australia. Dev. Sedimentol. 1976, 20, 543–563. [Google Scholar]
- Playford, P.E.; McLaren, D.J.; Orth, C.; Gilmore, J.S.; Goodfellow, W.D. Iridium anomaly in the Upper Devonian of the Canning Basin, Western Australia. Science 1984, 226, 437–439. [Google Scholar] [CrossRef]
- Nicoll, R.S.; Playford, P.E. Upper Devonian iridium anomalies, conodont zonation and the Frasnian-Fammenian boundary in the Canning Basin, Western Australia. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1993, 104, 105–113. [Google Scholar] [CrossRef]
- Walter, M.R.; Awramik, S.M. Frutexites from stromatolites of the Gunflint Iron Formation of Canada, and its biological affinities. Precambrian Res. 1979, 9, 23–33. [Google Scholar] [CrossRef]
- Hofmann, H.J.; Grotzinger, J.P. Shelf-facies microbiotas from the Odjick and Rocknest foramtions (Epworth Group; 1.89 Ga), northwesttern Canada. Can. J. Earth Sci. 1985, 30, 1781–1792. [Google Scholar] [CrossRef]
- Guido, A.; Rosso, A.; Sanfilippo, R.; Russo, F.; Mastandrea, A. Frutexites from microbial/metazoan bioscontructions of recent and Pleistocene marine caves (Sicily, Italy). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016, 453, 127–138. [Google Scholar] [CrossRef]
- Alexopoulos, C.J.; Mims, C.W.; Blackwell, M. 1996. Introductory Mycology; John Wiley & Sons Inc.: Toronto, ON, USA, 1996; p. 869. [Google Scholar]
- Bornhold, B.D.; Giresse, P. Glauconitic sediments on the continental shelf off Vancouver Island, British Columbia, Canada. J. Sed. Petrol. 1985, 55, 653–664. [Google Scholar]
- Tóth, E.; Weiszburg, T.G.; Jeffries, T.; Williams, C.T.; Bartha, A.; Bertalan, E.; Cora, I. Submicroscopic accessory minerals overprinting clay mineral REE patterns (celadonite-glauconite group examples). Chem. Geol. 2010, 269, 312–328. [Google Scholar] [CrossRef]
- Martín-Algarra, A.; Sánchez-Navas, A. Bacterially mediated authigenesis in Mesozoic stromatolites from condensed pelagic sediments (Betic Cordillera, Southern Spain). SEPM Spec. Pub. 2000, 66, 499–525. [Google Scholar]
- Fortin, D.; Langley, S. Formation and occurrence of biogenic iron-rich minerals. Earth-Sci. Rev. 2005, 72, 1–19. [Google Scholar] [CrossRef]
- Southam, G. Bacterial surface-mediated mineral formation. In Environmental Microbe-Mineral Interactions; Lovley, D.R., Ed.; ASM Press: Washington, DC, USA, 2000; pp. 257–276. [Google Scholar]
- Hanert, H.H. The genus Siderocapsa (and other iron- and manganese-oxidizing eubacteria). Prokaryotes 2006, 7, 1005–1015. [Google Scholar]
- Böhm, F.; Brachert, T.C. Deep-water stromatolites and Frutexites Maslov from the Early and Middle Jurassic of S-Germany and Austria. Facies 1993, 28, 145–168. [Google Scholar] [CrossRef]
- Chafetz, H.S.; Akdim, B.; Julia, R.; Reid, A. Mn- and Fe-rich black travertine shrubs: Bacterially (and nanobacterially) induced precipitates. J. Sed. Res. 1998, 68, 404–413. [Google Scholar] [CrossRef]
- Ehrlich, H.L. The formation of ores in the sedimentary environment of the deep sea with microbial participation: The case for ferromanganese concretions. Soil Sci. 1975, 119, 36–41. [Google Scholar] [CrossRef]
- Schaefer, M.O.; Gutzmer, J.; Beukes, N.J. Late Paleoproterozoic Mn-rich oncoids: Earliest evidence for microbially mediated Mn precipitation. Geology 2001, 29, 835–838. [Google Scholar] [CrossRef]
