Mineralogical Features of Ore Diagenites in the Urals Massive Sulfide Deposits, Russia
Abstract
:1. Introduction
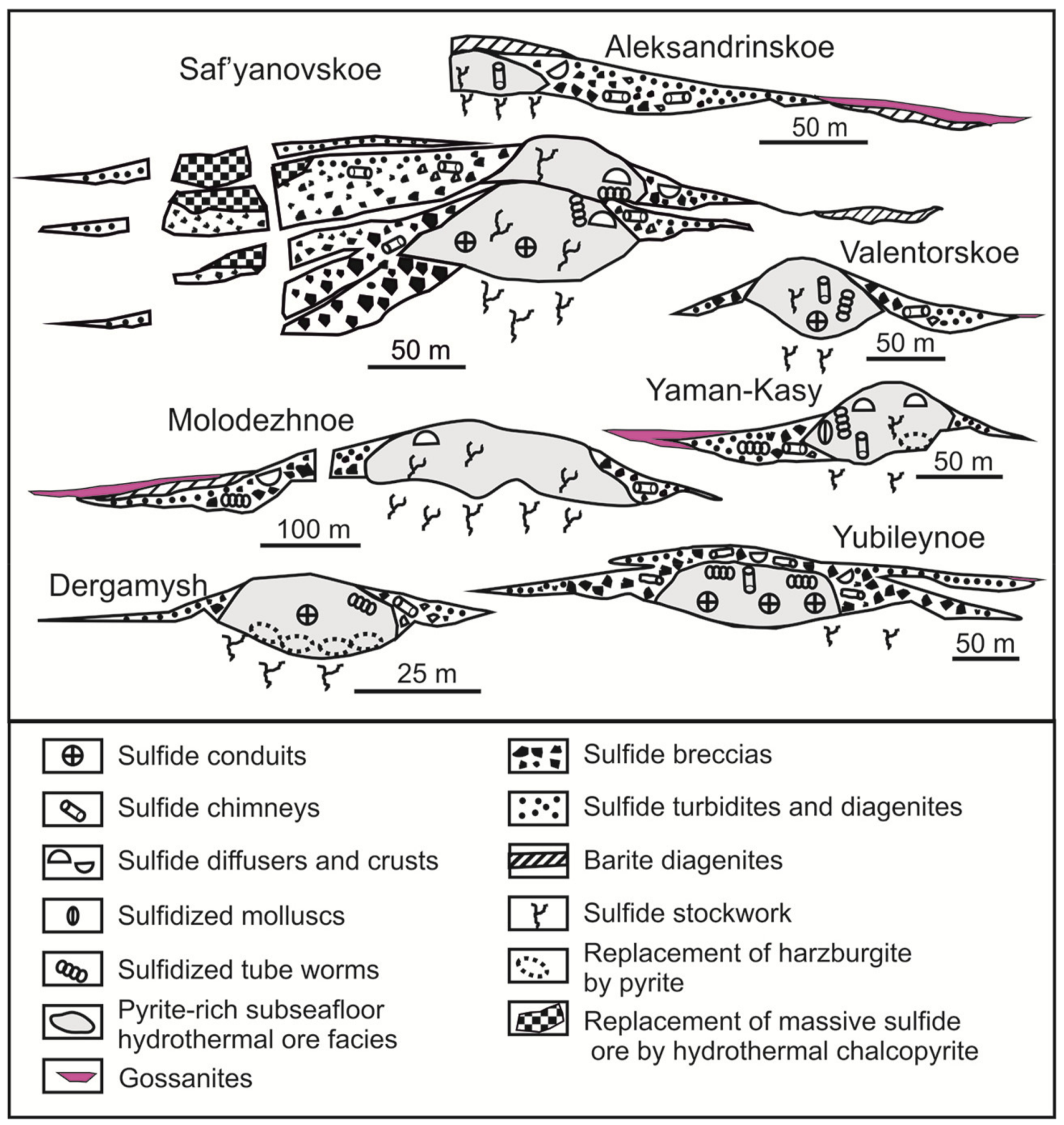
2. Geological Setting and Types of the Deposits Studied
3. Sampling and Analytical Techniques
4. Results
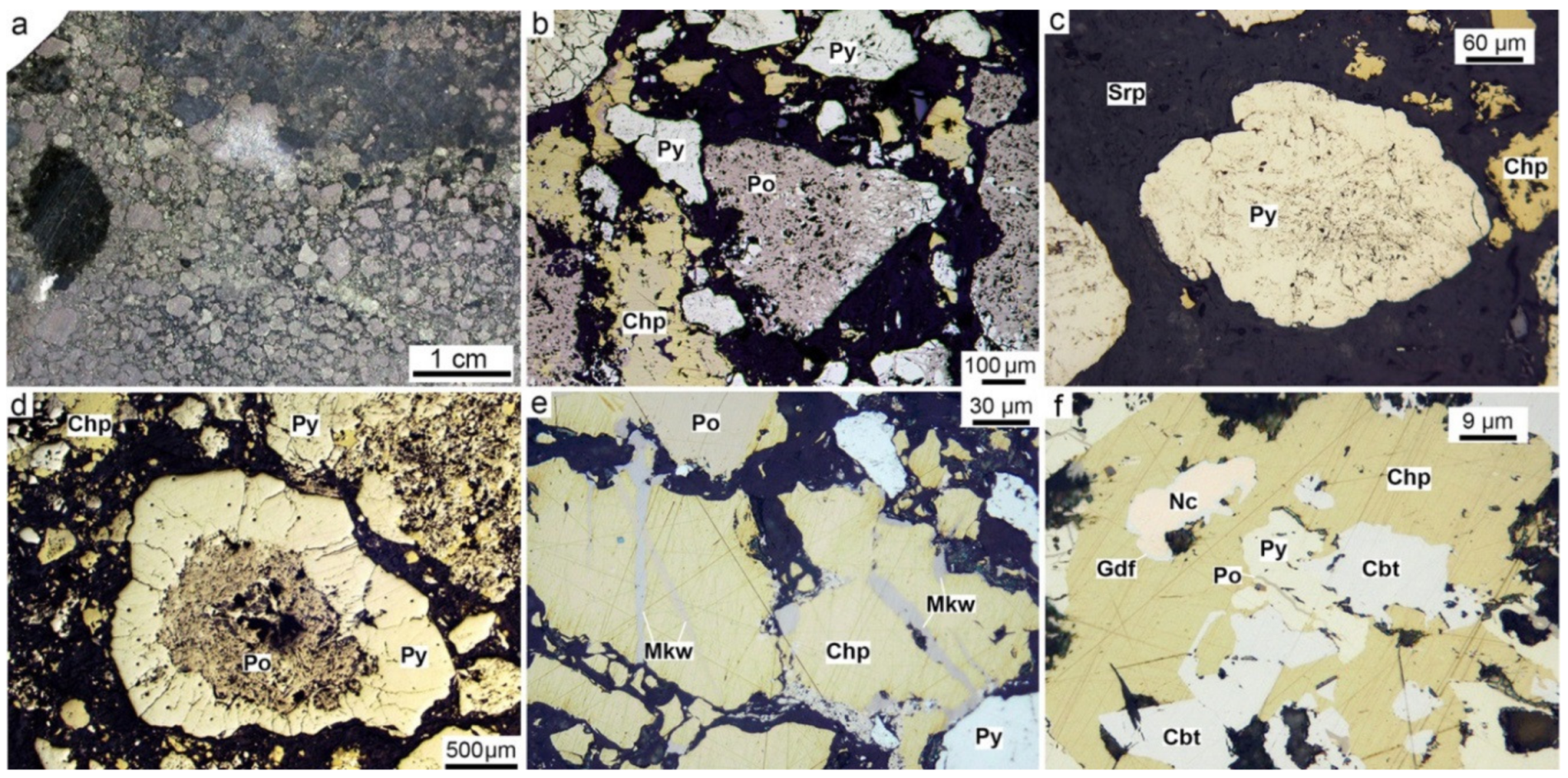
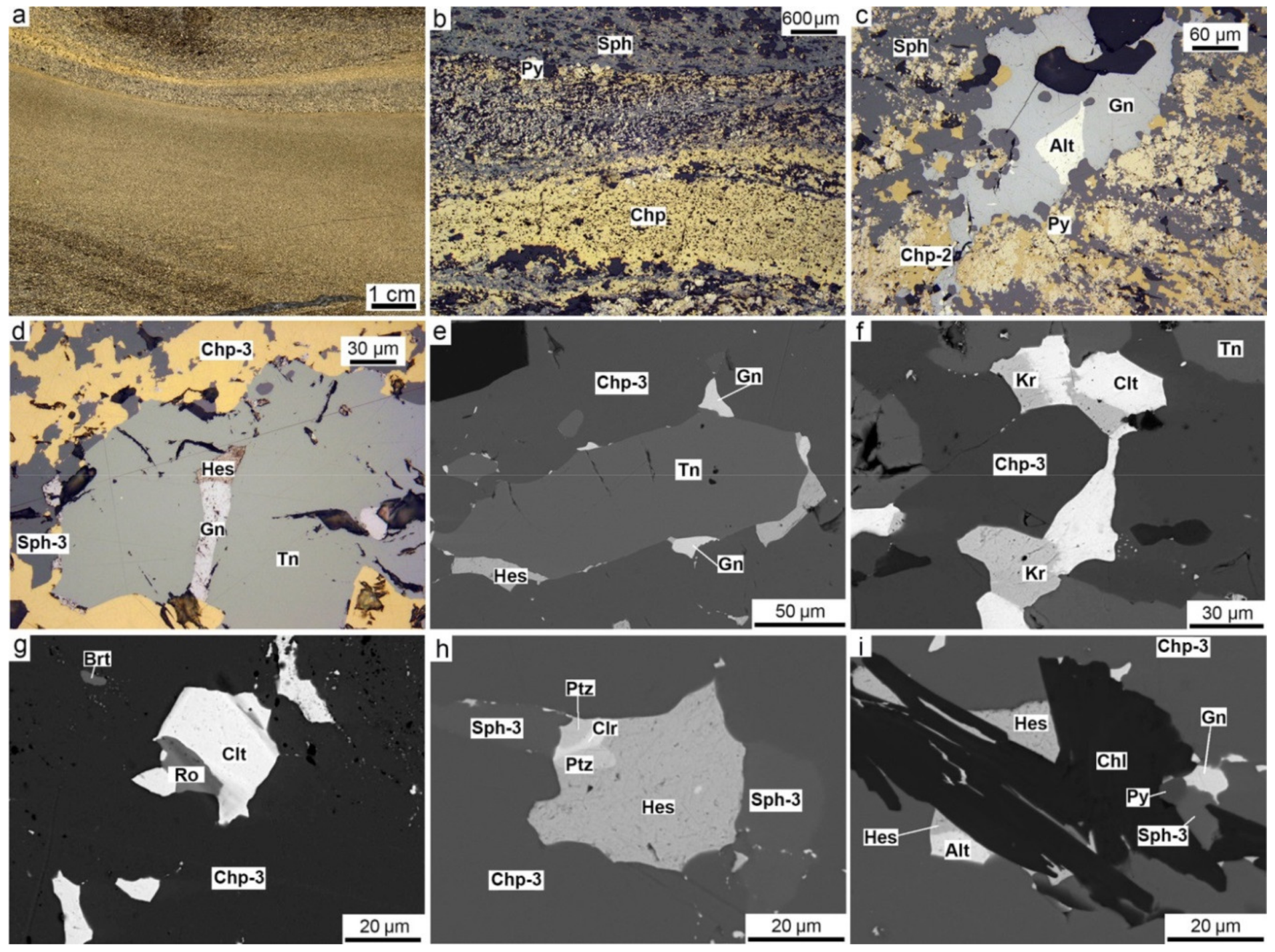
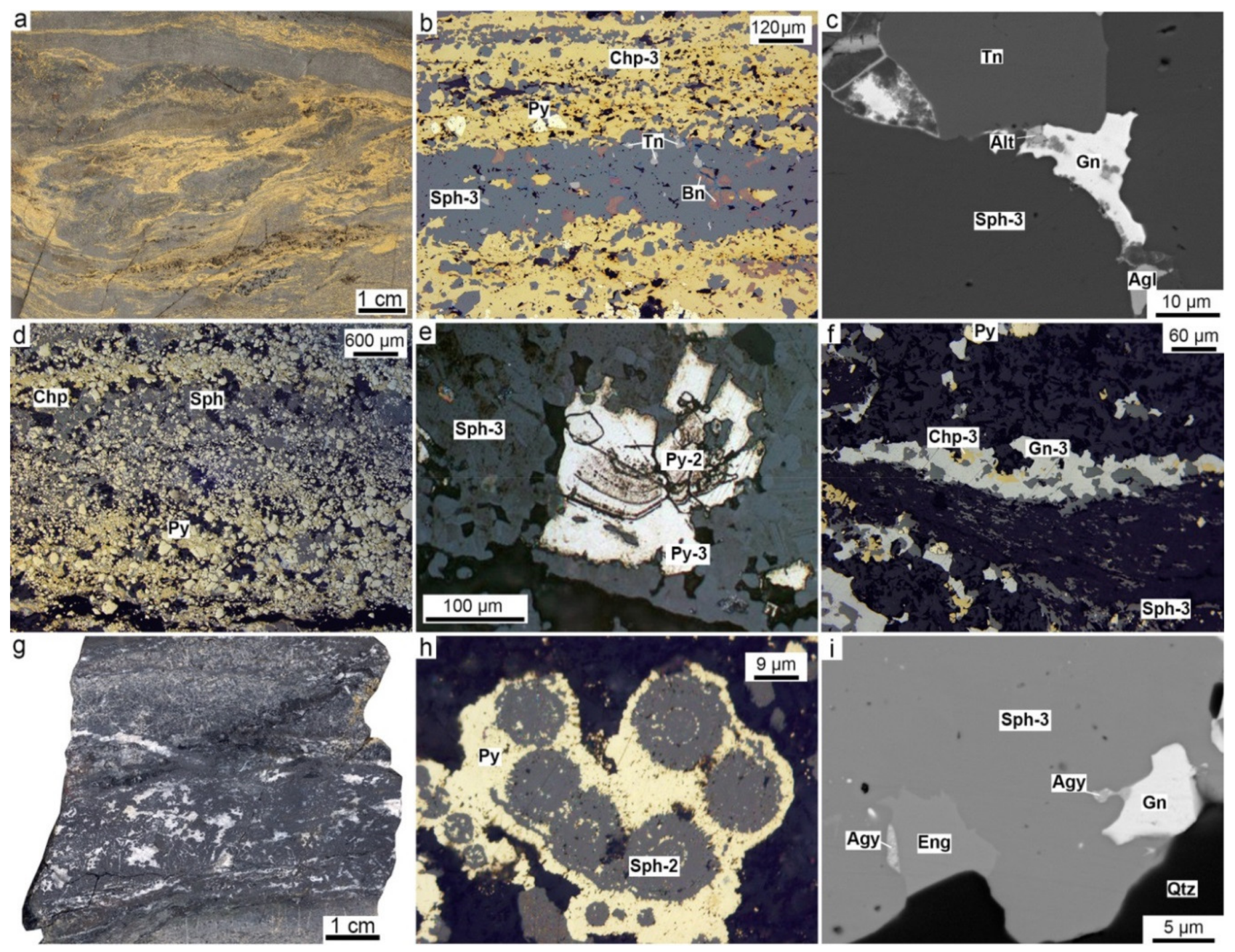
4.1. Pyrrhotite-Rich Diagenites
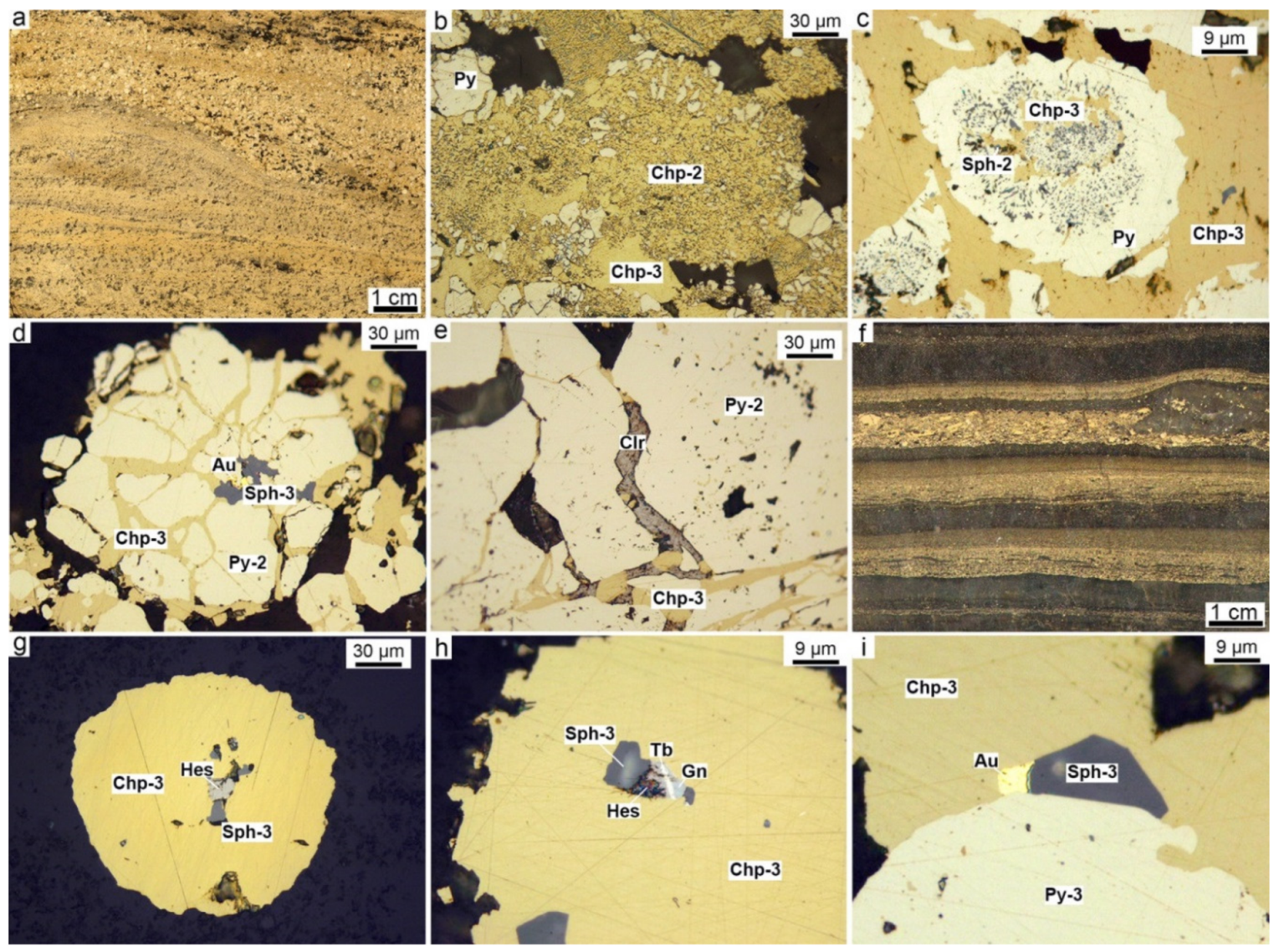
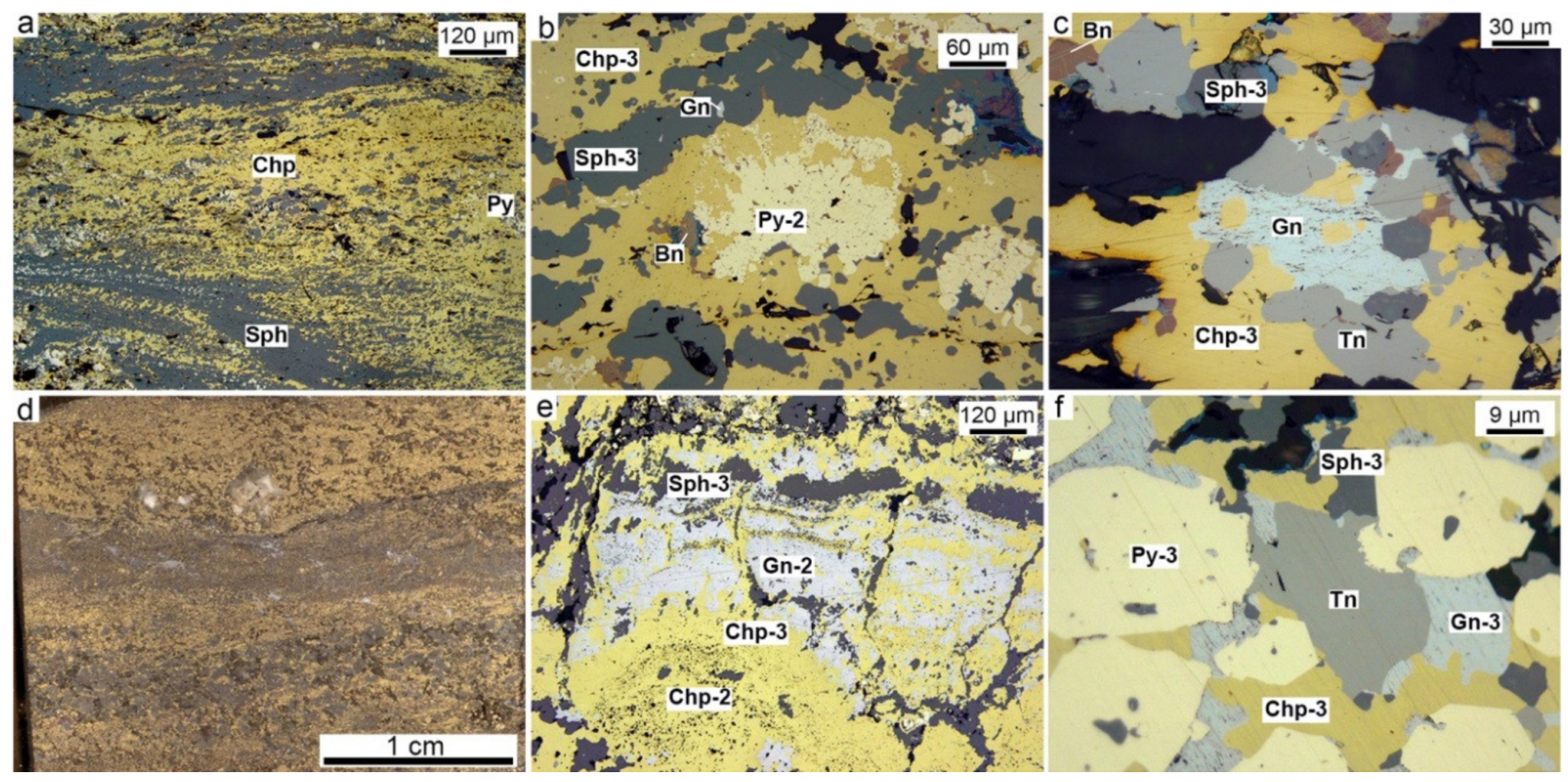
4.2. Chalcopyrite-Rich Diagenites
4.3. Bornite-Rich Diagenites
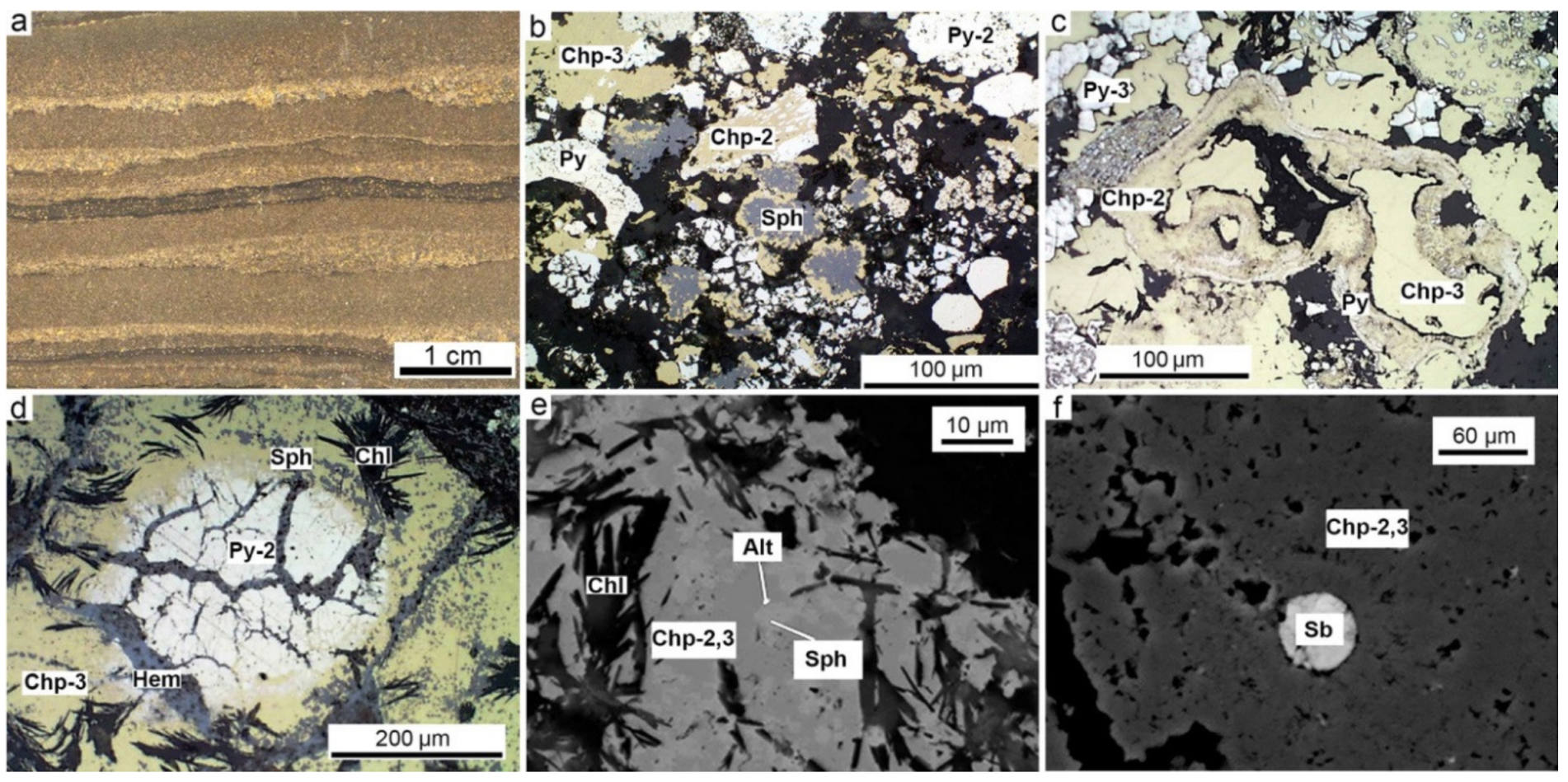
4.4. Sphalerite-Rich Diagenites
4.5. Pyrite-Rich Diagenites
4.6. Barite-Rich Diagenites
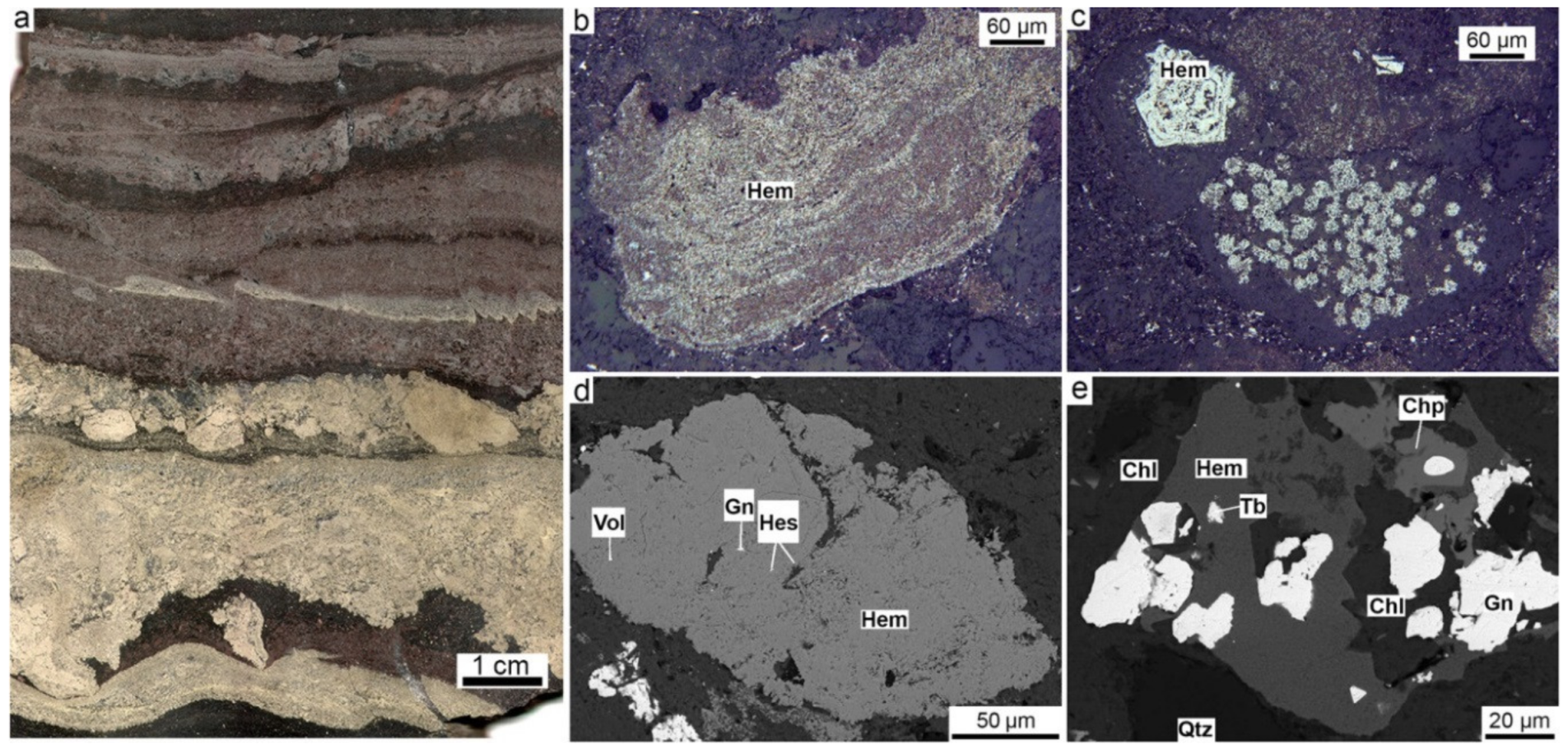
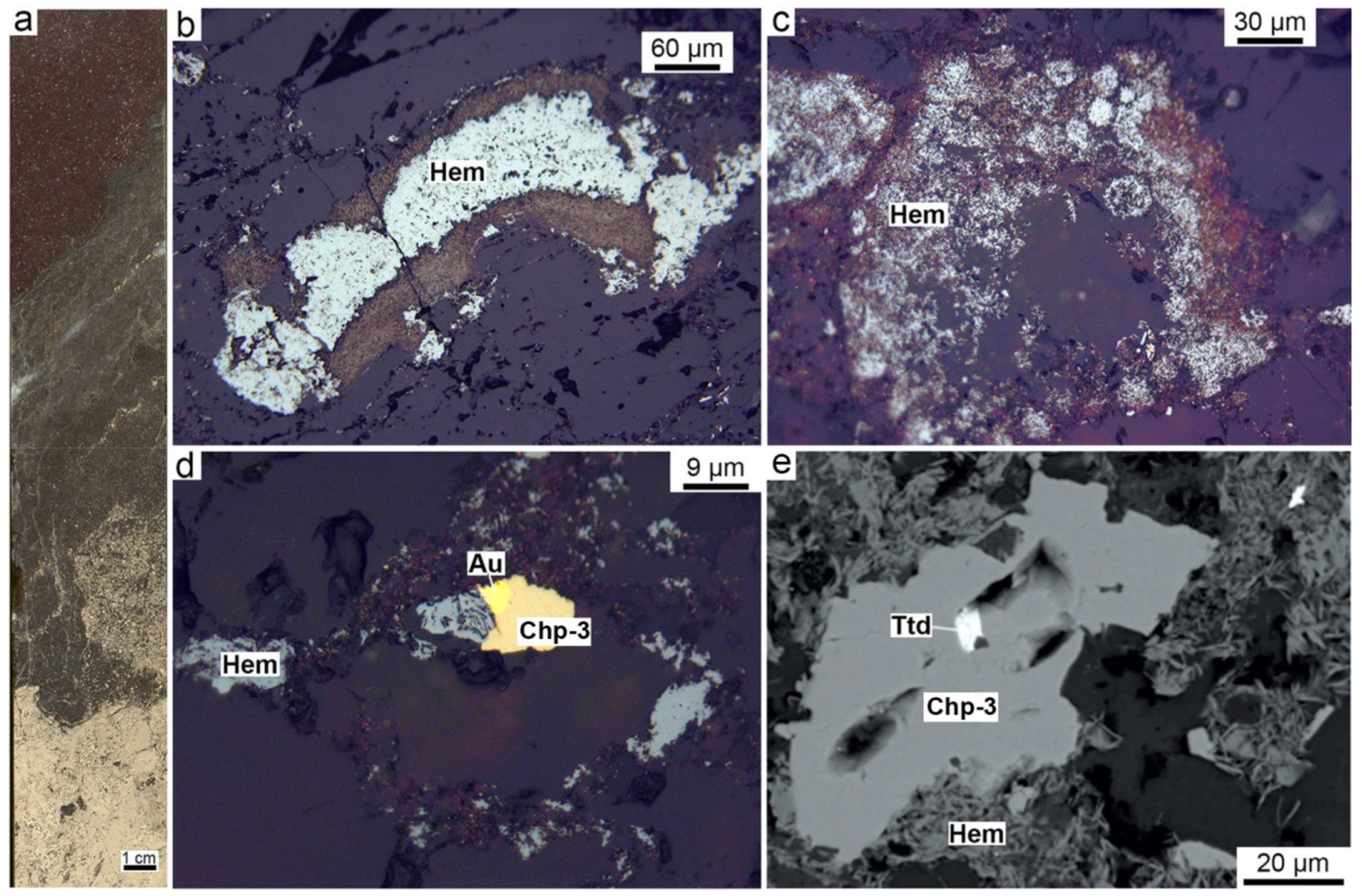
