Nanoscale Structure of Zoned Laurites from the Ojén Ultramafic Massif, Southern Spain
Abstract
:1. Introduction
2. Geological Background of the Samples
3. Methods
3.1. Sampling, Imaging and Chemical Characterization of the Platinum-Group Minerals (PGM)
3.2. Nanoscale Study of the Zoned Laurite Crystal
4. Results
4.1. Laurite Zoning Patterns in the Ojén Ultramafic Massif
4.2. Nanoscale Structure of Zoned Laurite
5. Discussion
5.1. Magmatic Origin of Laurites from the Ojén Chromitites
5.2. Nanostructure of Cores and Rims in Zoned Laurites from the Ojén Chromitites
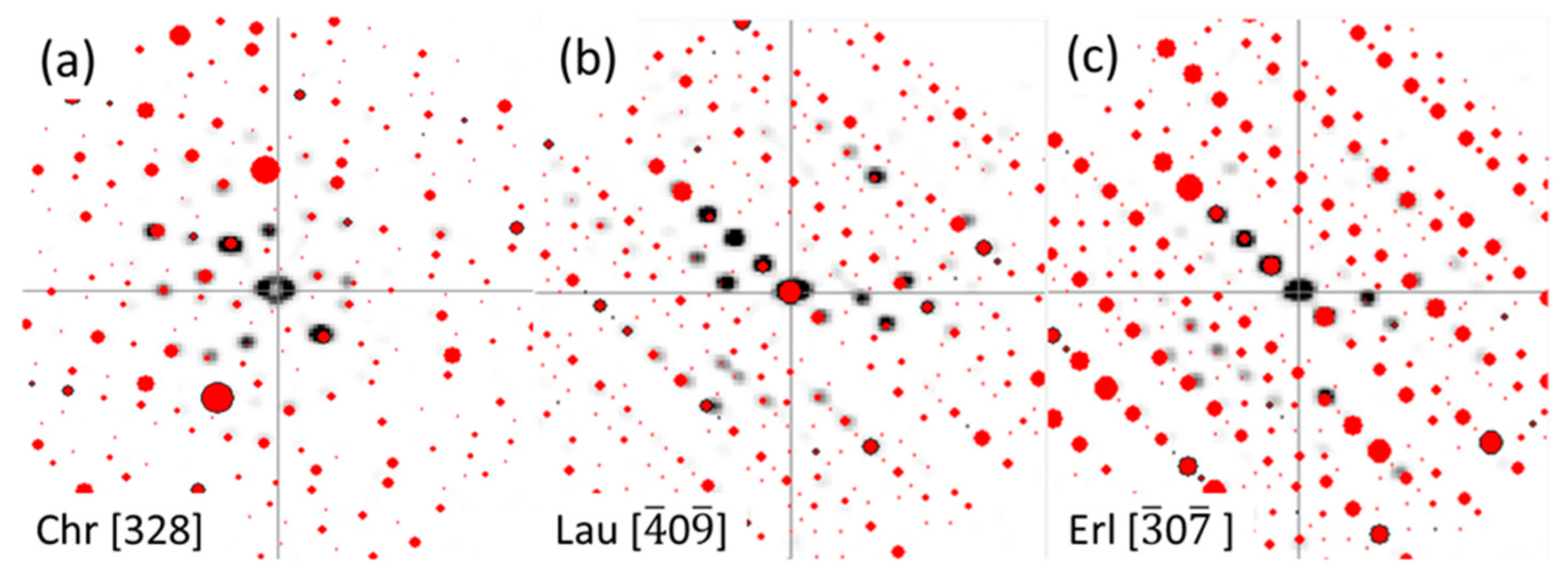
5.3. Nanoscale Coupling of Os and As in Erlichmanite
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- O’Driscoll, B.; González-Jiménez, J.M. Petrogenesis of the Platinum-Group Minerals. Rev. Mineral. Geochem. 2016, 81, 489–578. [Google Scholar] [CrossRef]
- Gervilla, F.; Proenza, J.A.; Frei, R.; González-Jiménez, J.M.; Garrido, C.J.; Melgarejo, J.C.; Meibom, A.; Díaz-Martínez, R.; Lavaut, W. Distribution of platinum-group elements and Os isotopes in chromite ores from Mayarí-Baracoa Ophiolitic Belt (eastern Cuba). Contrib. Mineral. Petrol. 2005, 150, 589–607. [Google Scholar] [CrossRef]
- Torres-Ruiz, J.; Garuti, G.; Gazzotti, M.; Gervilla, F.; Fenoll Hach-All, I.P. Platinum-group minerals in chromitites from the Ojén lherzolite massif (Serranía de Ronda, Betic Cordillera, Southern Spain). Mineral. Petrol. 1996, 56, 25–50. [Google Scholar] [CrossRef]
- Melcher, F.; Grum, W.; Simon, G.; Thalhammer, T.V.; Stumpfl, E.F. Petrogenesis of the ophiolitic giant chromite deposits of Kempirsai, Kazkhstan: A study of solid and fluid inclusions in chromite. J. Petrol. 1997, 38, 1419–1458. [Google Scholar] [CrossRef]
- Coggon, J.A.; Nowell, G.M.; Pearson, D.G.; Oberthür, T.; Lorand, J.P.; Melcher, F.; Parman, S.W. The 190Pt–186Os decay system applied to dating platinum-group element mineralization of the Bushveld Complex, South Africa. Chem. Geol. 2012, 302, 48–60. [Google Scholar] [CrossRef]
- Prichard, H.M.; Barnes, S.J.; Fisher, P.C.; Pagé, P.; Zientek, M.L. Laurite and Associated PGM in the Stillwater Chromitites: Implications for Processes of Formation, and Comparisons with Laurite in the Bushveld and Ophiolitic Chromitites. Can. Mineral. 2017, 55, 121–144. [Google Scholar] [CrossRef]
- Weiser, T.W. Platinum-group minerals (PGM) in placer deposits. In The Geology, Geochemistry, Mineralogy and Mineral Beneficiation of Platinum-Group Elements; Cabri, L.J., Ed.; Canadian Institute of Mining, Metallurgy and Petroleum: Montreal, QC, Canada, 2002; CIM Special Volume 54; pp. 721–756. [Google Scholar]