- Hasting, D.; Emerson, S. Oxidation of manganese by spores of a marine bacillus: Kinetic and thermodynamic considerations. Geochim. Cosmochim. Acta 1986, 50, 1819–1824. [Google Scholar] [CrossRef]
- Nealson, K.H.; Stahl, D.A. Microorganisms and biochemical cycles: What can we learn from layered microbial communities? Rev. Mineral. 1997, 35, 5–34. [Google Scholar]
- Francis, C.A.; Tebo, B.M. Enzymatic manganese (II) oxidation by metabolically dormant spores of diverse Bacillus species. Appl. Environ. Microbiol. 2002, 68, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Ghiorse, W.C.; Ehrlich, H.L. Microbial biomineralization of iron and manganese. Catena 1992, 21, 75–99. [Google Scholar]
- Lewin, R. How microorganisms transport iron. Science 1984, 225, 401–402. [Google Scholar] [CrossRef]
- Veillette, J.; Juniper, S.K.; Gooday, A.J.; Sarrazin, J. Influence of surface texture and microhabitat heterogeneity in structuring nodule faunal communities. Deep-Sea Res. I 2007, 54, 1936–1943. [Google Scholar] [CrossRef]
- Gooday, A.J.; Haynes, J.R. Abyssal foraminifers, including two new genera, encrusting the interior of Bathysiphon rusticus tubes. Deep-Sea Res. 1983, 30, 591–614. [Google Scholar] [CrossRef]
- Lipps, J.H. Biotic interactions in benthic foraminifera. In Biotic Interaction in Modern and Fossil Benthic Communities; Tevesz, M.J.S., McCall, P.L., Eds.; Plenum Press: New York, NY, USA, 1983; pp. 331–376. [Google Scholar]
- Kobluk, D.R. Cryptic faunas in reefs: Ecology and geologic importance. Palaios 1988, 3, 379–390. [Google Scholar] [CrossRef]
- Toscano, F.; Raspini, A. Epilithozoan fauna associated with ferromanganese crustgrounds on the continental slope segment between Capri and Li Galli Islands (Bay of Salerno, Northern Tyrrhenian Sea, Italy). Facies 2005, 50, 427–441. [Google Scholar] [CrossRef]
- Schlögl, J.; Michalík, J.; Zágoršek, A.; Atrops, F. Early Tithonian serpulid-dominated cavity-dwelling fauna and the recruitment pattern of the serpulid larvae. J. Paleontol. 2008, 82, 382–392. [Google Scholar] [CrossRef]
- Wallace, M.W.; Keays, R.R.; Gostin, V.A. Stromatolitic iron oxides: Evidence that sea-level changes can cause sedimentary iridium anomalies. Geology 1991, 19, 551–554. [Google Scholar] [CrossRef]
- Mamet, B.; Préat, A. Jurassic microfacies, Rosso Ammonitico limestone, Subbetic Cordillera, Spain. Rev. Española Micropal. 2006, 38, 219–228. [Google Scholar]
- Jakubowicz, M.; Belka, Z.; Berkowski, B. Frutexites encrustations on rugose corals (Middle Devonian, southern Morocco): Complex growth of microbial microstromatolites. Facies 2014, 60, 631–650. [Google Scholar] [CrossRef]
- Mišík, M.; Aubrecht, R. Some notes concerning mineralized hardgrounds (Jurassic and Cretaceous, Western Carpathians). Were all hardgrounds always hard from the beginning? Slovak Geol. Mag. 2004, 10, 183–202. [Google Scholar]
- Myrow, P.M.; Coniglio, M. Origin and diagenesis of cryptobiontic Frutexites in the Chapel Island Formation (Vendian to Early Cambrian) of southeast Newfoundland, Canada. Palaios 1991, 6, 572–585. [Google Scholar] [CrossRef]
