Au and Te Minerals in Seafloor Massive Sulphides from Semyenov-2 Hydrothermal Field, Mid-Atlantic Ridge
Abstract
:1. Introduction
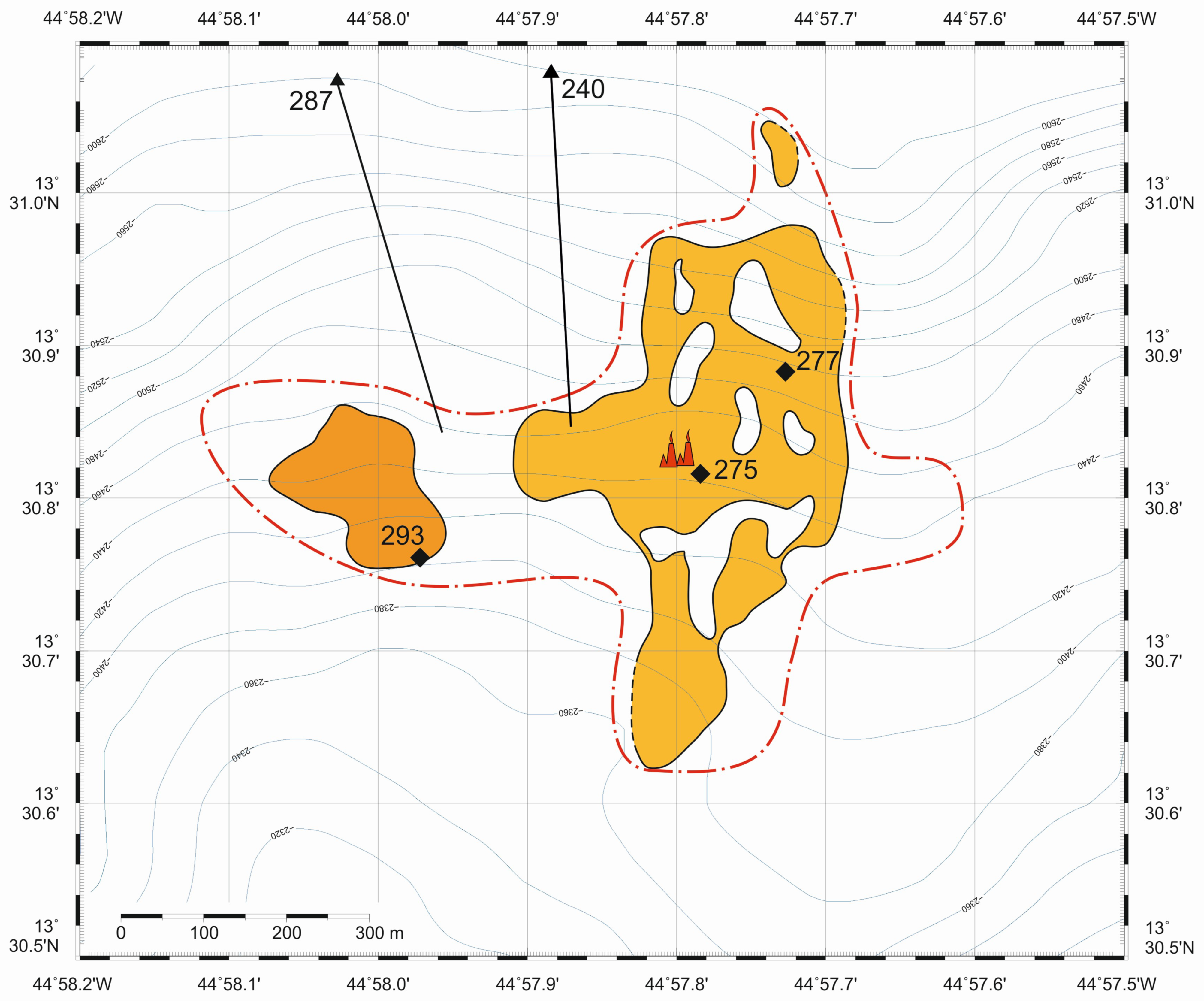
2. Geological Background
3. Materials and Methods
4. Results
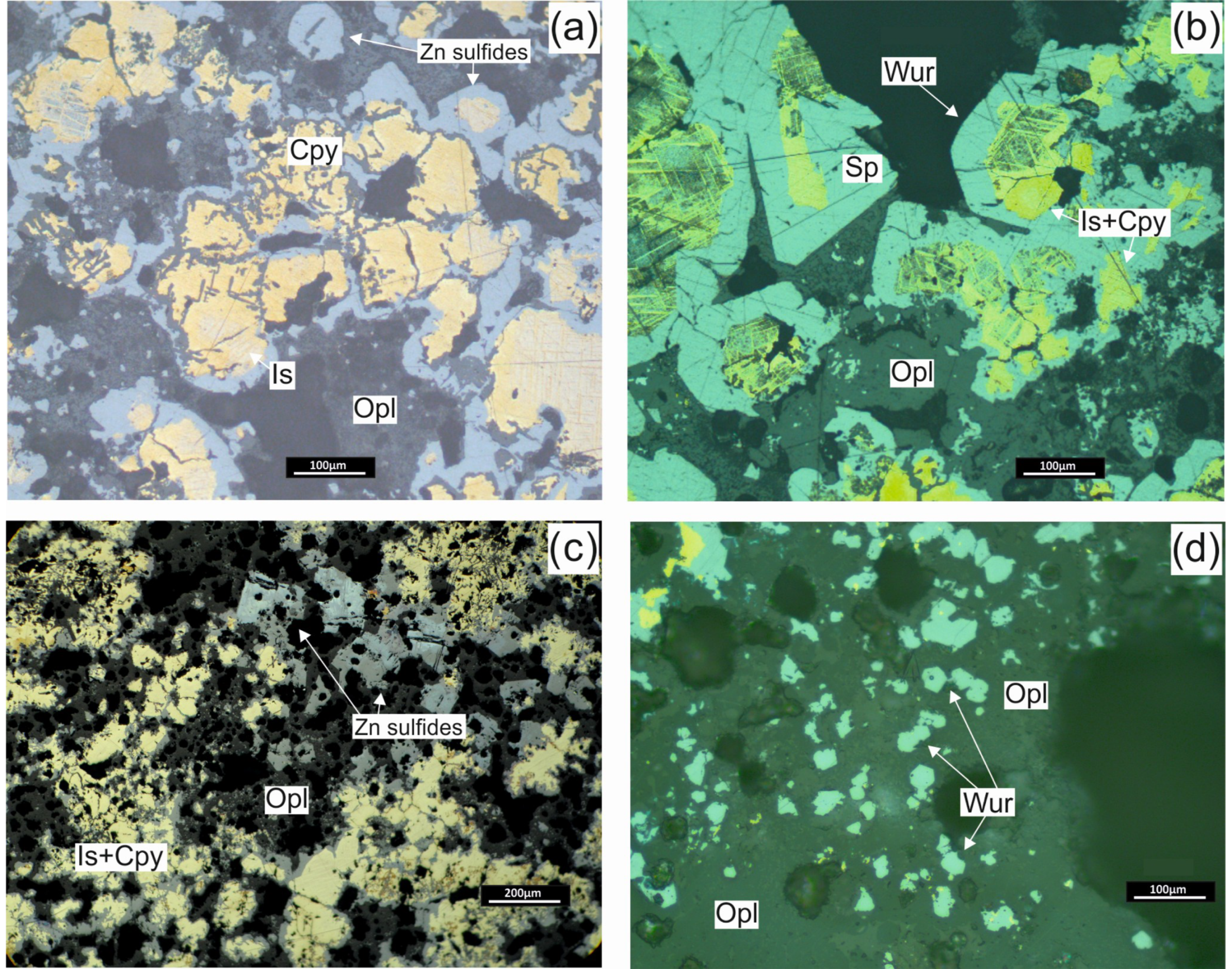
4.1. SMS Samples: Structures, Textures and Mineralogy
4.1.1. Chimneys
4.1.2. Massive Sulphides
4.1.3. Breccias
4.2. Composition and Isotopic Studies of Aragonite