4.7. Hematite-Rich and Magnetite-Rich Diagenites (Halmyrolytes)
5. Discussion
5.1. Arguments for Diagenetic Origin of Banded Sulfides and Barite
5.2. Mineralogical Diversity of Ore Diagenites
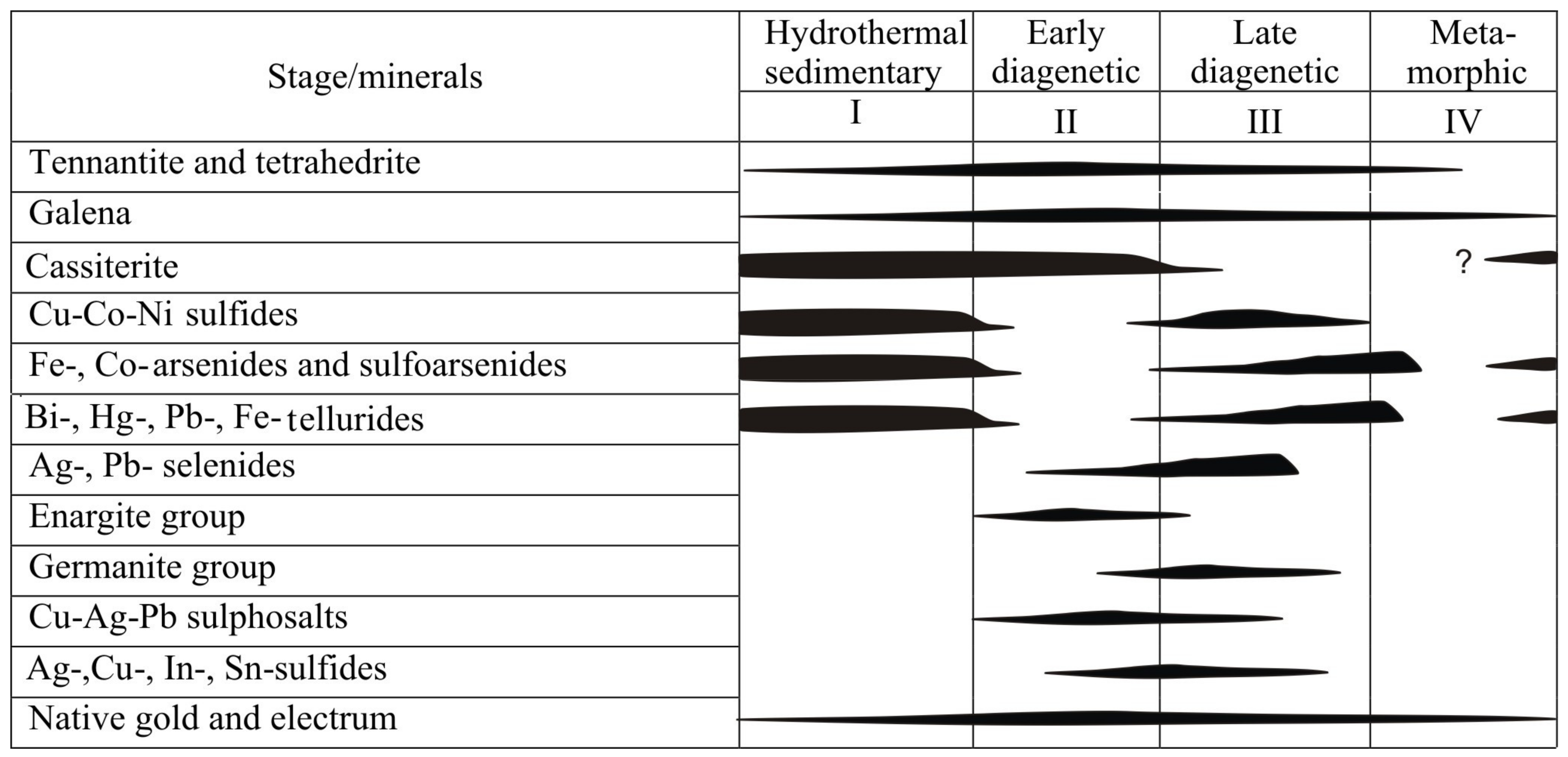
5.3. Microtextures and Mineral Evolution
6. Conclusions
- Each type of ore diagenites exhibits specific rare mineral assemblages and microtextures. Mineral diversity of the ore diagenites is explained by different primary compositions of ore clasts and the maturity of diagenesis. Tellurides, sulfarsenides, fahlores, galena, and native gold show a dual nature since they are formed both in primary smoker chimneys and in ore diagenites. Selenides, Cu–Ag and Cu–Sn sulfides and the minerals of the germanite group are observed only in mature bornite-rich diagenites.