- Allouc, J.; Harmelin, J.G. Les dépôts d’enduits manganoferrifères en environnement marin littoral. L’exemple de grottes sous-marines en Méditerrée nord-occidentale. Bull. Soc. Géol. Fr. 2001, 172, 765–778. [Google Scholar] [CrossRef]
- Guido, A.; Jiménez, C.; Achilleos, K.; Rosso, A.; Sanfilippo, R.; Hadjioannou, L.; Petrou, A.; Russo, F.; Mastandrea, A. Cryptic serpulid-microbialite bioconstructions in the Kakoskali submarine cave (Cyprus, Eastern Mediterranean). Facies 2017, 63. [Google Scholar] [CrossRef]
- Reitner, J.; Thiel, V.; Zankl, H.; Michaelis, W.; Wörheide, G.; Gautret, P. Organic and biogeochemical patterns in cryptic microbialites. In Microbial Sediments; Riding, R., Awramik, S.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 149–160. [Google Scholar]
- Heim, C.; Queric, N.V.; Lonescu, D.; Schafer, N.; Reitner, J. Frutexites-like structures formed by iron oxidizing biofilms in the continental subsurface (Aspo Hard Rock Laboratory, Sweden). PLoS ONE 2017, 12, E0177542. [Google Scholar] [CrossRef] [PubMed]
- Olivier, N. Microbialites dans les Bioconstructions du Jurassique: Morphologies, rôles Edificateurs et Significations Paléoenvironnementales. Ph.D. Thesis, Université Claude-Bernard Lyon 1, Lyon, France, 2004. [Google Scholar]
- Reolid, M.; Gaillard, C. Microtaphonomy of bioclasts and paleoecology of microencrusters from Upper Jurassic spongiolithic limestones (External Prebetic, Southern Spain). Facies 2007, 53, 97–112. [Google Scholar] [CrossRef]
- Corliss, J.B.; Lyle, M.; Dymond, J.; Crane, K. The chemistry of hydrothermal mounds near Galapagos Rift. Earth Planet. Sci. Lett. 1978, 40, 12–24. [Google Scholar] [CrossRef]
- Usui, A.; Bau, M.; Yamazaki, T. Manganese microchimneys buried in the Central Pacific pelagic sediments: Evidence of intraplate water circulation? Mar. Geol. 1997, 141, 269–285. [Google Scholar] [CrossRef]
- Matsumoto, R.; Minai, Y.; Iijima, A. Manganese content, Cerium anomaly, and rate of sedimentation as clues to characterize and classify deep sea sediments. In Advances in Earth and Planetary Sciences, Formation of Oceanic Margin; Terra Science Publications: Tokyo, Japan, 1985; pp. 913–939. [Google Scholar]
- Mills, R.A.; Eldefield, H. Rare earth element geochemistry of hydrothermal deposits from the active TAG mound, 26°N Mid-Atlantic Ridge. Geochim. Cosmochim. Acta 1995, 59, 3511–3524. [Google Scholar] [CrossRef]
- Kuhn, T.; Bau, M.; Blum, N.; Halbach, P. Origin of negative Ce anomalies in mixed hydrothermal-hydrogenetic Fe-Mn crusts from the central Indian Ridge. Earth Planet. Sci. Lett. 1998, 163, 207–220. [Google Scholar] [CrossRef]
- Lonsdale, P.; Burns, V.M.; Fisk, M. Nodules of hydrothermal birnessite in the caldera of a young seamount. Jour. Geol. 1980, 88, 611–618. [Google Scholar] [CrossRef]
- Glasby, G.P.; Papavassiliou, C.T.; Mitsis, J.; Valsani-Jones, E.; Liakopoulos, A.; Renner, R.M. The Vani manganese deposits, Milos Island, Greece: A fossil stratabound Mn-Ba-Pb-Zn-As-Sb-W-rich hydrothermal deposit. Dev. Volcanol. 2005, 7, 255–291. [Google Scholar]
- Canet, C.; Prol-Ledesma, R.M. Mineralizing processes at shallow submarine hydrothermal vents: Examples from México. GSA Spec. Pap. 2007, 422, 359–376. [Google Scholar]