- González-Jiménez, J.M.; Gervilla, F.; Proenza, J.A.; Kerestedjian, T.; Augé, T.; Bailly, L. Zoning of laurite (RuS2)–erlichmanite (OsS2): Implications for the genesis of PGM in ophiolite chromitites. Eur. J. Mineral. 2009, 21, 419–432. [Google Scholar] [CrossRef]
- Proenza, J.A.; Zaccarini, F.; Escayola, M.; Cábana, C.; Schalamuk, A.; Garuti, G. Composition and textures of chromite and platinum-group minerals in chromitites of the western ophiolitic belt from Pampeans Ranges of Córdoba, Argentina. Ore Geol. Rev. 2008, 33, 32–48. [Google Scholar] [CrossRef]
- Oberthür, T.; Melcher, F.; Weiser, T.W. Detrital platinum-group minerals and gold in placers of Southeastern Samar Island, Philippines. Can. Mineral. 2017, 55, 45–62. [Google Scholar] [CrossRef]
- González-Jiménez, J.M.; Griffin, W.L.; Proenza, J.A.; Gervilla, F.; O’Reilly, S.Y.; Akbulut, M.; Pearson, N.J.; Arai, S. Chromitites in ophiolites: How, where, when, why? Part II. The crystallization of chromitites. Lithos 2014, 189, 140–158. [Google Scholar] [CrossRef]
- Fonseca, R.O.C.; Brückel, K.; Bragagni, A.; Leitzke, F.P.; Speelmanns, I.M.; Wainwright, A.N. Fractionation of Rhenium from Osmium during noble metal alloy formation in association with sulfides: Implications for the interpretation of model ages in alloy-bearing magmatic rocks. Geochim. Cosmochim. Acta 2017, 216, 184–200. [Google Scholar] [CrossRef]
- Malitch, K.N.; Augé, T.; Badanina, I.Y.; Goncharov, M.M.; Junk, S.A.; Pernicka, E. Os-rich nuggets from Au-PGE placers of the Maimecha-Kotui Province, Russia: A multi-disciplinary study. Mineral. Petrol. 2002, 76, 121–148. [Google Scholar] [CrossRef]
- Ahmed, A.H.; Hanghøj, K.; Kelemen, P.B.; Hart, S.R.; Arai, S. Osmium isotope systematics of the Proterozoic and Phanerozoic ophiolitic chromitites: In situ ion probe analysis of primary Os-rich PGM. Earth Planet. Sci. Lett. 2006, 245, 777–791. [Google Scholar] [CrossRef] [Green Version]
- Marchesi, C.; González-Jiménez, J.M.; Gervilla, F.; Garrido, C.J.; Griffin, W.L.; O’Reilly, S.Y.; Proenza, J.A.; Pearson, N.J. In situ Re-Os isotopic analysis of platinum-group minerals from the Mayarí Cristal ophiolitic massif (Mayarí-Baracoa Ophioitic Belt, eastern Cuba): Implications for the origin of Os-isotope heterogeneities in podiform chromitites. Contrib. Mineral. Petrol. 2011, 161, 977–990. [Google Scholar] [CrossRef]
- González-Jiménez, J.M.; Gervilla, F.; Griffin, W.L.; Proenza, J.A.; Augé, T.; O’Reilly, S.Y.; Pearson, N.J. Os-isotope variability within sulfides from podiform chromitites. Chem. Geol. 2012, 291, 224–235. [Google Scholar] [CrossRef]
- Brenan, J.M.; Cherniak, D.J.; Rose, L.A. Diffusion of osmium in pyrrhotite and pyrite; implications for closure of the Re–Os isotopic system. Earth Planet. Sci. Lett. 2000, 180, 399–413. [Google Scholar] [CrossRef]