- Mineral evolution of clastic sulfide sediments is coeval with their textural evolution. The ore diagenites exhibit no hydrothermal features in contrast to dominant replacement and nodular microtextures. Several trends of diagenetic replacements are constrained in ore diagenites. The style and intensity of alteration depend on variable primary composition, sizes and proportions between hydrothermal ore clasts and serpentinite, hyaloclastite, carbonaceous and calcareous material in terms of influencing the pH and oxidation conditions during mineral evolution. In ultramafic-hosted deposits, the chalcopyrite- and pyrrhotite-rich diagenites contain Cu-Co-Ni sulfides. Tellurides and selenides are typical of some chalcopyrite-rich diagenites in the Uralian type of the deposits. The minerals of the germanite group, Cu-Ag and Cu-Sn sulfides are characteristic of mature bornite-rich diagenites. The barite- and sphalerite-rich diagenites host abundant galena and sulfosalts. Diverse tellurides are disseminated in hematite-rich diagenites, the extreme products of halmyrolysis. All ore diagenites are enriched in native gold.
- In general, the ore diagenites are characterized by higher proportions of SO42−-, As3+-, As5+-, Sb3+-, Sb5+-, V5+-, Fe3+- and Ge4+-bearing minerals versus reduced forms. These reflect the influence of oxygenated seawater on the seafloor supergene alteration of sulfide turbidites. Halmyrolysis triggered enrichment in economically important Cu, Co, Se, Ge, Zn, Bi, Pb, Au and Ag in some of the ore diagenites.