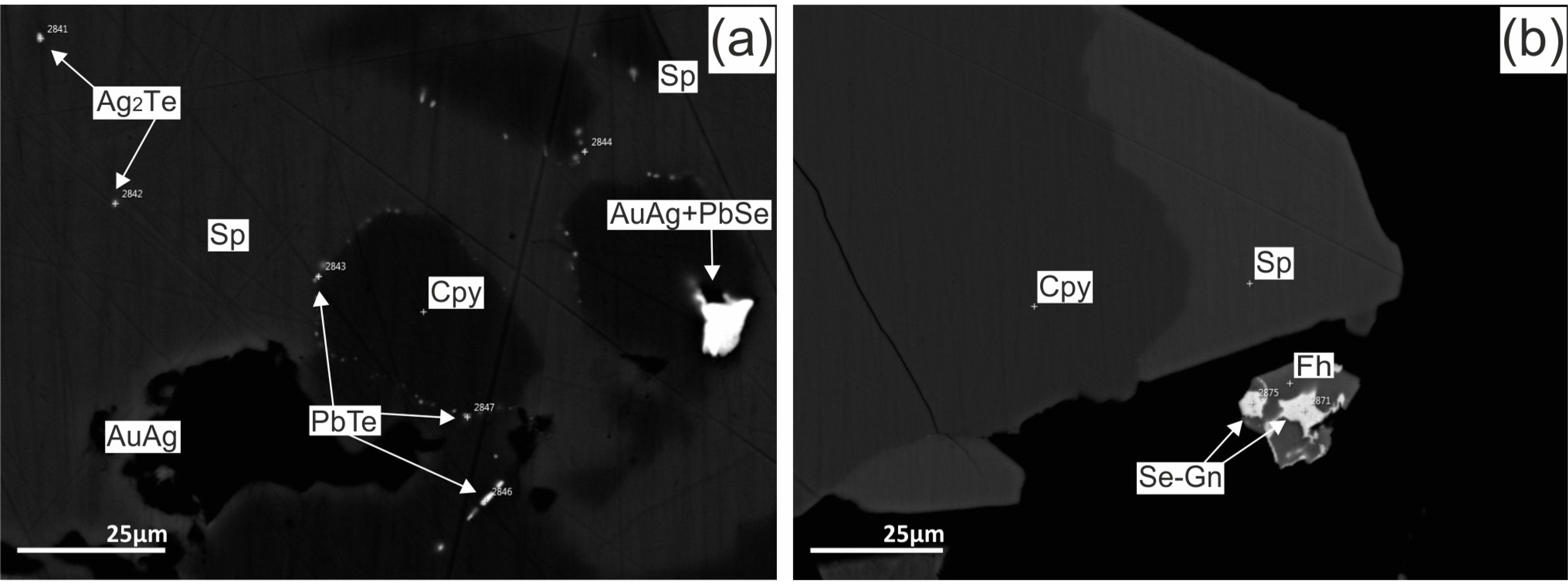
4.3. Sulfide Composition
4.3.1. Chimneys
4.3.2. Massive Sulphides
4.3.3. Breccia
4.3.4. Au Content in Host Rocks from the Semyenov-2 Hydrothermal Field
5. Discussion
5.1. The Condition of Formation SMS
- (a)
- chalcopyrite with isocubanite and predominantly chalcocite (high Cu-sulphide)
- (b)
- chalcopyrite with mainly covellite (low Cu-sulphide)
- (c)
- chalcopyrite-sphalerite.