- Koschinsky, A.; Hein, J.R. Uptake of elements from seawater by ferromanganese crusts: Solid-phase association and seawater speciation. Mar. Geol. 2003, 198, 331–351. [Google Scholar] [CrossRef]
- Canet, C.; Prol-Ledesma, R.M.; Bandy, W.L.; Schaaf, P.; Linares, C.; Camprubí, A.; Tauler, E.; Mortera-Gutiérrez, C. Mineralogical and geochemical constraints on the origin of ferromanganese crusts from the Rivera Plate (western margin of Mexico). Mar. Geol. 2008, 251, 47–59. [Google Scholar] [CrossRef]
- Corona-Esquivel, R.; Ortega-Gutiérrez, F.; Reyes-Salas, M.; Lozano-Santacruz, R.; Miranda-Gasca, M.A. Mineralogical study of the La Hueca Cretaceous iron-manganese deposit, Michoacán, Southwestern Mexico. Rev. Mex. Cienc. Geol. 2000, 17, 143–153. [Google Scholar]
- Camprubí, A.; Canet, C.; Rodríguez-Díaz, A.A.; Prol-Ledesma, R.M.; Blanco-Florido, D.; Villanueva, R.E.; López-Sánchez, A. Geology, ore deposits and hydrothermal venting in Bahía Concepción, Baja California Sur, Mexico. Island Arc. 2008, 17, 6–25. [Google Scholar] [CrossRef]
- Ehrlich, H.L. Geomicrobiology of manganese. In Geomicrobiology; Marcel Dekker: New York, NY, USA, 1996; pp. 389–489. [Google Scholar]
- Tebo, B.M.; Bargar, J.R.; Clement, B.G.; Dick, G.J.; Murray, K.J.; Parker, D.; Verity, R.; Webb, S.M. Biogenic manganese oxides: Properties and mechanisms of formation. Ann. Rev. Earth Planet. Sci. 2004, 32, 287–328. [Google Scholar] [CrossRef]
- Connell, L.; Barret, A.; Templeton, A.; Staudigel, H. Fungal diversity associated with Active Deep Sea Volcano: Vailulu’u Seamount, Samoa. Geomicrobiol. J. 2009, 26, 597–605. [Google Scholar] [CrossRef]
- Santelli, C.M. Life in the deep sea. Nature Geoscience 2009, 2, 825–826. [Google Scholar] [CrossRef]
- Le Calvez, T.; Burgaud, G.; Mahé, S.; Barbier, G.; Vandenkoornhuyse, P. Fungal diversity in deep-sea hydrothermal ecosystem. App. Environ. Microbiol. 2009, 75, 6415–6421. [Google Scholar] [CrossRef] [PubMed]
- Dick, G.J.; Clement, B.G.; Webb, S.M.; Fodrie, F.J.; Bargar, J.R.; Tebo, B.M. Enzymatic microbial Mn(II) oxidation and Mn biooxide production in the Guaymas Basin deep-sea hydrothermal plume. Geochim. Cosmochim. Acta 2009, 73, 6517–6530. [Google Scholar] [CrossRef]
- Peck, S.B. Bacterial deposition of iron and manganese oxides in North American caves. Nat. Assoc. Speleol. Bull. 1986, 48, 26–30. [Google Scholar]
- Corbin, J.C.; Person, A.; Iatzoura, A.; Ferré, B.; Renard, M. Manganese in pelagic carbonates: Indication of major tectonic events during the geodynamic evolution of a passive continental margin (the Jurassic European Margin of the Tethys-Ligurian Sea). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2000, 156, 123–138. [Google Scholar] [CrossRef]
- Rankama, K.; Sahama, T.G. Geochemistry; University of Chicago Press: Chicago, IL, USA, 1960; p. 912. [Google Scholar]
- Millot, G. Géologie des Argiles; Masson: Paris, France, 1964; p. 499. [Google Scholar]
- Odin, G.S.; Fullagar, P.D. Geological significance of the glaucony facies. Dev. Sedimentol. 1988, 45, 295–332. [Google Scholar]
- Odin, G.S.; Matter, A. De glauconiarum origine. Sedimentology 1981, 28, 611–641. [Google Scholar] [CrossRef]
- Amorosi, A. Glaucony and sequence stratigraphy: A conceptual framework of distribution in siliciclastic sequences. J. Sed. Res. 1995, 65, 419–425. [Google Scholar]