Supplementary Materials
Author Contributions
Funding
Acknowledgements
Conflicts of Interest
References
- Peter, J.M.; Scott, S.D. Windy Craggy, Northwestern British Columbia: The world’s largest Besshi-type deposits. Rev. Econ. Geol. 1999, 8, 261–295. [Google Scholar] [CrossRef]
- Solomon, M.; Tornos, F.; Large, R.R.; Badham, J.N.P.; Both, R.A.; Kin, Z. Zn-Pb-Cu volcanic-hosted massive sulfide deposits criteria for distinguishing brine pool-type from black smoker-type sulfide deposition. Ore Geol. Rev. 2004, 25, 259–283. [Google Scholar] [CrossRef]
- Tornos, F.; Peter, J.M.; Allen, R.; Conde, C. Controls on the siting and style of volcanogenic massive sulphide deposits. Ore Geol. Rev. 2015, 68, 142–163. [Google Scholar] [CrossRef]
- Genna, D.; Gaboury, D. Deciphering the hydrothermal evolution of a VMS system by LA-ICP-MS using trace elements in pyrite: An example from the Bracemac-McLeod deposits, Abitibi, Canada, and implication for exploration. Econ. Geol. 2015, 110, 2087–2108. [Google Scholar] [CrossRef]
- Kajiwara, Y.; Hyrayama, H. Diagenetic chemical differentiation of Kuroko ore deposits. Min. Geol. 1983, 33, 149–164. (In Japanese) [Google Scholar]
- Peter, J.M.; Kjarsgaard, M.I.; Goodfellow, W.D. Hydrothermal sedimentary rocks of the Heath Steel Belt, Bathurst Mining Camp. New Brunswick: Part 1. Mineralogy and mineral chemistry. In Massive Sulfide Deposits of the Bathurst Mining Camp, New Brunswick, and Nothern Maine; Goodfellow, W.D., McCutcheon, S.R., Peter, J.M., Eds.; Economic Geology Monograph; Society of Economic Geologist: Littleton, CO, USA, 2003; Volume 11, pp. 361–390. [Google Scholar] [CrossRef]
- Solomon, M.; Walshe, D. Formation of massive sulfide deposits on sea floor. Econ. Geol. 1979, 69, 947–973. [Google Scholar] [CrossRef]
- Zlotnik-Khotkevich, A.G. Diagenetic transformations of massive sulfide ores. Geol. Rudn. Mestorozh. 1992, 34, 83–98. (In Russian) [Google Scholar]
- Yarosh, P.Y. Diagenesis and Metamorphism of Massive Sulfide Ores in the Urals; Nauka: Moscow, Russia, 1973; 240p. (In Russian) [Google Scholar]
- Constantinou, G.; Govett, J.S. Geology, geochemistry and genesis of Cyprus sulfide deposits. Econ. Geol. 1973, 68, 843–858. [Google Scholar] [CrossRef]
- Large, R. Australian volcanic-hosted massive sulfide deposits: Features, styles, and genetic models. Econ. Geol. 1992, 87, 113–128. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Zaykov, V.V. Erosion and oxidation of sulfide mounds on the bottom of the Uralian Paleoocean. Dokl. Acad. Nauk USSR 1991, 319, 1434–1437. (In Russian) [Google Scholar]
- Gablina, I.F.; Gorkova, N.V.; Semkova, T.A.; Stepanova, T.V. Diagenetic alteration of copper sulfides in modern ore-bearing sediments of the Logachev-1 hydrothermal field (Mid-Atlantic ridge 14 45 N). Lithol. Miner. Resour. 2006, 41, 27–44. [Google Scholar] [CrossRef]
- Hannington, M.D.; Thompson, G.; Rona, P.A.; Scott, S.D. Gold and native copper in supergene sulphides from the Mid-Atlantic Ridge. Nature 1988, 333, 64–66. [Google Scholar] [CrossRef]
- Herzig, P.M.; Hannington, M.D.; Scott, S.D.; Maliotis, G.; Rona, P.A.; Tompson, G. Gold-rich sea-floor gossans in the Troodos ophiolite and on the Atlantic Ridge. Econ. Geol. 1991, 86, 1747–1755. [Google Scholar] [CrossRef]
- Ayupova, N.R.; Melekestseva, I.Y.; Maslennikov, V.V.; Tseluyko, A.S.; Blinov, I.A.; Beltenev, V.E. Uranium accumulation in modern and ancient Fe-oxide sediments: Examples from the Ashadze-2 hydrothermal sulfide field (Mid-Atlantic ridge) and Yubileynoe massive sulfide deposit (South Urals, Russia). Sediment. Geol. 2018, 367, 164–174. [Google Scholar] [CrossRef]
- Herrington, R.J.; Zaykov, V.V.; Maslennikov, V.V.; Brown, D.; Puchkov, V. Mineral deposits of the Urals and links to geodynamic evolution. In Economic Geology One Hundredth Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologist: Littleton, CO, USA, 2005; pp. 1069–1095. [Google Scholar] [CrossRef]
- Maslennikov, V.V. Sedimentogenesis, Halmyrolysis and Ecology of Massive Sulfide Paleohydrothermal Fields; Geotur: Miass, Russia, 1999; 348p. (In Russian) [Google Scholar]
- Maslennikov, V.V. Lithogenesis and Formation of Massive Sulfide Deposits; IMin UB RAS: Miass, Russia, 2006; 384p. (In Russian) [Google Scholar]
- Maslennikov, V.V.; Ayupova, N.R.; Herrington, R.J.; Danyushevskiy, L.V.; Large, R.R. Ferruginous and manganiferous haloes around massive sulphide deposits of the Urals. Ore Geol. Rev. 2012, 47, 5–41. [Google Scholar] [CrossRef]
- Rusakov, V.Y.; Ryzhenko, B.N.; Roshchina, I.A.; Kononkova, N.N.; Karpukhina, V.S. Devonian ore clastic turbidites of the Molodezhnoe massive copper deposit, Southern Urals. Geochem. Int. 2015, 53, 624–650. [Google Scholar] [CrossRef]
- Safina, N.P.; Maslennikov, V.V. Litologo-mineralogical zonality of sulfide cyclites of the Yaman-Kasy and Safyanovskoye massive sulfide deposits. Dokl. Earth Sci. 2008, 419, 804–806. [Google Scholar] [CrossRef]
- Safina, N.P.; Maslennikov, V.V. Clastic Ores from the Yaman-Kasy and Saf’yanovskoe Massive Sulfide Deposits (Urals); Ural Branch of RAS: Miass, Russia, 2009; 260p. (In Russian) [Google Scholar]
- Lein, A.Y.; Bogdanov, Y.A.; Maslennikov, V.V.; Li, S.; Ulyanova, N.V.; Maslennikova, S.P.; Ulyanov, A.A. Sulfide Minerals in the Menez Gwen Nonmetallic Hydrothermal Field (Mid_Atlantic Ridge). Lithol. Miner. Resour. 2010, 45, 305–323. [Google Scholar] [CrossRef]
- Melekestseva, I.Y.; Maslennikov, V.V.; Safina, N.P.; Nimis, P.; Maslennikova, S.P.; Beltenev, V.; Rozhdestvenskaya, I.; Danyushevsky, L.; Large, R.; Artemyev, D.A.; et al. Sulfide breccias from the Semenov-3 hydrothermal field, Mid-Atlantic Ridge: Authigenic mineral formation and trace element pattern. Minerals 2018, 8, 321. [Google Scholar] [CrossRef]
- Ridley, W.I. Weathering Processes. In Volcanogenic Massive Sulfide Occurrence Model; Pat Shanks, W.C., Thurston, R., Eds.; Scientific Investigation Report 2010-50-70-C; U.S. Geological Survey: Reston, VA, USA, 2012; Chapter 13; pp. 195–201. [Google Scholar]
- Fairbridge, R.W. Syndiagenesis–anadiagenesis–epidiagenesis: Phases of lithogenesis. In Diagenesis in Sediments and Sedimentary Rocks; Larsen, G., Chilingar, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; Volume 2, pp. 17–113. [Google Scholar] [CrossRef]
- Larsen, G.; Chilingarian, G.V. Introduction—Diagenesis of sediments and rocks. In Diagenesis in Sediments and Sedimentary Rocks; Larsen, G., Chilingar, G.V., Eds.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; New York, NY, USA, 1979; pp. 1–29. [Google Scholar] [CrossRef]
- Singer, A.; Müller, G. Diagenesis in Argillaceous Sediments. In Diagenesis in Sediments and Sedimentary Rocks; Larsen, G., Chilingar, G.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1983; Volume 2, pp. 115–211. [Google Scholar] [CrossRef]
- Hümmel, K. Die Enstehung eisenreicher Gestein durch Halmyrolyse (=submarine Gesteinszersetzung). Geol. Rundsch. 1922, 13, 40–81. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Zaykov, V.V.; Zaykova, E.V. Paleohydrothermal fields and ore formation conditions at massive sulfide deposits in the Uralian paleoocean. In Geodynamics and Metallogeny: Theory and Implications for Aplied Geology; Mezhelovsky, N.V., Morozov, A.F., Gusev, G.S., Popov, V.S., Eds.; Geokart: Moscow, Russia, 2000; pp. 339–358. (In Russian) [Google Scholar]
- Zaykov, V.V.; Maslennikov, V.V.; Zaykova, E.V.; Herrington, R.J. Hydrothermal activity in the segments of the rift valley on the marginal Urals palaeoocean. In Tectonic, Magmatic, Hydrothermal and Biological Segmentation at the Mid-Ocean Ridges; MacLeod, C.J., Tyler, P.A., Walker, C.L., Eds.; Geological Society Special Publication: London, UK, 1996; Volume 118, pp. 199–210. [Google Scholar]
- Fersman, A.E. Geochemistry of Russia; Nauchnoe Khimicheskoe Tekhnicheskoe Izdatel’stvo: St. Petersburg, FL, USA, 1922; 220p. (In Russian) [Google Scholar]
- Zaykov, V.V.; Herrington, R. Mawsonite from products of submarine oxidation of the Molodezhnoe copper-sulfide deposit (South Urals). In Ural’skiy Mineralogicheskiy Sbornik; IMin UB RAS: Miass, Russia, 1998; Volume 8, pp. 40–48. (In Russian) [Google Scholar]