5.2. Factors Controlling High Content of Au and Te
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| MAR | Mid-Atlantic Ridge |
| EPR | East Pacific Rise |
| OCC | Oceanic Core Complex |
| SMS | Seafloor Massive Sulfides |
| VMS | Volcanogenic Massive Sulphides |
| PDB | Pee Dee Belemnite |
| SMOW | Standard Mean Ocean Water |
| BSE | Back-Scattered Electron Detector |
| ICP-MS | Inductively Coupled Plasma-Mass Spectroscopy |
References
- Hannington, M.D.; Thompson, G.; Rona, P.A.; Scott, S.D. Gold and native copper in supergene sulphides from the Mid-Atlantic Ridge. Nature 1988, 333, 64–66. [Google Scholar] [CrossRef]
- Hannington, M.; Herzig, P.; Scott, S.; Thompson, G.; Rona, P. Comparative mineralogy and geochemistry of goldbearing sulphide deposits on the mid-ocean ridges. Mar. Geol. 1991, 101, 217–248. [Google Scholar] [CrossRef]
- Herzig, P.M.; Hannington, M.D.; Scott, S.D.; Maliotis, G.; Rona, P.A.; Thompson, G. Gold-rich sea-floor gossans in the Troodos ophiolite and on the Mid-Atlantic Ridge. Econ. Geol. 1991, 86, 1747–1755. [Google Scholar] [CrossRef]
- Hannington, M.D.; Tivey, M.K.; Larocque, A.C.L.; Petersen, S.; Rona, P. The occurrence of gold in sulphide deposits of the TAG hydrothermal field, Mid-Atlantic Ridge. Can. Miner. 1995, 33, 1285–1310. [Google Scholar]
- Petersen, S.; Kuhn, T.; Herzig, P.M.; Hannington, M.D. Factors controlling precious and base-metal enrichments at the ultramafic-hosted Logatchev hydrothermal field, 14°45′N on the MAR; new insights from cruise M60/3. In Mineral Deposit Research: Meeting the Global Challenge, Proceedings of the 8th Biennial SGA Meeting, Beijing, China, 18–21 August 2005; Mao, J., Bierlein, F.P., Eds.; Springer: Berlin, Germany, 2005; pp. 679–682. [Google Scholar]
- Hannington, M.D.; de Ronde, C.E.J.; Petersen, S. Sea-Floor Tectonics and Submarine Hydrothermal Systems; Economic Geology 100th Anniversary Volume; Society of Economic Geologists: Littelton, CO, USA, 2005; pp. 111–141. [Google Scholar] [CrossRef]
- Rona, P.A.; Hannington, M.D.; Raman, C.V.; Thompson, G.; Tivey, M.K.; Humphris, S.E.; Lalou, C.; Petersen, S. Active and relict seafloor hydrothermal mineralization at the TAG hydrothermal field, Mid-Atlantic Ridge. Econ. Geol. 1993, 88, 1989–2017. [Google Scholar] [CrossRef]
- Lein, A.Yu.; Cherkashov, G.A.; Ul’yanov, A.A.; Ul’yanova, N.V.; Stepanova, T.V.; Sagalevich, A.M.; Bogdanov, Yu.A.; Gurvich, E.G.; Torokhov, M.P. Mineralogy and geochemistry of sulphide ores from the Logachev-2 and Rainbow fields: Similar and distinctive features. Geochem. Int. 2003, 41, 271–294. [Google Scholar]
- Fouquet, Y.; Cambon, P.; Etoubleau, J.; Charlou, J.; Ondréas, H.; Barriga, F.; Cherkashov, G.; Semkova, T.; Poroshina, I.; Bohn, M.; et al. Geodiversity of hydrothermal processes along the Mid-Atlantic Ridge and ultramafic-hosted mineralization: A new type of oceanic Cu-Zn-Co-Au volcanogenic massive sulphide deposit. In Diversity of Hydrothermal Systems on Slow Spreading Ocean Ridges, Geophysical Monograph Series; Rona, P., Devey, C., Dyment, J., Murton, B., Eds.; American Geophysical Union: Washington, DC, USA, 2010; Volume 188, pp. 321–368. [Google Scholar]
- Firstova, A.; Stepanova, T.; Cherkashov, G.; Goncharov, A.; Babaeva, S. Composition and Formation of Gabbro-Peridotite Hosted Seafloor Massive Sulphide Deposits from the Ashadze-1 Hydrothermal Field, Mid-Atlantic Ridge. Minerals 2016, 6, 19. [Google Scholar] [CrossRef]
- Hannington, M.D.; Peter, J.M.; Scott, S.D. Gold in seafloor polymetallic sulphide deposits. Econ. Geol. 1986, 81, 1867–1883. [Google Scholar] [CrossRef]
- Melekestseva, I.Y.; Maslennikov, V.V.; Tret’yakov, G.A.; Nimis, P.; Beltenev, V.E.; Rozhdestvenskaya, I.I.; Maslennikova, S.P.; Belogub, E.V.; Danyushevsky, L.; Large, R.; et al. Gold- and Silver-Rich Massive Sulphides from the Semenov-2 Hydrothermal Field, 13°31.13′N, Mid-Atlantic Ridge: A Case of Magmatic Contribution? Econ. Geol. 2017, 112, 741–773. [Google Scholar] [CrossRef]
- Ivanov, V.N.; Beltenev, V.E.; Stepanova, T.V.; Lazareva, L.I.; Samovarov, M.L. Sulphide ores of the new hydrothermal field at the 13°31′N of the MAR. In Metallogeny of Ancient and Modern Ocean–2008: Ore Bearing Complexes and Ore Facies; Zaykov, V.V., Ed.; Institute of Mineralogy: Miass, Russia, 2008; pp. 19–22. (in Russian) [Google Scholar]