- Eder, V.; Martín-Algarra, A.; Sánchez-Navas, A.; Zanin, Y.N.; Zamirailova, A.G.; Lebedevs, Y.N. Depositional controls on glaucony texture and composition, Upper Jurassic, West Siberian Basin. Sedimentology 2007, 54, 1365–1387. [Google Scholar] [CrossRef]
- Rieder, M.; Cavazzini, G.; D’Yakonov, Y.S.; Frank-Lamenetskii, V.A.; Gottardi, G.; Guggenheim, S.; Koval, P.V.; Müller, G.; Neiva, A.M.R.; Radoslovich, E.W.; et al. Nomenclature of the micas. Can. Mineral. 1998, 36, 905–912. [Google Scholar] [CrossRef]
- Andrews, A.J. Saponite and celadonite in layer 2 basalts DSDP Leg 37. Contrib. Miner. Petrol. 1980, 73, 323–340. [Google Scholar] [CrossRef]
- Delmont, P. Smectites et Produtis D’altération des Basaltes Tertiaires des iles Faeroe (Atlantique Nord Est). Genèse, Évolution et Contribution à la Sédimentation Océanique. Ph.D. Thesis, Université de Bordeaux I, Bordeaux, France, 1985; 490p. [Google Scholar]
- Staudigel, M.; Gillis, K.; Duncan, R. K-Ar and Rb-Sr ages of celadonites from the Troodos ophiolite, Cyprus. Geology 1986, 14, 72–75. [Google Scholar] [CrossRef]
- Odin, G.S.; Desprairies, A.; Fullagar, P.D.; Bellon, H.; Decarreau, A.; Frohlich, F.; Zelvelder, M. Nature and geological significance of celadonite. Dev. Sedimentol. 1988, 45, 337–398. [Google Scholar]
- Tazaki, K.; Fyfe, W. Microbial green marine clay from Izu-Bonin (west pacific) deep-sea sediments. Chem. Geol. 1992, 102, 105–118. [Google Scholar] [CrossRef]
- Renac, C.; Kyser, K.; Bowden, P.; Moine, B.; Cottin, J.Y. Hydrothermal fluid interaction in basaltic lava units, Kerguelen Archipelago (SW Indian Ocean). Eur. J. Miner. 2010, 22, 215–234. [Google Scholar] [CrossRef]
- D’Antonio, M.; Kristensen, M.B. Hydrothermal alteration of oceanic crust in the West Philippine Sea Basin (Ocean Drilling Program Leg 195, Site 1201): Inferences from a mineral chemistry investigation. Mineral. Petrol. 2005, 83, 87–112. [Google Scholar] [CrossRef]
- Clayton, T.; Pearce, R.B. Alteration mineralogy of Cretaceous basalt from ODP Site 1001, Leg 165 (Caribbean Sea). Clay Miner. 2000, 35, 719–733. [Google Scholar] [CrossRef]
- Clauer, N.; O’Neil, J.R.; Honnorez, J.; Buatier, M. 87Sr/86Sr and 18O/16O ratios of clays from a hydrothermal area near the Galapagos rift as records of origin, crystallization temperature and fluid composition. Mar. Geol. 2011, 288, 32–42. [Google Scholar] [CrossRef]
- Stackes, D.S.; O’Neil, J.R. Mineralogy and stable isotope geochemistry of hydrothermaly altered oceanic rocks. Earth Planet. Sci. Lett. 1982, 57, 285–304. [Google Scholar] [CrossRef]
- Desprairies, A.; Bonnot, C.; Jehanno, C.; Vernhet, S.; Joron, J.L. Mineralogy and Geochemistry of Alteration Products in Leg 81 Basalts; Initial Report DSDP 81; United States Government Publishing Office: Washington, DC, USA, 1984; pp. 733–742. [Google Scholar]
- Alt, J.C. Very low-grade hydrothermal metamorphism of basic igneous rocks. In Low-Grade Metamorphism; Frey, M., Robinson, D., Eds.; Blackwell: Oxford, UK, 1999; pp. 169–201. [Google Scholar]