- Herrington, R.J.; Armstrong, R.N.; Zaykov, V.V.; Maslennikov, V.V.; Tessalina, S.G.; Orgeval, J.-J.; Taylor, R.N. Massive sulfide deposits in the southern Urals: Geological setting within the Framework of the Uralide Orogen. In Mountain Building in the Uralides: Pangea to the Present; Brown, D., Ed.; Geophisical Monograph; American Geophysical Union: Washington, DC, USA, 2002; Volume 132, pp. 155–182. [Google Scholar] [CrossRef]
- Herrington, R.J.; Maslennikov, V.V.; Zaykov, V.V.; Seravkin, I.B.; Kosarev, A.S.; Bushmann, B.; Orgeval, J.-J.; Holland, N.; Tessalina, S.G.; Nimis, P.; et al. Classification of VHMS deposits: Lessons from the Uralides. Ore Geol. Rev. 2005, 27, 203–237. [Google Scholar] [CrossRef]
- Kontar, E.S.; Libarova, L.E. Geological Economic Types of Cu, Zn, and Pb Deposits of the Urals: Geological Setting, Evolution, and Prospects; Uralgeolkom: Yekaterinburg, Russian, 2013; 199p. (In Russian) [Google Scholar]
- Koroteev, V.A.; de Boorder, H.; Necheukhin, V.M.; Sazonov, V.N. Geodynamic setting of the mineral deposits of the Urals. Tectonophysics 1997, 276, 291–300. [Google Scholar] [CrossRef]
- Prokin, V.A.; Buslaev, F.P. Massive copper-zinc sulfide deposits in the Urals. Ore Geol. Rev. 1999, 14, 1–69. [Google Scholar] [CrossRef]
- Puchkov, V.N. Geology of the Urals and Cis-Urals (Topical Problems of Stratigraphy, Tectonics, Geodynamics, and Metallogeny); DizainPoligraphServis: Ufa, Russia, 2010; 280p. (In Russian) [Google Scholar]
- Seravkin, I.B. Correlation between compositions of ores and host rocks in volcanogenic massive sulfide deposits of the Southern Urals. Geol. Ore Depos. 2013, 55, 207–224. [Google Scholar] [CrossRef]
- Seravkin, I.B.; Kosarev, A.M.; Puchkov, V.M. Geodynamic conditions of formation of massive sulfide deposits in the Magnitogorsk megazone, Southern Urals, and prospection criteria. Geol. Ore Depos. 2017, 59, 227–243. [Google Scholar] [CrossRef]
- Spadea, P.; Kabanova, L.Y.; Scarrow, J. Petrology, geochemistry, and geodinamic significance of Mid-Devonian Boninitic rocks from the Baimak-Buribai area (Magnitogorsk zone, Southern Urals). Ofioliti 1998, 23, 17–36. [Google Scholar]
- Tessalina, S.G.; Herrington, R.J.; Taylor, R.N.; Sundblad, K.; Maslennikov, V.V.; Orgeval, J.J. Lead isotope systematic of massive sulphide deposits in the Urals: Application for geodynamic setting and metal sources. Ore Geol. Rev. 2016, 72, 22–36. [Google Scholar] [CrossRef]
- Zaykov, V.V. Volcanism and Sulfide Mounds of Paleocean Margins (after Example of the Ural’s and Siberia’s Massive Sulfide-Bearing Zones); Nauka: Moscow, Russia, 2006; 428p. (In Russian) [Google Scholar]
- Kontar, E.S. Quantitative estimation of massive sulfide ore formation. Geol. Ore Depos. 2002, 44, 543–555. [Google Scholar]
- Eremin, N.I.; Dergachev, A.L.; Sergeeva, N.E.; Pozdnyakova, N.V. Types of massive sulfide deposits of volcanic association. Geol. Ore Depos. 2000, 42, 177–190. [Google Scholar]
- Seravkin, I.B. The Metallogeny of the Southern Urals and the Central Kazakhstan; Gilem: Ufa, Russia, 2010; 281p. (In Russian) [Google Scholar]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevskiy, L.V.; Herrington, R.J.; Stanley, C.J. Tellurium-bearing minerals in zoned sulfide chimneys from Cu-Zn massive sulfide deposits of the Urals, Russia. Mineral. Petrol. 2013, 107, 67–99. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Ayupova, N.R.; Zaykov, V.V.; Tseluyko, A.S.; Melekestseva, I.Y.; Large, R.R.; Danyushevsky, L.V.; Herrington, R.J.; Lein, A.T.; et al. Chimneys in Paleozoic massive sulfide mounds of the Urals VMS deposits: Mineral and trace element comparison with modern black, grey, white and clear smokers. Ore Geol. Rev. 2017, 85, 64–106. [Google Scholar] [CrossRef]
- Franklin, J.M.; Gibson, H.L.; Jonasson, I.R.; Galley, A.G. Volcanogenic massive sulfide deposits. In Economic Geology One Hundredth Anniversary Volume; Hedenquist, J.W., Thompson, J.F.H., Goldfarb, R.J., Richards, J.P., Eds.; Society of Economic Geologist: Littleton, CO, USA, 2005; pp. 523–560. [Google Scholar] [CrossRef]
- Zaykov, V.V.; Melekestsev, I.Y.; Artemyev, D.A.; Yuminov, A.M.; Simonov, V.A.; Dunaev, A.Y. Geology and Massive Sulfide Mineralization of the Southern Flank of the Main Uralian Fault; Geotur: Miass, Russia, 2009; 376p. (In Russian) [Google Scholar]
- Melekestseva, I.Y.; Zaykov, V.V.; Nimis, P.; Tret’yakov, G.A.; Tesalina, S.G. Cu-(Ni-Co-Au)-bearing massive sulfide deposits associated with mafic-ultramafic rocks of the Main Urals Fault, South Urals: Geological structures, ore textural and mineralogical features, comparison with modern analogs. Ore Geol. Rev. 2013, 52, 18–37. [Google Scholar] [CrossRef]
- Prokin, V.A.; Seravkin, I.B.; Vinogradov, A.M. Geological conditions of distribution and prognostic perspectives of large massive copper-sulfide deposits in the Urals. Litosfera 2011, 6, 123–133. (In Russian) [Google Scholar]
- Zaykov, V.V.; Maslennikov, V.V. Seafloor sulfide mounds of massive sulfide deposits of the Urals. Dokl. Akad. Nauk USSR 1987, 293, 181–184. (In Russian) [Google Scholar]
- Zhabin, A.G.; Sharfman, V.S.; Samsonova, N.S. Interpretation of conditions of Devonian volcanosedimentary sulfide deposition. Geol. Rudn. Mestorozh. 1974, 13, 60–75. (In Russian) [Google Scholar]
- Eldridge, C.S.; Barton, P.B.; Ohmoto, H. Mineral textures and their bearing on formation of the Kuroko orebodies. In The Kuroko and Related Volcanogenic Sulfide Deposits; Ohmoto, H., Skinner, B.J., Eds.; Economic Geology Monograph; Society of Economic Geologist: Littleton, CO, USA, 1983; Volume 5, pp. 241–281. [Google Scholar]
- Ayupova, N.R.; Maslennikov, V.V.; Maslennikova, S.P.; Blinov, I.A.; Danyushevsky, L.V.; Large, R.R. Rare mineral and trace element assemblages in submarine supergene zone at the devonian Molodezhnoye VMS deposit, the Urals, Russia. In Proceedings of the 13 SGA Biennial Meeting, Nancy, France, 24–27 August 2015; Volume 5, pp. 2051–2054. [Google Scholar]
- Eremin, N.I.; Sergeeva, N.E.; Dergachev, A.L. Rare minerals from massive sulfide ores: Typomorphic features and geochemical trend. Mosc. Univ. Geol. Bull. 2007, 62, 85–106. (In Russian) [Google Scholar] [CrossRef]
- Grabezhev, A.I.; Moloshag, V.P.; Sotnikov, V.I.; Murzin, V.V.; Korovko, A.V.; Zhukhlistov, A.P. Metasomatic halo of the Saf’yanovskoe Zn–Cu deposit (Central Urals). Petrologiya 2001, 9, 294–312. (In Russian) [Google Scholar]
- Vikentyev, I.V.; Belogub, E.V.; Novoselov, R.A.; Moloshag, V.P. Metamorphism of volcanogenic massive sulphide deposits in the Urals. Ore Geol. Rev. 2017, 85, 30–63. [Google Scholar] [CrossRef]
- Herrington, R.J.; Maslennikov, V.V.; Spiro, B.; Zaykov, V.V.; Little, C.T.S. Ancient vent chimney structures in the Silurian massive sulfides of the Urals. In Modern Ocean Floor Processes and the Geological Record; Mills, R.A., Harrison, K., Eds.; Geological Society Special Publication: London, UK, 1998; Volume 148, pp. 241–257. [Google Scholar] [CrossRef]
- Little, C.T.S.; Herrington, R.J.; Maslennikov, V.V.; Morris, N.J.; Zaykov, V.V. Silurian hydrothermal-vent community from the southern Urals, Russia. Nature 1997, 385, 146–148. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V. Study of trace element zonation in vent chimneys from the Silurian Yaman-Kasy VMS (the Southern Urals, Russia) using laser ablation inductively coupled plasma mass spectrometry (LA-ICP MS). Econ. Geol. 2009, 104, 1111–1141. [Google Scholar] [CrossRef]
- Shadlun, T.N. Some sulfide intergrowths in modern and ancient massive sulfide ores. Geol. Rudn. Mestorozh. 1991, 33, 110–118. (In Russian) [Google Scholar]
- Ayupova, N.R. Marsturite from the Uzelga massive sulfide field (Southern Urals). Zap. Vseross. Miner. Obshch. 2003, 4, 58–61. (In Russian) [Google Scholar]
- Tseluyko, A.S.; Maslennikov, V.V.; Artem’yev, D.A. Microtopochemistry of pyrite nodules of siliceous siltstones from the Yubileinoe massive sulfide deposit (the Southern Urals) according to LA-ICP-MS data. Litosfera 2018, 18, 621–641. (In Russian) [Google Scholar] [CrossRef]