- Zierenberg, R.A.; Koski, R.A.; Morton, J.L.; Bouse, R.M. Genesis of massive sulphide deposits on a sediment-covered spreading centre, Escanaba trough, southern Gorda Ridge. Econ. Geol. 1993, 88, 2069–2098. [Google Scholar] [CrossRef]
- Maslennikov, V.V.; Maslennikova, S.P.; Large, R.R.; Danyushevsky, L.V.; Herrington, R.J.; Stanley, C.J. Tellurium-bearing minerals in zoned sulphide chimneys from Cu-Zn massive sulphide deposits of the Urals, Russia. Mineral. Petrol. 2013, 107, 67–99. [Google Scholar] [CrossRef]
- Firstova, A.; Cherkashov, G.; Stepanova, T.; Babaeva, S. Rare elements in seafloor massive sulphides of the Semyenov hydrothermal cluster, Mid-Atlantic Ridge. In Proceedings of the UMI 2014—Harvesting Seabed Mineral Resources in Harmony with Nature: 43rd Underwater Mining Institute, Lisbon, Portugal, 21–28 September 2014; pp. 131–139. [Google Scholar]
- Smith, D.K.; Cann, J.R.; Escartín, J. Widespread active detachment faulting and core complex formation near 13°N on the Mid-Atlantic Ridge. Nature 2006, 443, 440–444. [Google Scholar] [CrossRef]
- Smith, D.K.; Escartín, J.; Schouten, H.; Cann, J.R. Fault rotation and core complex formation: Significant processes in seafloor formation at slow-spreading mid-ocean ridges (Mid-Atlantic Ridge, 13°–15°N). Geochem. Geophys. Geosystems 2008, 9, Q03003. [Google Scholar] [CrossRef]
- MacLeod, C.J.; Searle, R.C.; Casey, J.F.; Mallows, C.; Unsworth, M.; Achenbach, K.; Harris, M. Life cycle of oceanic core complexes. Earth Planet. Sci. Lett. 2009, 287, 333–344. [Google Scholar] [CrossRef]
- Mallows, C.; Searle, R.C. A geophysical study of oceanic core complexes and surrounding terrain, Mid-Atlantic Ridge 13°N–14°N. Geochem. Geophys. Geosystems 2012, 13, Q0AG08. [Google Scholar] [CrossRef]
- Escartín, J.; Mevel, C.; Petersen, S.; Bonnemains, D.; Cannat, M.; Andreani, M.; Godard, M. Tectonic structure, evolution and the nature of oceanic core complexes and their detachment fault zones (13°20′N and 13°30′N, Mid Atlantic Ridge). Geochem. Geophys. Geosystems 2017, 18, 1451–1482. [Google Scholar] [CrossRef]
- Cann, J.R.; Blackman, D.K.; Smith, D.K.; McAllister, E.; Janssen, B.; Mello, S.; Avgerinos, E.; Pascoe, A.R.; Escartín, J. Corrugated slip surfaces formed at North Atlantic ridge-transform intersections. Nature 1997, 385, 329–332. [Google Scholar] [CrossRef]
- Tucholke, B.E.; Lin, J.; Kleinrock, M.C. Megamullions and mullion structure defining oceanic metamorphic core complexes on the Mid-Atlantic Ridge. J. Geophys. Res. 1998, 103, 9857–9866. [Google Scholar] [CrossRef] [Green Version]
- MacLeod, C.J.; Escartin, J.; Banerji, D.; Banks, G.J.; Gleeson, M.; Irving, D.H.B.; Smith, D.K. Direct geological evidence for oceanic detachment faulting: The Mid-Atlantic Ridge, 15°45′N. Geology 2002, 30, 879–882. [Google Scholar] [CrossRef]
- Escartín, J.; Mevel, C.; MacLeod, C.J.; McCaig, A.M. Constraints on deformation conditions and the origin of oceanic detachments: The Mid-Atlantic Ridge core complex at 15°45′N. Geochem. Geophys. Geosystems 2003, 4, 1067. [Google Scholar] [CrossRef]
- Pertsev, A.N.; Bortnikov, N.S.; Vlasov, E.A.; Beltenev, V.E.; Dobretsova, I.G.; Ageeva, O.A. Recent massive sulphide deposits of the Semenov ore district, Mid-Atlantic Ridge, 13°31′ N: Associated rocks of the oceanic core complex and their hydrothermal alteration. Geol. Ore Depos. 2012, 54, 334–346. [Google Scholar] [CrossRef]
- Beltenev, V.; Ivanov, V.; Rozhdestvenskaya, I.; Cherkashov, G.; Stepanova, T.; Shilov, V.; Davydov, M.; Laiba, A.; Kaylio, V.; Narkevsky, E.; et al. New data about hydrothermal fields on the Mid-Atlantic Ridge between 11°–14°N: 32nd cruise of R/V Professor Logatchev. InterRidge News 2009, 18, 14–18. [Google Scholar]
- PMGE. Unpublished PMGE report on regional works on a scale of 1:500,000–1:1,000,000 for deep-sea polymetallic sulphides in the axial zone of the Mid-Atlantic Ridge and searching works at Ashadze hydrothermal field: Polar Marine Geosurvey Expedition, Lomonosov-St-Petersburg. 2007; Unpublished report. 67p. (in Russian) [Google Scholar]
- PMGE. Unpublished PMGE report on regional works for deep-sea polymetallic sulphides in the axial zone of the Mid-Atlantic Ridge: Polar Marine Geosurvey Expedition, Lomonosov-St-Petersburg. 2009; Unpublished report. 75p. (in Russian) [Google Scholar]