- Wirth, R.; Reid, D.; Schreiber, A. Nanometer-sized platinum-group minerals (PGM) in base metal sulfides: New evidence for an orthomagmatic origin of the merensky reef pge ore deposit, bushveld complex, South Africa. Can. Mineral. 2013, 51, 143–155. [Google Scholar] [CrossRef]
- Junge, M.; Wirth, R.; Oberthür, T.; Melcher, F.; Schreiber, A. Mineralogical siting of platinum-group elements in pentlandite from the Bushveld Complex, South Africa. Miner. Depos. 2014, 50, 41–54. [Google Scholar] [CrossRef] [Green Version]
- González-Jiménez, J.M.; Reich, M. An overview of the platinum-group element nanoparticles in mantle-hosted chromite deposits. Ore Geol. Rev. 2017, 81, 1236–1248. [Google Scholar] [CrossRef]
- Gutierrez-Narbona, R.; Lorand, J.P.; Gervilla, F.; Gros, M. New data on base metal mineralogy and platinum group minerals in the Ojén chromitites (Serranía de Ronda, Betic Cordillera, southern Spain). J. Mineral. Geochem. 2003, 2, 143–173. [Google Scholar] [CrossRef]
- Gervilla, F.; Leblanc, M. Magmatic ores in high temperature alpine-type Lherzolite massifs (Ronda, Spain, and Beni Bousera, Morocco). Econ. Geol. 1990, 85, 112–132. [Google Scholar] [CrossRef]
- González-Jiménez, J.M.; Marchesi, C.; Griffin, W.L.; Gutiérrez-Narbona, R.; Lorand, J.P.; O’Reilly, S.Y.; Garrido, C.J.; Gervilla, F.; Pearson, N.J.; Hidas, K. Transfer of Os isotopic signatures from peridotite to chromitite in the subcontinental mantle: Insights from in situ analysis of platinum-group and base-metal minerals (Ojén peridotite massif, southern Spain). Lithos 2013, 164–167, 74–85. [Google Scholar] [CrossRef]
- González-Jiménez, J.M.; Marchesi, C.; Griffin, W.L.; Gervilla, F.; Belousova, E.A.; Garrido, C.J.; Romero, R.; Talavera, C.; Leisen, M.; O’Reilly, S.Y.; et al. Zircon recycling and crystallization during formation of chromite- and Ni-arsenide ores in the subcontinental lithospheric mantle (Serranía de Ronda, Spain). Ore Geol. Rev. 2017, 90, 193–209. [Google Scholar] [CrossRef]
- Obata, M. The Ronda Peridotite: Garnet-, Spinel-, and Plagioclase-Lherzolite Facies and the P–T trajectories of a high-temperature mantle intrusion. J. Petrol. 1980, 21, 533–572. [Google Scholar] [CrossRef]
- Van der Wal, D.; Vissers, R.L.M. Uplift and emplacement of upper-mantle rocks in the western Mediterranean. Geology 1993, 21, 1119–1122. [Google Scholar] [CrossRef]
- Van der Wal, D.; Vissers, R.L.M. Structural petrology of the Ronda Peridotite, SW Spain: Deformation history. J. Petrol. 1996, 37, 23–43. [Google Scholar] [CrossRef]
- Garrido, C.J.; Bodinier, J.L. Diversity of mafic rocks in the Ronda peridotite: Evidence for pervasive melt-rock reaction during heating of subcontinental lithosphere by upwelling asthenosphere. J. Petrol. 1999, 40, 729–754. [Google Scholar] [CrossRef]