- Edwards, K.J.; Bach, W.; McCollom, T.M. Geomicrobiology in oceanography: Microbe-mineral interactions at and below the sea-floor. Trends Microbiol. 2005, 13, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Santelli, C.M.; Orcutt, B.N.; Banning, E.; Bach, W.; Moyer, C.L.; Sogin, M.L.; Staudigel, H.; Edwards, K.J. Abundance and diversity of microbial life in ocean crust. Nature 2008, 453, 653–656. [Google Scholar] [CrossRef] [PubMed]
- Bach, W.; Edwards, K.J. Iron and sulphide oxidation within the basaltic ocean crust: Implications for chemolithoautotrophic microbial biomass production. Geochim. Cosmochim. Acta 2003, 67, 3871–3887. [Google Scholar] [CrossRef]
- Juniper, S.K.; Fouquet, Y. Filaments iron-silica deposits from modern and ancient hydrothermal sites. Can. Mineral. 1988, 26, 859–869. [Google Scholar]
- Vanko, D.A.; Milby, B.J.; Heinzquith, S.W. Massive sulphides with fluid-inclusion-bearing quartz from a young seamount on the East Pacific Rise. Can. Mineral. 1991, 29, 453–460. [Google Scholar]
- Geptner, A.R.; Ivanovskaya, T.A. Glauconite from Lower Cretaceous marine terrigenous rocks of England: A concept of biochemogenic origin. Lithol. Miner. Resour. 2000, 35, 487–499. [Google Scholar] [CrossRef]
- Zanin, Y.N.; Eder, V.G.; Zamirailova, A.G. Bacterial forms in glauconites from Upper Jurassic deposits of the West Siberian Plate. Russ. Geol. Geophys. 2004, 45, 774–777. [Google Scholar]
- Zanin, Y.N.; Eder, V.G.; Zamirailova, A.G. Mn-carbonates, glauconites and phosphorites in the Upper Jurassic Georgiev Formation of the West Siberian marine basin. Geophys. Res. Abstracts 2006, 8, A-00271. [Google Scholar]
- Baldermann, A.; Warr, L.N.; Grathoff, G.H.; Dietzel, M. The rate and mechanism of deep-sea glauconite formation at the Ivory Coast-Ghana Marginal Ridge. Clays Clay Miner. 2013, 61, 258–276. [Google Scholar] [CrossRef]
- Geptner, A.R.; Ivanovskaya, T.A.; Pokrovskaya, E.V.; Kuralenko, N.P. Glauconite from Paleogene volcano-terrigenous rocks in Western Kamchatka. Lithol. Miner. Resour. 2008, 43, 228–249. [Google Scholar] [CrossRef]
- Beveridge, T.J.; Makin, S.A.; Kadurugamuwa, J.L.; Li, Z.S. Interactions between biofilms and the environments. FEMS Microbiol. Rev. 1997, 20, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Konhauser, K.O. Diversity of bacterial iron mineralization. Earth-Sci. Rev. 1998, 43, 91–121. [Google Scholar] [CrossRef]
- Eickmann, B.; Bach, W.; Kiel, S.; Reitner, J.; Peckmann, J. Evidence for cryptoendolithic life in Devonian pillow basalts of Variscan orogens, Germany. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2009, 283, 120–125. [Google Scholar] [CrossRef]


















© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reolid, M.; Abad, I. Jurassic Non-Carbonate Microbialites from the Betic-Rifian Cordillera (Tethys Western End): Textures, Mineralogy, and Environmental Reconstruction. Minerals 2019, 9, 88. https://doi.org/10.3390/min9020088
Reolid M, Abad I. Jurassic Non-Carbonate Microbialites from the Betic-Rifian Cordillera (Tethys Western End): Textures, Mineralogy, and Environmental Reconstruction. Minerals. 2019; 9(2):88. https://doi.org/10.3390/min9020088
Chicago/Turabian StyleReolid, Matías, and Isabel Abad. 2019. "Jurassic Non-Carbonate Microbialites from the Betic-Rifian Cordillera (Tethys Western End): Textures, Mineralogy, and Environmental Reconstruction" Minerals 9, no. 2: 88. https://doi.org/10.3390/min9020088