- Melekestseva, I.Y.; Kotlyarov, V.A.; Khvorov, P.V.; Ivanov, V.N.; Beltenev, V.E.; Dobretsova, I.G. Noble-metal mineralization in the Semenov-2 hydrothermal field (13°31′N), Mid-Atlantic Ridge. Geol. Ore Depos. 2010, 52, 800–810. [Google Scholar] [CrossRef]
- Böhm, F.; Dullo, W.-Ch.; Joachimski, M.M.; Eisenhauer, A. Oxygen isotope fractionation in marine aragonite of coralline sponges. Geochim. Cosmochim. Acta 2000, 64, 1695–1703. [Google Scholar] [CrossRef] [Green Version]
- Bonatti, E.; Lawrence, J.R.; Hamlyn, P.R.; Breger, D. Aragonite from deep-sea ultramafic rocks. Geochim. Cosmochim. Acta 1980, 44, 1207–1214. [Google Scholar] [CrossRef]
- Thompson, G.; Livingston, H.D. Strontium and uranium concentrations in aragonite precipitated by some modern corals. Earth Planet. Sci. Lett. 1970, 8, 439–442. [Google Scholar] [CrossRef]
- Dubinina, E.O.; Chernyshov, I.V.; Bortnikov, N.S.; Lein, A.Y. Isotopic and geochemical features of hydrothermal field Lost-City. Geochemistry 2007, 11, 1223–1236. (in Russian). [Google Scholar]
- Früh-Green, G.L.; Kelley, D.S.; Bernasconi, S.M.; Karson, J.A.; Ludwig, K.A.; Butterfield, D.A.; Boschi, C.; Proskurowski, G. 30,000 years of hydrothermal activity at the Lost City vent field. Science 2003, 301, 495–498. [Google Scholar] [CrossRef]
- Lein, A.Yu.; Galkin, S.V.; Maslennikov, V.V. New type of carbonate rocks on the ocean floor. Rep. Acad. Sci. 2007, 412, 535–539. (in Russian). [Google Scholar]
- Mozgova, N.N.; Trubkin, N.V.; Borodaev, Y.S.; Cherkashov, G.A.; Stepanova, T.V.; Semkova, T.A.; Uspenskaya, T.Y. Mineralogy of massive sulphides from the Ashadze hydrothermal field, 13°N, Mid-Atlantic Ridge. Can. Miner. 2008, 46, 545–567. [Google Scholar] [CrossRef]
- Taylor, S.R. Trace element abundances and the chondritic Earth model. Geochim. Cosmochim. Acta 1964, 28, 1989–1998. [Google Scholar] [CrossRef]
- Vinogradov, A.P. The average content of chemical elements in the main types of igneous rocks of the earth’s crust. Geochemistry 1962, 7, 641–664. (in Russian). [Google Scholar]
- Turekian, K.K.; Wedepohl, K.H. Distribution of the elements in some major units of the Earth’s crust. Geol. Soc. Am. Bull. 1961, 72, 175–192. [Google Scholar] [CrossRef]
- Foster, R.P. Gold metallogeny and Exploration; Springer: New York, NY, USA, 1991; 432p. [Google Scholar]
- Silantyev, S.A.; Kubrakova, I.V.; Tyutyunnik, O.A. The distribution pattern of siderophilic and chalcophilic elements in the serpentinites of the oceanic lithosphere is a reflection of the magmatic and intracrustal evolution of the mantle substrate. Geochemistry 2016, 12, 1059–1075. (in Russian). [Google Scholar]
- Maslennikov, V.V.; Maslennikova, S.P. Rare mineral assemblages in black and white smoker vent chimneys from Uralian VHMS deposits, Russia. In Mineral Deposit Research: Meeting the Global Challenge, Proceedings of the Eighth Biennial SGA Meeting, Beijing, China, 18–21 August 2005; Mao, J., Bierlein, F.P., Eds.; Springer: Berlin, Germany, 2005; pp. 647–650. [Google Scholar]
- Afifi, A.M.; Kelly, W.C.; Essene, E.J. Phase relations among tellurides, sulphides and oxides: I. Thermodynamical data and calculated equilibria. Econ. Geol. 1988, 83, 377–394. [Google Scholar] [CrossRef]
- Jaireth, S. Hydrothermal geochemistry of Te, Ag2Te and AuTe2 in epithermal precious metal deposits. EGRU Contrib. 1991, 37, 1–21. [Google Scholar]
- Fouquet, Y.; Wafik, A.; Cambon, P.; Mevel, C.; Meyer, G.; Gente, P. Tectonic setting and mineralogical and geochemical zonation in the Snake Pit sulphide deposit (Mid-Atlantic Ridge at 23° N). Econ. Geol. 1993, 88, 2018–2036. [Google Scholar] [CrossRef]
- Petrovskaya, N.V. Native Gold; Science: Moscow, Russia, 1973; 350p. [Google Scholar]
- Prokin, V.A.; Buslaev, F.P. Massive copper-zinc sulphide deposits in the Urals. Ore Geol. Rev. 1998, 14, 1–69. [Google Scholar] [CrossRef]
- Helgeson, H.C. Thermodynamics of hydrothermal systems at elevated temperatures and pressures. Am. J. Sci. 1969, 267, 729–804. [Google Scholar] [CrossRef]
- Henley, R.W. Solubility of gold in hydrothermal chloride solutions. Chem. Geol. 1973, 11, 73–87. [Google Scholar] [CrossRef]
- Shernberger, D.M.; Barnes, H.L. Solubility of gold in aqueous sulphide solutions from 150 to 250 °C. Geochim. Cosmochim. Acta 1989, 53, 269–278. [Google Scholar] [CrossRef]