- Lenoir, X.; Garrido, C.J.; Bodinier, J.L.; Dautria, J.M.; Gervilla, F. The recrystallization front of the Ronda peridotite: Evidence for melting and thermal erosion of subcontinental lithospheric mantle beneath the Alboran Basin. J. Petrol. 2001, 42, 141–158. [Google Scholar] [CrossRef]
- Booth-Rea, G.; Ranero, C.R.; Martinez-Martinez, J.M.; Grevemeyer, I. Crustal types and Tertiary tectonic evolution of the Alboran sea, western Mediterranean. Geochem. Geophys. Geosyst. 2007, 8, 1–25. [Google Scholar] [CrossRef]
- Garrido, C.J.; Gueydan, F.; Booth-Rea, G.; Precigout, J.; Hidas, K.; Padron-Navarta, J.A.; Marchesi, C. Garnet lherzolite and garnet-spinel mylonite in the Ronda peridotite: Vestiges of Oligocene backarc mantle lithospheric extension in the western Mediterranean. Geology 2011, 39, 927–930. [Google Scholar] [CrossRef]
- Marchesi, C.; Garrido, C.J.; Bosch, D.; Bodinier, J.-L.; Hidas, K.; Padrón-Navarta, J.A.; Gervilla, F.A. Late Oligocene Suprasubduction Setting in the Westernmost Mediterranean Revealed by Intrusive Pyroxenite Dikes in the Ronda Peridotite (Southern Spain). J. Geol. 2012, 120, 237–247. [Google Scholar] [CrossRef]
- Precigout, J.; Gueydan, F.; Gapais, D.; Garrido, C.J.; Essaifi, A. Strain localisation in the subcontinental mantle—A ductile alternative to the brittle mantle. Tectonophysics 2007, 445, 318–336. [Google Scholar] [CrossRef]
- Platt, J.P.; Soto, J.I.; Whitehouse, M.J.; Hurford, A.J.; Kelley, S.P. Thermal evolution, rate of exhumation, and tectonic significance of metamorphic rocks from the floor of the Alboran extensional basin, western Mediterranean. Tectonics 1998, 17, 671–689. [Google Scholar] [CrossRef] [Green Version]
- Gervilla, F.; Kojonen, K. The platinum-group minerals in the upper section of the Keivitsansarvi Ni-Cu-PGE deposit, Northern Finland. Can. Mineral. 2002, 40, 377–394. [Google Scholar] [CrossRef]
- Wirth, R. Focused Ion Beam (FIB) combined with SEM and TEM: Advanced analytical tools for studies of chemical composition, microstructure and crystal structure in geomaterials on a nanometre scale. Chem. Geol. 2009, 261, 217–229. [Google Scholar] [CrossRef]
- Roqué-Rosell, J.; Portillo-Serra, J.; Aiglsperger, T.; Plana-Ruiz, S.; Trifonov, T.; Proenza, J.A. Au crystal growth on natural occurring Au—Ag aggregate elucidated by means of precession electron diffraction (PED). J. Cryst. Growth. 2018, 483, 228–235. [Google Scholar] [CrossRef]
- Zou, X.; Hovmöller, S.; Oleynikov, P. Electron Crystallography: Electron Microscopy and Electron Diffraction; International Union of Crystallography Book Series, 16; Oxford University Press: Oxford, UK, 2011; ISBN 978-0-19-958020-0. [Google Scholar]