- Maslennikova, S.P.; Maslennikov, V.V. Paleozoic Black Smoker Sulfide Chimneys; Ural Branch of RAS: Yekaterinburg, Russia, 2007; 312p. (In Russian) [Google Scholar]
- Criddle, A.J.; Chisholm, J.E.; Stanley, C.J. Cervelleite, Ag4TeS, a new mineral from the Bambolla mine, Mexico, and description of a photo-chemical reaction involving cervelleite, acantite and hessite. Eur. J. Mineral. 1989, 1, 371–380. [Google Scholar] [CrossRef]
- Kolotov, S.V.; Gmyra, V.G. Rare minerals of the Molodeyzhnoe massive sulfide deposit. In Yezhegodnik–1989; Institute of Geology and Geochemistry UB RAS: Yekaterinburg, Russia, 1989; pp. 78–80. (In Russian) [Google Scholar]
- Moloshag, V.P.; Vikentiev, I.V. Germanium mineralization of massive sulfide deposits of the Urals. Vestnik Ural’skogo Otdeleniya RMO 2009, 6, 79–84. (In Russian) [Google Scholar]
- Trudu, A.G.; Knittel, U. Crystallography, mineral chemistry and chemical nomenclature of goldfieldite, the tellurian member of the tetrahedrite solid-solution series. Can. Mineral. 1998, 36, 1115–1137. [Google Scholar] [CrossRef]
- Tessalina, S.G.; Maslennikov, V.V.; Surin, T.N. Aleksandrinskoe Copper-Zinc Massive Sulfide Deposit (East-Magnitogorsk Paleoisland Arc, Urals); Institute of Mineralogy UB RAS: Miass, Russia, 1998; 228p. (In Russian) [Google Scholar]
- Dobrovolskaya, M.U.; Distler, V.V. Platinum minerals in ores of the Urals copper massive sulfide deposits. Rudy Met. 1998, 4, 56–64. (In Russian) [Google Scholar]
- Moloshag, V.P.; Vikentiev, I.V.; Gulyaeva, T.Y.; Tesalina, S.G. Precious and rare metals of bornite in ores from massive sulfide deposits of the Urals. Zap. Vseros. Miner. Obshch. 2005, 134, 53–69. (In Russian) [Google Scholar]
- Arkhireeva, N.S.; Kotlyarov, V.A. Mineral composition of Fe-poor ferruginous and carbonaceous shales from the Aleksandrinskoe and Saf′yanovskoe deposits (the Urals). Mineralogiya 2016, 2, 60–69. (In Russian) [Google Scholar]
- Safina, N.P.; Ayupova, N.R. Diagenetic bournonite in clastic ores of the Saf’yanovskoe copper-zinc massive sulfide deposit, the Central Urals. Zap. Vseross. Miner. Obshch 2017, CXLVI, 73–87. (In Russian) [Google Scholar]
- Zaykov, V.V.; Maslennikov, V.V.; Zaykova, E.V.; Herrington, R. Ore-Formational and Ore-Facies Analysis of Massive Sulfide Deposits of Ural Paleo-Ocean; Imin UB RAS: Miass, Russia, 2001; 315p. (In Russian) [Google Scholar]
- Safina, N.P.; Melekestseva, I.Y.; Nimis, P.; Ankusheva, N.N.; Yuminov, A.M.; Kotlyarov, V.A.; Sadykov, S.A. Barite from the Saf’yanovka VMS deposit (Central Urals) and Semenov-1 and Semenov-3 hydrothermal sulfide fields (Mid-Atlantic Ridge): A comparative analysis of formation conditions. Miner. Depos. 2016, 54, 491–507. [Google Scholar] [CrossRef]
- Ayupova, N.R.; Maslennikov, V.V. Halmyrolytites of the Uzelga Massive Sulfide Bearing Field, Southern Urals; Urals Branch of the Russian Academy of Science: Miass, Russia, 2005; 199p. (In Russian) [Google Scholar]
- Hannington, M.D.; Bleeker, W.; Kjarsgaard, I. Sulfide mineralogy, geochemistry and ore genesis of the Kidd Creek Deposit: Part I. Noth, Central and South orebodies. In The Giant Kidd Creek Volcanogenic Massive Sulfide Deposit, Western Abitibi Subprovince, Canada; Hannington, M.D., Barrie, T.C., Eds.; Economic Geology Monograph; Society of Economic Geologist: Littleton, CO, USA, 1999; Volume 10, pp. 163–224. [Google Scholar] [CrossRef]
- Safina, N.P.; Maslennikov, V.V.; Maslennikova, S.P.; Kotlyarov, V.A.; Danyushevsky, L.V.; Large, R.R.; Blinov, I.A. Banded sulfide–magnetite ores of Mauk copper massive sulfide deposit, Central Urals: Composition and genesis. Geol. Ore Depos. 2015, 57, 197–212. [Google Scholar] [CrossRef]
- Hannington, M.D.; Bleeker, W.; Kjarsgaard, I. Sulfide Mineralogy, Geochemistry, and Ore Genesis of the Kidd Creek Deposit: Part II. The Bornite Zone. In The Giant Kidd Creek Volcanogenic massive Sulfide Deposit, Western Abitibi Subprovince, Canada; Hannington, M.D., Barrie, T.C., Eds.; Economic Geology Monograph; Society of Economic Geologist: Littleton, CO, USA, 1999; pp. 225–266. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Zaykov, V.V. Method of Ore-Facies Analysis in Geology of Massive Sulfide Deposits; SUSU: Chelyabinsk, Russia, 2006; 224p. (In Russian) [Google Scholar]
- Goodfellow, W.D.; Blaise, B. Sulfide formation and hydrothermal alteration of hemipelagic sediment in Middle Valley, Northern Juan de Fuca Ridge. Can. Mineral. 1988, 26, 675–696. [Google Scholar]
- Goodfellow, W.D.; Franklin, J.M. Geology, mineralogy, and geochemistry of sediment-hosted clastic massive sulfides in shallow cores, Middle Valley, northern Juan-de-Fuca ridge. Econ. Geol. 1993, 88, 2037–2068. [Google Scholar] [CrossRef]
- Skripchenko, N.S. Hydrothermal-Sedimentary Sulfide Ores of Basaltic Complexes; Nauka: Moscow, Russia, 1972; 217p. (In Russian) [Google Scholar]
- Skripchenko, N.S. Hydrothermal-Sedimentary Polymetallic Ores of Lime-Shale Complexes; Nauka: Moscow, Russia, 1980; 215p. (In Russian) [Google Scholar]
- Safina, N.P.; Maslennikov, V.V. Sequence of mineral formation in clastic ores of the Safyanovka volcanic-hosted copper massive sulfide deposit, the Central Urals. Geol. Ore Depos. 2009, 51, 633–643. [Google Scholar] [CrossRef]
- Skinner, B.J. The system Cu-Ag-S. Econ. Geol. 1966, 61, 1–26. [Google Scholar] [CrossRef]
- Vogan, D.; Kreig, D. Chemistry of Sulphide Minerals; Mir: Moscow, Russia, 1981; 575p. (In Russian) [Google Scholar]
- Auclair, G.; Fouquet, Y.; Bohn, M. Distribution of selenium in high-temperature hydrothermal sulfide deposits at 13° North, East Pacific Rise. Can. Mineral. 1987, 87, 577–587. [Google Scholar]
- Hannington, M.D.; Jonasson, I.R.; Herzig, P.M.; Petersen, S. Physical, chemical processes of seafloor mineralization at Mid-Ocean Ridges. In Seafloor Hydrothermal Systems: Physical, Chemical, Biological, and Geological Interactions; Humphris, R.A., Zierenberg, R., Mullineau, L., Thompson, R., Eds.; Geophysical Monograph; American Geophysical Union: Washington, DC, USA, 1995; Volume 91, pp. 115–157. [Google Scholar] [CrossRef]
- Butler, I.B.; Nesbitt, R.V. Trace element distribution in the chalcopyrite wall of a black smoker chimney: Insights from laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). Earth Planet. Sci. Lett. 1999, 167, 335–345. [Google Scholar] [CrossRef]
- Keith, M.; Häckel, F.; Haase, K.M.; Schwarz-Schampera, U.; Klemd, R. Trace element systematics of pyrite from submarine hydrothermal vents. Ore Geol. Rev. 2016, 72, 728–745. [Google Scholar] [CrossRef]
- Wohlgemuth-Ueberwasser, C.C.; Viljoen, F.; Petersen, S.; Vorster, C. Distribution and solubility limits of trace elements in hydrothermal black smoker sulfides: An in-situ LA-ICP-MS study. Geochim. Cosmochim. Acta 2015, 159, 16–41. [Google Scholar] [CrossRef]
- Rouxel, O.; Fouquet, Y.; Ludden, J.N. Subsurface processes at the Lucky Strike hydrothermal field, Mid-Atlantic ridge: Evidence from sulfur, selenium, and iron isotopes. Geochim. Cosmochim. Acta 2004, 68, 2295–2311. [Google Scholar] [CrossRef]
- Ayupova, N.R.; Maslennikov, V.V.; Kotlyarov, V.A.; Maslennikova, S.P.; Danyushevsky, L.V.; Large, R. Se and in minerals of in the Submarine Oxidation Zone of the Molodezhnoe Copper–Zinc Massive Sulfide Deposit, Southern Urals. Dokl. Earth Sci. 2017, 473, 318–322. [Google Scholar] [CrossRef]
- Akinfiev, N.N.; Tagirov, B.R. Effect of selenium on silver transport and precipitation by hydrothermal solution: Thermodynamic description of the Ag-Se-S-Cl-OH system. Geol. Ore Depos. 2006, 48, 402–413. [Google Scholar] [CrossRef]
- Large, R.R.; Bull, S.W.; Maslennikov, V.V. A carbonaceous sedimentary source-rock model for Carlin-type and Orogenic Gold deposits. Econ. Geol. 2011, 106, 331–358. [Google Scholar] [CrossRef]
- Thomas, H.V.; Large, R.R.; Bull, S.W.; Maslennikov, V.V.; Berry, R.F.; Fraser, R.; Froud, S.; Moye, R. Pyrite and pyrrhotite textures and composition in Sedimentary rocks, laminated quartz veins, and gold reefs, at Bendigo Mine, Austarlia: Insights for ore genesis. Econ. Geol. 2011, 105, 1–40. [Google Scholar] [CrossRef]