- Hannington, M.D.; Scott, S.D. Sulfidation equilibria as guides to gold mineralization in volcanogenic massive sulphides; evidence from sulphide mineralogy and the composition of sphalerite. Econ. Geol. 1989, 84, 1978–1995. [Google Scholar] [CrossRef]
- Seward, T.M. Thio complexes of gold and the transport of gold in hydrothermal ore solutions. Geochim. Cosmochim. Acta 1973, 37, 379–399. [Google Scholar] [CrossRef]
- Oudin, E.; Constantinou, G. Black smoker chimney fragments in Cyprus sulphide deposits. Nature 1984, 308, 349–353. [Google Scholar] [CrossRef]
- Little, C.T.S.; Maslennikov, V.V.; Morris, N.J.; Gubanov, A.P. Two Palaezoic hydrothermal vent communities from the Southern Ural Mountains, Russia. Palaentology 1999, 42, 1043–1078. [Google Scholar] [CrossRef]
- Eremin, N.I. Differentiation of Volcanogenic Sulphide Mineralization; Moscow University: Moscow, Russia, 1983; 256p. [Google Scholar]













| Location | Au, ppm (n) | Te, ppm (n) | Reference |
|---|---|---|---|
| Mid-Ocean Ridges | |||
| 21°N, EPR | 0.12 (46) | 3.2 (46) | [6] |
| 13°N, EPR | 0.45 (71) | 0.3 (71) | [6] |
| 11°N, EPR | 0.15 (18) | 0.4 (18) | [6] |
| TAG, MAR | 6.1 | <0.1 (310) | [6,7] |
| Snakepit, MAR | 1.66 (93) | 4.2 (93) | [6] |
| Broken Spur, MAR | 1.64 (76) | 13.3 (76) | [6] |
| Rainbow * MAR | 5.10 | - | [8] |
| Krasnov, MAR | 0.8 (162) | 8.1 (8) | ** |
| Logatchev-1 *, MAR | 13 (53) | 17 (17) | ** |
| Logatchev-2 *, MAR | 25.9 | - | [9] |
| Ashadze-1 *, MAR | 3.0 (120) | 6.8 (53) | [10] ** |
| Ashadze-2 *, MAR | 10.0 (53) | 7.6 (18) | ** |
| Semyenov-2 *, MAR | 21 (29) | 40 (29) | This study |
| SMS | Chimneys | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral Composition | Chalcopyrite | Sphalerite-Chalcopyrite | |||||||||||||||
| Station | 293 | 277 | 277 | 275 | |||||||||||||
| Sample | Т-1 | Т-1/2 | Т-6 | Т-9 | Т-9/1а | Т-9/2 | Т-2 | Т-3 | Т-3/1 | Т-5 | Т-7 | Т-7/1 | Т-4 | Т-4b | Т-8 | Т-1 | |
| Mineral | |||||||||||||||||
| Chalcopyrite, CuFeS2 | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | +++ | |
| Isocubanite, CuFe2S3 | ++ | ++ | + | ||||||||||||||
| Sphalerite, (Zn,Fe)S | ++ | + | + | + | +++ | +++ | ++ | +++ | |||||||||
| Wurtzite, (Zn,Fe)S | |||||||||||||||||
| Pyrite, FeS2 | + | + | + | ++ | + | ||||||||||||
| Marcasite, FeS2 | |||||||||||||||||
| Bornite, Cu5FeS4 | ++ | ++ | ++ | ++ | ++ | + | + | + | + | ||||||||
| Idaite, Cu3FeS4 | + | ++ | + | + | ++ | ++ | |||||||||||
| Chalcocite, Cu2S | ++ | + | ++ | ++ | |||||||||||||
| Djurleite, Cu31S16 | + | + | + | ++ | + | ||||||||||||
| Roxbyite, Cu9S5 | + | + | + | + | |||||||||||||
| Digenite, Cu9S5 | + | + | ++ | + | ++ | + | |||||||||||
| Geerite, Cu8S5 | + | + | + | + | + | ||||||||||||
| Spionkopite, Cu1.4S | + | + | |||||||||||||||
| Yarrowite, Cu9S8 | + | + | + | ++ | |||||||||||||
| Covellite, CuS | . | ++ | ++ | +++ | +++ | ++ | ++ | ++ | ++ | + | + | ||||||
| Opal, SiO2·nH2O | + | + | |||||||||||||||
| Aragonite, CaCO3 | |||||||||||||||||
| Barite, BaSO4 | + | + | + | ||||||||||||||
| Atakamite, Cu2Cl(OH)3 | |||||||||||||||||
| Fe oxides and hydroxides | |||||||||||||||||
| SMS | Massive | Breccia | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral Composition | Isocubanite-Chalcopyrite- Sphalerite-Wurtzite-Opal | Opal Cement | Aragonite Cement | |||||||||
| Sphalerite-Wurtzite- Chalcopyrite-Isocubanite | Cu-sulphide | Isocubanite, Cu-sulphide | Cu-sulphide | |||||||||
| Station | 287 | 287 | 287 | 240 | 293-КА | |||||||
| Sample | М-1/1 | М-1/1а | B-1 | B-1/1 | B-1/2 | Т-1, T-1a | Т-1 | Т-2 | М-1 | М-1/1 | KA-1,2 | |
| Mineral | ||||||||||||
| Chalcopyrite, CuFeS2 | ++ | ++ | + | + | + | + | + | + | + | + | + | |
| Isocubanite, CuFe2S3 | ++ | ++ | +++ | +++ | +++ | + | + | + | + | |||
| Sphalerite, (Zn,Fe)S | +++ | +++ | ++ | ++ | ++ | + | + | + | + | |||
| Wurtzite, (Zn,Fe)S | + | |||||||||||
| Pyrite, FeS2 | + | +++ | + | |||||||||
| Marcasite, FeS2 | ++ | + | + | |||||||||
| Bornite, Cu5FeS4 | + | + | + | + | + | |||||||
| Idaite, Cu3FeS4 | + | + | ||||||||||
| Chalcocite, Cu2S | ++ | ++ | ||||||||||
| Djurleite, Cu31S16 | + | |||||||||||
| Roxbyite, Cu9S5 | ||||||||||||
| Digenite, Cu9S5 | + | ++ | + | |||||||||
| Geerite, Cu8S5 | + | |||||||||||
| Spionkopite, Cu1.4S | + | + | ||||||||||
| Yarrowite, Cu9S8 | ||||||||||||