- Lutz, H.D.; Mueller, B.; Schmidt, T.; Stingl, T. Structure refinement of pyrite-type ruthenium disulfide, RuS2, and ruthenium diselenide, RuSe2. Acta Crystallogr. Sect. C-Cryst. Struct. Commun. 1990, 46, 2003–2005. [Google Scholar] [CrossRef]
- Stingl, T.; Müller, Β.; Lutz, H.D. Crystal structure refinement of osmium (II) disulfide, OsS2. Z. Kristallogr. Cryst. Mater. 1992, 202, 161–162. [Google Scholar] [CrossRef]
- Lenaz, D.; Braidotti, R.; Princivalle, F.; Garuti, G.; Zaccarini, F. Crystal chemistry and structural refinement of chromites from different chromitite layers and xenoliths of the Bushveld Complex. Eur. J. Mineral. 2007, 19, 599–609. [Google Scholar] [CrossRef]
- Augé, T.; Johan, Z. Comparative study of chromite deposits from Troodos, Vourinos, North Oman and New Caledonia ophiolites. In Mineral Deposits within the European Community; Boissonnas, J., Omenetto, P., Eds.; Society for Geology Applied to Mineral Deposits, Spec. Publ.; Springer: Berlin, Germany, 1988; Volume 6, pp. 267–288. ISBN 978-3-642-51860-7. [Google Scholar]
- Brenan, J.M.; Andrews, D. High-temperature stability of laurite and Ru–Os–Ir alloy and their role in PGE fractionation in mafic magmas. Can. Minerl. 2001, 39, 341–360. [Google Scholar] [CrossRef]
- Andrews, D.; Brenan, J.M. Phase-equilibrium constraints on the magmatic origin of laurite + Ru-Os-Ir alloy. Can. Mineral. 2002, 40, 1705–1716. [Google Scholar] [CrossRef]
- Bockrath, C.; Ballhaus, C.; Holzheid, A. Stabilities of laurite RuS2 and monosulphide liquid solution at magmatic temperature. Chem. Geol. 2004, 208, 265–271. [Google Scholar] [CrossRef]
- Finnigan, C.S.; Brenan, J.M.; Mungall, J.E.; McDonough, W.F. Experiments and models bearing on the role of chromite as a collector of platinum group minerals by local reduction. J. Petrol. 2008, 49, 1647–1665. [Google Scholar] [CrossRef]
- Augé, T.; Legendre, O.; Maurizot, P. The distribution of Pt and Ru–Os–Ir minerals in the New Caledonia ophiolite. In Proceedings of the 8th Internationa Platinum, Rustenburg, South Africa, 29 June–3 July 1998; pp. 129–132. [Google Scholar]
- Garuti, G.; Zaccarini, F.; Economou-Eliopoulos, M. Paragenesis and composition of laurite from the chromitites of Othrys (Greece): Implications for Os–Ru fractionation in ophiolitic upper mantle of the Balkan Peninsula. Miner. Depos. 1999, 34, 312–319. [Google Scholar] [CrossRef]
- Zaccarini, F.; Tredoux, M.; Miller, D.E.; Garuti, G.; Aiglsperger, T.; Proenza, J.A. The occurrence of platinum-group element and gold minerals in the Bon Accord Ni-oxide body, South Africa. Am. Mineral. 2014, 99, 1774–1782. [Google Scholar] [CrossRef] [Green Version]