- Ismagilov, M.I.; Loginov, V.P.; Vasil’eva, G.L. Zonality of ore deposition of the Ozernoe massive sulfide deposit (the Southern Urals) and some formation conditions of its pyrrhotite ores. In The Problems of Physicochemical Petrology; Nauka: Moscow, Russia, 1979; Volume 2, pp. 184–200. (In Russian) [Google Scholar]
- Roberts, A.P.; Zhao, X.; Harrison, R.J.; Helslop, D.; Muxworthy, A.R.; Roman, C.J.; Larrasoana, J.-C.; Florindo, F. Signature of reductive magnetic mineral diagenesis from unmixxing of first-order reversal curves. J. Geophys. Res. 2018, 123, 4500–4522. [Google Scholar] [CrossRef]
- Hannington, M.D.; Scott, S.D. Mineralogy and geochemistry of hydrothermal silica-sulfide-sulfate spire in the Caldera of Axial Seamount, Juan de Fuca Ridge. Can. Mineral. 1988, 26, 603–625. [Google Scholar]
- Törmänen, T.O.; Koski, R.A. Gold enrichment and the Bi-Au association in pyrrhotite-rich massive sulfide deposits, Escanaba Trough, southern Gorda Ridge. Econ.Geol. 2005, 100, 1135–1150. [Google Scholar] [CrossRef]
- Pshenichny, G.N. Textures and Structures of Ores of Massive Sulfide Deposits of the Southern Urals; Nauka: Moscow, Russia, 1984; 207p. (In Russian) [Google Scholar]
- Vikentyev, I.V.; Belenkaya, Y.A.; Ageev, B.I. Aleksandrinskoe polymetallic massive sulfide deposit (the Urals, Russia). Geol. Ore Depos. 2000, 42, 221–246. [Google Scholar]
- Vikentyev, I.V. Precious metal and telluride mineralogy of large vocanic-hosted massive sulfide deposits in the Urals. Mineral. Petrol. 2006, 87, 305–326. [Google Scholar] [CrossRef]
- Moloshag, V.P.; Grabezhev, A.I.; Vikentiev, I.V.; Gulyaeva, T. Ore formation of massive sulfide ore deposits and copper-gold-porphyry deposits of the Urals. Litosfera 2004, 2, 30–51. (In Russian) [Google Scholar]
- Moloshag, V.P.; Grabezhev, A.I.; Gulyaeva, T.Y. Conditions of telluride formation in ores of the Urals massive sulfide and copper-gold-porphyry deposits. Zap. Vseros. Miner. Obshch 2002, 131, 40–53. (In Russian) [Google Scholar]
- Belogub, E.V.; Novoselov, K.A.; Kotlyarov, V.A. Germanium minerals in ores of the Babaryk massive sulfide deposit, South Urals. In Proceedings of the Metalogeny of modern and ancient oceans, Miass, Russia, 25–30 April 2005; Imin UB RAS: Miass, Russia, 2005; pp. 123–128. (In Russian). [Google Scholar]
- Novoselov, K.A.; Belogub, E.V.; Kotlyarov, V.A. Germanium minerals in sulfide ores of the deposits of the Aleksandrinsky ore district. In Ural’skiy Mineralogicheskiy Sbornik; IMin UB RAS: Miass, Russia, 2007; Volume 14, pp. 96–104. (In Russian) [Google Scholar]
- Novoselov, K.A.; Belogub, E.V.; Zaykov, V.V.; Yakovleva, V.A. Silver sulfotellurides from volcanic-hosted massive sulfide deposits in the Southern Urals. Mineral. Petrol. 2006, 83, 327–349. [Google Scholar] [CrossRef]
- Ayupova, N.R.; Novoselov, K.A.; Belogub, E.V. Ferruginous-siliceous sediments as the indicators of massive-sulfide deposits (on example of the Babaryk ore field, Southern Urals). Litosfera 2011, 3, 117–134. (In Russian) [Google Scholar]
- Belogub, E.V.; Novoselov, K.A.; Yakovleva, V.A.; Spiro, B. Supergene sulphides and related minerals in the supergene profiles of VHMS deposits from the South Urals. Ore Geol. Rev. 2008, 33, 239–254. [Google Scholar] [CrossRef]
- Ohmoto, H. Formation of volcanogenic massive sulfide deposits: The Kuroko perspective. Ore Geol. Rev. 1996, 10, 135–177. [Google Scholar] [CrossRef]
- Feely, R.A.; Lewison, M.; Massoth, J.W.; Massoth, G.J.; Robert-Baldo, G.; Lavelle, J.W.; Byrne, R.H.; Von Damm, K.L.; Curl, H.C. Composition and dissolution of black smoker particulates from active vents on the Juan de Fuca Ridge. J. Geophys. Res. 1987, 92, 11347–11363. [Google Scholar] [CrossRef]
- Eremin, N.I.; Sergeeva, N.E.; Shishakov, V.O. Finding a Pd-bearing melonite in copper-rich sulfide ores of the Pyshminsko-Kluchevskoe deposit in the Urals. Dokl. Earth Sci. 1997, 355, 795–797. [Google Scholar]
- Belogub, E.V.; Moloshag, V.P.; Novoselov, K.A.; Kotlyarov, V.A. Native bismuth, tsumoite, and Pb-bearing tsumoite from the Tarn’er copper–zinc massive sulfide deposit, Northern Urals. Geol. Ore Depos. 2011, 53, 798–805. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Tretyakov, G.A. Physicochemical modeling of the sequence of mineral formation with submarine hypergenesis of massive sulfide deposits of the Urals and Kuroko types. In Ural’skiy Mineralogicheskiy Sbornik; IMin UB RAS: Miass, Russia, 2008; Volume 15, pp. 9–17. (In Russian) [Google Scholar]











| Deposit | Cu | Zn | Pb | S | Se | Te | Au | Ag | Co | Size |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | ppm | Mt | ||||||||
| Dergamysh | 1.3 | 0.3 | <0.005 | 47 | 54 | 16 | 2.0 | 12 | 0.01–0.25 | 1.0 |
| Yubileynoe | 1.4 | 1.0 | <0.005 | 43.8 | 50 | 30 | 1.6 | 18 | <0.01 | 108.8 |
| Yaman-Kasy | 2.6 | 5.6 | 0.03 | 42.4 | 22 | 325 | 3.3 | 34 | <0.01 | 1.7 |
| Molodezhnoe | 2.1 | 3.3 | 0.17 | 29.4 | 82 | 89 | 1.6 | 46 | <0.01 | 15.9 |
| Valentorskoe | 6.0 | 3.2 | 0.24 | 17.1 | 22 | 28 | 1.72 | 26 | <0.01 | 1.6 |
| Aleksandrinskoe | 2.7 | 4.6 | 0.52 | 27.4 | 8 | 39 | 1.1 | 37 | <0.01 | 3.8 |
| Saf’yanovskoe | 2.2 | 0.7 | 0.13 | 25.44 | 60 | 9.2 | 0.5 | 20 | <0.01 | 19.4 |
| Mineral Types of Diagenites | Authigenic Subordinate and Rare Mineral Assemblages |
|---|---|
| Pyrrhotite-rich | Chalcopyrite, “isocubanite”, arsenopyrite, pentlandite, gersdorffite, nickeline, cobaltite, native gold |
| Chalcopyrite-rich | Sphalerite, pyrite, Te- and Bi-tennantite, galena, phase between cattierite and pyrite, cobaltpentlandite, mackinawite, pyrite, pyrrhotite, cubanite, cobaltite, carrolite, cassiterite, coloradoite, altaite, tellurobismuthite, tetradymite, hessite, petzite, stützite, volynskite, calaverite, rucklidgeite, bornite, native antimony, naumannite, clausthalite, kurilite, bohdanowiczite, roquesite, magnetite, native gold, native tellurium, bornite, arsenopyrite |
| Bornite-rich | Sphalerite, pyrite, Te- and Bi-tennantite, goldfieldite, galena, digenite, chalcocite, enargite, jalpaite, stromeyerite, germanite, renierite, mackinstryite, stannite, stannoidite, mawsonite, colusite, sulvanite, hessite, cervelleite, native gold |
| Sphalerite-rich | Chalcopyrite, pyrite, tennantite–tetrahedrite, barite, galena, tetrahedrite, barite, enargite, covellite, argyrodite, native gold, tennantite, enargite, bornite, galena, acanthite |
| Pyrite-rich | Tennantite, bornite, enargite, galena, tetrahedrite, bournonite, famatinite, native gold, luzonite, petzite, hessite |
| Barite-rich | Tennantite, tetrahedrite, galena, acanthite, polybasite, diaphorite, pyrargyrite, native gold, jordanite |
| Hematite-rich | magnetite, Se-rich galena, uraninite, acanthite, clausthalite, bornite, digenite, coloradoite, hessite, cervelleite, altaite, tellurobismuthite, volynskite, tetradymite, monheimite, native gold |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslennikov, V.V.; Ayupova, N.R.; Safina, N.P.; Tseluyko, A.S.; Melekestseva, I.Y.; Large, R.R.; Herrington, R.J.; Kotlyarov, V.A.; Blinov, I.A.; Maslennikova, S.P.; et al. Mineralogical Features of Ore Diagenites in the Urals Massive Sulfide Deposits, Russia. Minerals 2019, 9, 150. https://doi.org/10.3390/min9030150
Maslennikov VV, Ayupova NR, Safina NP, Tseluyko AS, Melekestseva IY, Large RR, Herrington RJ, Kotlyarov VA, Blinov IA, Maslennikova SP, et al. Mineralogical Features of Ore Diagenites in the Urals Massive Sulfide Deposits, Russia. Minerals. 2019; 9(3):150. https://doi.org/10.3390/min9030150
Chicago/Turabian StyleMaslennikov, Valeriy V., Nuriya R. Ayupova, Nataliya P. Safina, Aleksandr S. Tseluyko, Irina Yu. Melekestseva, Ross R. Large, Richard J. Herrington, Vasiliy A. Kotlyarov, Ivan A. Blinov, Svetlana P. Maslennikova, and et al. 2019. "Mineralogical Features of Ore Diagenites in the Urals Massive Sulfide Deposits, Russia" Minerals 9, no. 3: 150. https://doi.org/10.3390/min9030150