| Covellite, CuS | + | + | ++ | +++ | ++ | ++ | ++ | ++ | ++ | + | ||
| Opal, SiO2·nH2O | +++ | +++ | +++ | ++ | ++ | + | ++ | + | ++ | + | + | |
| Aragonite, CaCO3 | +++ | +++ | +++ | +++ | +++ | +++ | ||||||
| Barite, BaSO4 | + | + | ||||||||||
| Atakamite, Cu2Cl(OH)3 | + | +++ | +++ | + | + | +++ | ||||||
| Fe oxides and hydroxides | ++ | + | + | + | + | |||||||
| SMS | Chimneys | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral Composition | Chalcopyrite | Sphalerite-Chalcopyrite | |||||||||||||||
| Station | 293 | 277 | 277 | 275 | |||||||||||||
| Sample | Т-1 | Т-1/2 | Т-6 | Т-9 | Т-9/1а | Т-9/2 | Т-2 | Т-3 | Т-3/1 | Т-5 | Т-7 | Т-7/1 | Т-4 | Т-4b | Т-8 | Т-1 | |
| Mineral | |||||||||||||||||
| Native gold, Au | + | + | + | + | + | + | + | ||||||||||
| Electrum, (AuAg)1 | + | + | + | + | + | + | |||||||||||
| Aurostibite, AuSb2 | |||||||||||||||||
| Calaverite, AuTe2 | + | + | + | + | + | + | + | ||||||||||
| Hessite, Ag2Te | + | ||||||||||||||||
| Melonite, NiTe2 | + | ||||||||||||||||
| Tellurobismuthite, Bi2Te3 | + | + | + | + | + | + | |||||||||||
| Tetradymite, Bi2Te2S | |||||||||||||||||
| Altaite, PbTe | + | ||||||||||||||||
| Clausthalite, PbSe | + | ||||||||||||||||
| Naumannite, Ag2Se | |||||||||||||||||
| Se-galena | + | + | + | ||||||||||||||
| Galena, PbS | + | + | + | ||||||||||||||
| Fahlore, Cu12 (Sb,As)4 S13 | + | + | + | + | + | + | + | + | + | + | + | ||||||
| Glaucodote, (Co,Fe)AsS | + | ||||||||||||||||
| Cobaltite, CoAsS | + | + | + | + | + | + | + | + | + | + | + | ||||||
| SMS | Massive | Breccia | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mineral Composition | Isocubanite-Chalcopyrite- Sphalerite-Wurtzite-Opal | Opal Cement | Aragonite Cement | |||||||
| Sphalerite-Wurtzite- Chalcopyrite-Isocubanite | Cu-sulphide | Isocubanite, Cu-sulphide | Cu-sulphide | |||||||
| Station | 287 | 287 | 287 | 240 | 293-КА | |||||
| Sample | М-1/1 | М-1/1а | B-1, B-1/1, B-1/2 | Т-1, T-1a | Т-1 | Т-2 | М-1 | М-1/1 | KA-1,2 | |
| Mineral | ||||||||||
| Native gold, Au | + | + | + | + | + | + | + | |||
| Electrum, (AuAg)1 | + | + | + | + | + | |||||
| Aurostibite, AuSb2 | + | |||||||||
| Calaverite, AuTe2 | ||||||||||
| Sylvanite, AuAgTe4 | ||||||||||
| Hessite, Ag2Te | + | + | + | |||||||
| Melonite, NiTe2 | ||||||||||
| Tellurobismuthite, Bi2Te3 | + | |||||||||
| Tetradymite, Bi2Te2S | + | |||||||||
| Altaite, PbTe | ||||||||||
| Clausthalite, PbSe | + | + | ||||||||
| Naumannite, Ag2Se | + | + | ||||||||
| Se-galena | + | + | + | |||||||
| Galena, PbS | + | + | + | |||||||
| Fahlore, Cu12 (Sb,As)4S13 | ||||||||||
| Glaucodote, (Co,Fe)AsS | + | + | ||||||||
| Cobaltite, CoAsS | + | + | + | |||||||
| Mineral (Number of Analyses) | Element (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bi | Te | Au | Ag | Ni | Cu | Fe | Totals | |
| Tellurobismuthite (10), Bi2Te3 | 51.69 | 48.00 | - | - | - | - | - | 99.69 |
| Calaverite (8), AuTe2 | 0.27 | 56.56 | 43.31 | - | - | 0.55 | - | 100.69 |
| Native gold (8), Au | - | - | 89.12 | 8.87 | - | 2.76 | 0.95 | 101.70 |
| Melonite (2), NiTe2 | - | 81.40 | - | - | 18.41 | - | - | 99.81 |
| Mineral (Number of Analyses) | Element (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Au | Ag | Te | Bi | Pb | Se | S | Totals | |
| Native gold (6), Au | 84.90 | 14.00 | - | - | - | - | - | 98.90 |
| Electrum (8), (Au,Ag)1 | 65.95 | 33.82 | - | - | - | - | - | 99.77 |
| Calaverite (6), AuTe2 | 45.03 | - | 54.69 | - | - | - | - | 99.72 |
| Hessite (13), Ag2Te | - | 63.77 | 35.86 | - | - | - | - | 99.63 |
| Tellurobismuthite (2), Bi2Te3 | - | - | 45.46 | 54.54 | - | - | - | 100.00 |
| Altaite (6), PbTe | - | - | 38.11 | - | 61.46 | - | - | 99.57 |
| Clausthalite (2), PbSe | - | - | - | - | 72.12 | 27.60 | - | 99.72 |
| Se-galena (9), Pb(S,Se) | - | - | - | - | 84.76 | 3.92 | 11.14 | 99.82 |
| Mineral (Number of Analyses) | Element (wt %) | ||||||
|---|---|---|---|---|---|---|---|
| Au | Ag | Te | Se | Mo | Bi | Totals | |
| Native gold (2), Au | 89.38 | 10.58 | - | - | 0.08 | 0.03 | 100.07 |
| Electrum (11), (Au,Ag)1 | 62.33 | 37.49 | - | - | - | - | 99.82 |
| Hessite (9), Ag2Te | - | 62.79 | 37.11 | - | - | - | 99.90 |
| Naumannite (24), Ag2Se | - | 72.06 | - | 27.66 | - | - | 99.72 |
| Mineral (Number of Analyses) | Element (wt %) | |||||||
|---|---|---|---|---|---|---|---|---|