- Bowles, J.F.W.; Atkin, D.; Lambert, J.L.M.; Deans, T.; Phillips, R. The chemistry, reflectance, and cell size of the erlichmanite (OsS2)–laurite (RuS2) series. Mineral. Mag. 1983, 47, 465–471. [Google Scholar] [CrossRef]
- Putnis, A. Introduction to Mineral Sciences, 2nd ed.; Cambridge University Press: Cambridge, MA, USA, 1992; pp. 282–284. ISBN 9780521429474. [Google Scholar]
- Ghiorso, M.S.; Carmichael, I.S.E.; Rivers, M.L.; Sack, R.O. The gibbs free energy of mixing of natural silicate liquids; an expanded regular solution approximation for the calculation of magmatic intensive variables. Contrib. Mineral. Petrol. 1983, 84, 107–145. [Google Scholar] [CrossRef]
- Tajčmanová, L.; Abart, R.; Wirth, R.; Habler, G.; Rhede, D. Intracrystalline Microstructures in Alkali Feldspars from Fluid-Deficient Felsic Granulites: A Mineral Chemical and TEM Study. Contrib. Mineral. Petrol. 2012, 164, 715–729. [Google Scholar] [CrossRef]
- Cahn, J.W. Spinodal decomposition. Trans. Metall. Soc. AIME 1968, 242, 166–180. [Google Scholar]
- Brown, W.L.; Parsons, I. Storage and release of elastic strain energy the driving force for low temperature reactivity and alteration of alkali feldspars. In Defects and Processes in the Solid State Geoscience Applications; Boland, J.N., Fitz-Gerald, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 1993; The McLaren Volume Developments in Petrology 14; pp. 267–290. ISBN 978-94-011-1106-5. [Google Scholar]
- Yund, R.A.; McCallister, R.H. Kinetics and Mechanisms of Exsolution. Chem. Geol. 1970, 6, 5–30. [Google Scholar] [CrossRef]
- Abart, R.; Petrishcheva, E.; Kässner, S.; Milke, R. Perthite microstructure in magmatic alkali feldspar with oscillatory zoning; Weinsberg Granite, Upper Austria. Mineral. Petrol. 2009, 97, 251–263. [Google Scholar] [CrossRef]
- Brizi, E.; Nazzareni, S.; Princivalle, F.; Zanazzi, P.F. Clinopyroxenes from mantle-related xenocrysts in alkaline basalts from Hannuoba (China): Augite-pigeonite exsolutions and their thermal significance. Contrib. Mineral. Petrol. 2003, 145, 578–584. [Google Scholar] [CrossRef]
- Harrison, R.J.; Putnis, A. Interaction between exsolution microstructures and magnetic properties of the magnetite-spinel solid solution. Am. Mineral. 1997, 82, 131–142. [Google Scholar] [CrossRef]
- Leute, V.; Behr, A.; Hünting, C.; Schmidtke, H.M. Phase diagram and diffusion properties of the quasibinary system (Sn, Pb)S. Solid State Ionics 1994, 68, 287–294. [Google Scholar] [CrossRef]
- Sack, R.O. Thermochemistry of Sulfide Mineral Solutions. Rev. Mineral. Geochem. 2006, 61, 265–364. [Google Scholar] [CrossRef]
- Huston, E.L.; Cahn, J.W.; Hilliard, J.E. Spinodal decomposition during continuous cooling. Acta Met. 1966, 14, 1053–1062. [Google Scholar] [CrossRef]