| Bi | Te | Au | Ag | Sb | S | Mo | Totals | |
| Tellurobismuthite (3), Bi2Te3 | 53.14 | 46.82 | - | - | - | - | - | 99.96 |
| Tetradymite (2), Bi2Te2S | 60.52 | 34.78 | - | - | - | 4.57 | - | 99.87 |
| Hessite (8), Ag2Te | - | 36.04 | - | 63.53 | - | - | - | 99.57 |
| Native gold (3), Au | - | - | 92.39 | 7.51 | - | - | 0.17 | 100.07 |
| Aurostibite (1), AuSb2 | - | - | 47.36 | 0.18 | 52.26 | - | - | 99.80 |
| Mineral (Number of Analyses) | Element (wt %) | ||
|---|---|---|---|
| Au | Ag | Totals | |
| Native gold (20), Au | 94.19 | 5.35 | 99.54 |
| Electrum (6), (Au,Ag)1 | 64.06 | 35.41 | 99.47 |
| Sample | Ca wt % | Mg wt % | Sr wt % | δ 13C ‰ (PDB) | δ 18O ‰ (PDB) | δ 18O ‰ (SMOW) | T (°C) Linear Equation |
|---|---|---|---|---|---|---|---|
| 293–KA-1 | 36.92 41.05 | 0.01 0.05 | 1.83 1.25 | +3.6 | +3.7 | +34.73 | 3.6 |
| 240-T 240-M | 38.66 39.48 41.80 49.70 | <LOD 0.01 <LOD 0.02 | 1.25 0.98 2.01 1.54 | n.d | n.d | n.d | n.d |
| 287-T-1 | 36.72 39.65 42.01 45.09 | 0.04 <LOD <LOD <LOD | 1.84 2.02 1.27 1.98 | +3.3 | +3.4 | +34.43 | 5.0 |
| SMS | Chimneys | Massive | Breccia | SMS Semyenov-2 | MAR * | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mineral Composition | Chalcopyrite | Chalcopyrite | Sphalerite- Chalcopyrite | Sphalerite- Chalcopyrite | Isocubanite- Chalcopyrite- Sphalerite- Wurtzite-Opal | Opal Cement | Aragonite Cement | ||||||
| Sphalerite-wurtzite- Chalcopyrite- Isocubanite | Cu-sulfide | Isocubanite, Cu-sulfide | Cu-sulfide | ||||||||||
| Station | 293 | 277 | 277 | 275 Active | 287-М | 287-B | 287-Т | 240 | 293-КА | 293, 277, 275, 287, 240 | |||
| Element | |||||||||||||
| Fe, wt % | 5.9 | 15.0 | 20.7 | 21.3 | 12.6 | 21.2 | 11.0 | 14.7 | 3.1 | 14.5 | 32.43 | ||
| S | 13.9 | 18.1 | 13.9 | 27.7 | 21.2 | 29.0 | 3.8 | 4.8 | 4.6 | 15.2 | 36.44 | ||
| Cu | 48.5 | 44.9 | 36.9 | 23.8 | 11.6 | 17.7 | 6.0 | 13.3 | 36.5 | 30.9 | 9.56 | ||
| Zn | 0.02 | 0.23 | 1.01 | 8.44 | 21.2 | 13.9 | 5.3 | 0.83 | 0.03 | 3.9 | 4.39 | ||
| SiO2 | 16.7 | 3.7 | 1.4 | 12.6 | 29.4 | 14.0 | 17.0 | 5.5 | 15.9 | 9.5 | 6.8 | ||
| CaO | 0.17 | 0.08 | 0.03 | 0.08 | 0.09 | <0.01 | 16.3 | 20.8 | 12.3 | 5.5 | 0.59 | ||
| MgO | 0.22 | 0.09 | 0.12 | 0.18 | 0.09 | 0.05 | 2.5 | 0.53 | 1.5 | 0.42 | 0.23 | ||
| Pb, ppm | 100 | 94 | 22 | 240 | 730 | 155 | 2400 | 36 | 155 | 312 | 157 | ||
| Cd | <2 | 7 | 23 | 280 | 670 | 545 | 120 | 7 | <2 | 190 | 110 | ||
| Ag | 26 | 50 | 16 | 127 | 371 | 294 | 1500 | 124 | 10 | 207 | 49 | ||
| Au | min/max | 0.8/2.9 | 0.7/4.7 | 5.8/14.4 | - | 15.6/32.9 | 17.1/60.5 | 128/188 | 0.4/20.6 | 0.9/1.7 | 0.4/188.2 | 0.1/66.0 * | |
| average | 1.8 | 2.9 | 10 | 22.3 | 24.3 | 39.2 | 158 | 8.6 | 1.3 | 20.8 | 3.2 | ||
| Te | min/max | 15/29 | 12/96 | 56/59 | - | 20/22 | 25/28 | 23/24 | 22/25 | 14/16 | 12/96 | 9.4/250 * | |
| average | 22 | 61 | 57 | 28 | 21 | 26 | 24 | 24 | 15 | 40 | 8.0 ** | ||
| N | 2 | 10 | 3 | 1 | 2 | 3 | 2 | 4 | 2 | 29 | 1051 * | ||
| Rocks | Au, ppb | References |
|---|---|---|
| Abundance of element in the earth crust, Clarke | 5 | [38] |
| Mafic rocks | 4 | [39] |
| 4 | [40] | |
| MORB | 1.2 | [41] |
| Basalt | 2.2 | This study |
| Basalt | 5.9 | This study |
| Layered gabbroic complex | 4.6 | [41] |
| Gabbro | 8 | This study |
| Ultramafic rocks | 5 | [39] |
| 6 | [40] | |
| Peridotites from Logatchev hydrothermal field | 4 | [42] |
| Serpentinized peridotite | 4.6 | This study |
| Felsic rocks | 4.5 | [39] |
| 4.0 | [40] | |
| Plagiogranite | 4.5 | This study |
| Plagiogranite | 6.8 | This study |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Firstova, A.; Stepanova, T.; Sukhanova, A.; Cherkashov, G.; Poroshina, I. Au and Te Minerals in Seafloor Massive Sulphides from Semyenov-2 Hydrothermal Field, Mid-Atlantic Ridge. Minerals 2019, 9, 294. https://doi.org/10.3390/min9050294
Firstova A, Stepanova T, Sukhanova A, Cherkashov G, Poroshina I. Au and Te Minerals in Seafloor Massive Sulphides from Semyenov-2 Hydrothermal Field, Mid-Atlantic Ridge. Minerals. 2019; 9(5):294. https://doi.org/10.3390/min9050294
Chicago/Turabian StyleFirstova, Anna, Tamara Stepanova, Anna Sukhanova, Georgy Cherkashov, and Irina Poroshina. 2019. "Au and Te Minerals in Seafloor Massive Sulphides from Semyenov-2 Hydrothermal Field, Mid-Atlantic Ridge" Minerals 9, no. 5: 294. https://doi.org/10.3390/min9050294