- Gervilla, F.; González Jiménez, J.; Roqué-Rosell, J.; Baurier-Amat, S.; Proenza, J.A. Nanoscale Structure of Zoned Laurites; Goldschmidt: Boston, MA, USA, 2018. [Google Scholar]
- Helmy, H.M.; Bragagni, A. Platinum-group elements fractionation by selective complexing, the Os, Ir, Ru, Rh-arsenide-sulfide systems above 1020 °C. Geochim. Cosmochim. Acta 2017, 216, 169–183. [Google Scholar] [CrossRef]

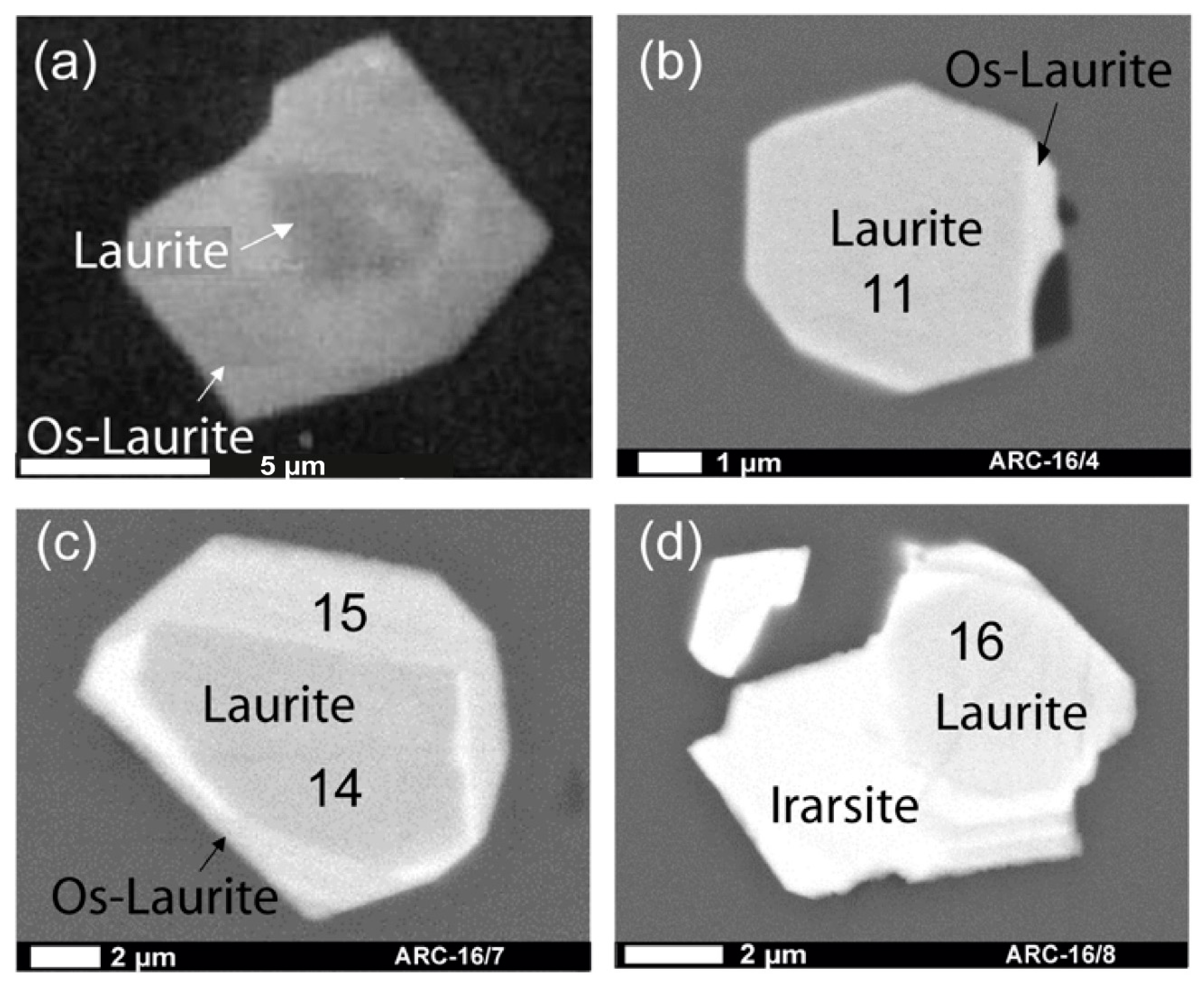





| Grain | Phase (wt.%) | # | Os | Ir | Ru | Pt | Pd | Rh | Fe | Ni | Cu | S | As | Cr | Total |
| ACA-4/1 | OsP-La | 1 | 13.81 | 13.51 | 34.93 | b.l.d | b.l.d | 1.06 | 0.76 | 0.25 | 0.04 | 30.63 | 2.33 | 2.11 | 99.43 |
| ACA-4/2 | OsP-La | 2 | 21.04 | 9.25 | 34.09 | b.l.d | b.l.d | 0.37 | 0.52 | 0.08 | 0.03 | 30.76 | 1.08 | 1.18 | 98.40 |
| ACA-4/5 | OsP-La | 3 | 22.48 | 7.85 | 33.41 | b.l.d | b.l.d | 0.43 | 0.71 | 0.05 | 0.03 | 30.45 | 0.79 | 1.70 | 97.90 |
| ACA-4/6 | OsR-La | 4 | 22.77 | 9.79 | 23.45 | b.l.d | b.l.d | 2.33 | 0.57 | 0.05 | 0.08 | 26.86 | 3.60 | 1.44 | 90.94 |
| ACA-4/9C | OsP-La | 5 | 17.26 | 8.39 | 32.68 | 0.01 | 0.26 | 4.06 | 0.67 | - | b.l.d | 29.46 | 4.09 | 1.71 | 98.60 |
| ACA-4/9 | OsP-La | 6 | 21.66 | 9.09 | 30.50 | b.l.d | 0.08 | 3.78 | 0.69 | 0.02 | b.l.d | 28.92 | 4.01 | 1.71 | 100.46 |
| ACA-4/9R | OsP-La | 7 | 17.31 | 6.04 | 37.23 | 0.23 | 0.14 | 3.08 | 0.73 | 0.04 | b.l.d | 31.17 | 2.23 | 1.89 | 100.09 |
| ACA-4/10 | OsP-La | 8 | 5.82 | 5.46 | 44.77 | 0.35 | 0.34 | 3.68 | 0.94 | 0.05 | b.l.d | 32.47 | 1.38 | 2.59 | 97.86 |
| ACA-4/14 | OsR-La | 9 | 26.33 | 9.26 | 28.61 | 0.32 | 0.37 | 1.32 | 0.61 | 0.08 | b.l.d | 29.70 | 2.51 | 1.58 | 100.69 |
| ACA-4/16 | OsP-La | 10 | 21.04 | 9.25 | 34.09 | b.l.d | b.l.d | 0.37 | 0.52 | 0.08 | 0.03 | 30.76 | 1.08 | 1.18 | 98.40 |
| ARC-16/4C | OsP-La | 11 | 0.01 | 0.50 | 54.66 | 0.21 | b.l.d | 5.00 | 0.39 | 0.03 | b.l.d | 35.32 | 1.62 | 1.23 | 98.97 |
| ARC-16/5 | OsP-La | 12 | 25.68 | 3.21 | 32.13 | 0.65 | 0.33 | 1.12 | 0.55 | 0.03 | b.l.d | 30.45 | 1.17 | 1.68 | 97.01 |
| ARC-16/6 | OsP-La | 13 | 7.97 | 0.98 | 50.99 | 0.31 | b.l.d | 1.67 | 0.47 | 0.05 | b.l.d | 34.50 | b.l.d | 1.06 | 98.00 |
| ARC-16/7C | OsP-La | 14 | 0.12 | 0.28 | 53.98 | 0.38 | b.l.d | 4.54 | 0.48 | 0.02 | b.l.d | 34.25 | 1.75 | 1.48 | 97.28 |
| ARC-16/7R | OsP-La | 15 | 7.34 | 0.93 | 47.54 | 0.46 | 0.25 | 3.86 | 0.56 | 0.02 | 0.02 | 33.48 | 1.73 | 1.72 | 97.91 |
| ARC-16/8 | OsP-La | 16 | 16.21 | 10.14 | 32.00 | 0.02 | 0.12 | 3.80 | 0.50 | 0.06 | 0.04 | 29.18 | 5.38 | 1.44 | 98.89 |
| CD-32/9 | OsR-La | 17 | 27.30 | 6.55 | 32.18 | b.l.d | 0.23 | b.l.d | 0.64 | 0.06 | b.l.d | 31.54 | b.l.d | 1.49 | 99.99 |
| Apfu (atoms per formula unit) | Os | Ir | Ru | Pt | Pd | Rh | Fe | Ni | Cu | S | As | Ru/(Ru + Os) | |||
| ACA-4/1 | OsP-La | 1 | 0.14 | 0.14 | 0.69 | - | - | 0.02 | 0.03 | - | - | 1.91 | 0.06 | 0.83 | |
| ACA-4/2 | OsP-La | 2 | 0.22 | 0.10 | 0.68 | - | - | 0.01 | 0.02 | - | - | 1.94 | 0.03 | 0.75 | |
| ACA-4/5 | OsP-La | 3 | 0.24 | 0.08 | 0.68 | - | - | 0.01 | 0.03 | - | - | 1.94 | 0.02 | 0.74 | |
| ACA-4/6 | OsR-La | 4 | 0.27 | 0.12 | 0.53 | - | - | 0.05 | 0.02 | - | - | 1.90 | 0.11 | 0.66 | |
| ACA-4/9C | OsP-La | 5 | 0.18 | 0.09 | 0.65 | - | - | 0.08 | 0.02 | - | - | 1.86 | 0.11 | 0.78 | |
| ACA-4/9 | OsP-La | 6 | 0.23 | 0.10 | 0.62 | - | - | 0.08 | 0.03 | - | - | 1.84 | 0.11 | 0.73 | |
| ACA-4/9R | OsP-La | 7 | 0.18 | 0.06 | 0.72 | - | - | 0.06 | 0.03 | - | - | 1.90 | 0.06 | 0.80 | |
| ACA-4/10 | OsP-La | 8 | 0.06 | 0.05 | 0.84 | - | - | 0.07 | 0.03 | - | - | 1.91 | 0.03 | 0.94 | |
| ACA-4/14 | OsR-La | 9 | 0.29 | 0.10 | 0.58 | - | - | 0.03 | 0.02 | - | - | 1.91 | 0.07 | 0.67 | |
| ACA-4/16 | OsP-La | 10 | 0.22 | 0.10 | 0.68 | - | - | 0.01 | 0.02 | - | - | 1.94 | 0.03 | 0.75 | |
| ARC-16/4C | OsP-La | 11 | - | - | 0.94 | - | - | 0.08 | 0.01 | - | - | 1.92 | 0.04 | 1.00 | |
| ARC-16/5 | OsP-La | 12 | 0.28 | 0.03 | 0.65 | 0.01 | - | 0.02 | 0.02 | - | - | 1.95 | 0.03 | 0.70 | |
| ARC-16/6 | OsP-La | 13 | 0.08 | 0.01 | 0.91 | - | - | 0.03 | 0.02 | - | - | 1.95 | - | 0.92 | |
| ARC-16/7C | OsP-La | 14 | - | - | 0.95 | - | - | 0.08 | 0.02 | - | - | 1.90 | 0.04 | 1.00 | |
| ARC-16/7R | OsP-La | 15 | 0.07 | 0.01 | 0.87 | - | - | 0.07 | 0.02 | - | - | 1.92 | 0.04 | 0.92 | |
| ARC-16/8 | OsP-La | 16 | 0.17 | 0.11 | 0.64 | - | - | 0.07 | 0.02 | - | - | 1.84 | 0.15 | 0.79 | |
| CD-32/9 | OsR-La | 17 | 0.29 | 0.07 | 0.64 | - | - | - | 0.02 | - | - | 1.98 | - | 0.69 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baurier-Aymat, S.; Jiménez-Franco, A.; Roqué-Rosell, J.; González-Jiménez, J.M.; Gervilla, F.; Proenza, J.A.; Mendoza, J.; Nieto, F. Nanoscale Structure of Zoned Laurites from the Ojén Ultramafic Massif, Southern Spain. Minerals 2019, 9, 288. https://doi.org/10.3390/min9050288
Baurier-Aymat S, Jiménez-Franco A, Roqué-Rosell J, González-Jiménez JM, Gervilla F, Proenza JA, Mendoza J, Nieto F. Nanoscale Structure of Zoned Laurites from the Ojén Ultramafic Massif, Southern Spain. Minerals. 2019; 9(5):288. https://doi.org/10.3390/min9050288
Chicago/Turabian StyleBaurier-Aymat, Sandra, Abigail Jiménez-Franco, Josep Roqué-Rosell, José María González-Jiménez, Fernando Gervilla, Joaquín A. Proenza, Joan Mendoza, and Fernando Nieto. 2019. "Nanoscale Structure of Zoned Laurites from the Ojén Ultramafic Massif, Southern Spain" Minerals 9, no. 5: 288. https://doi.org/10.3390/min9050288





