Numerical Simulation on Authigenic Barite Formation in Marine Sediments
Abstract
:1. Introduction
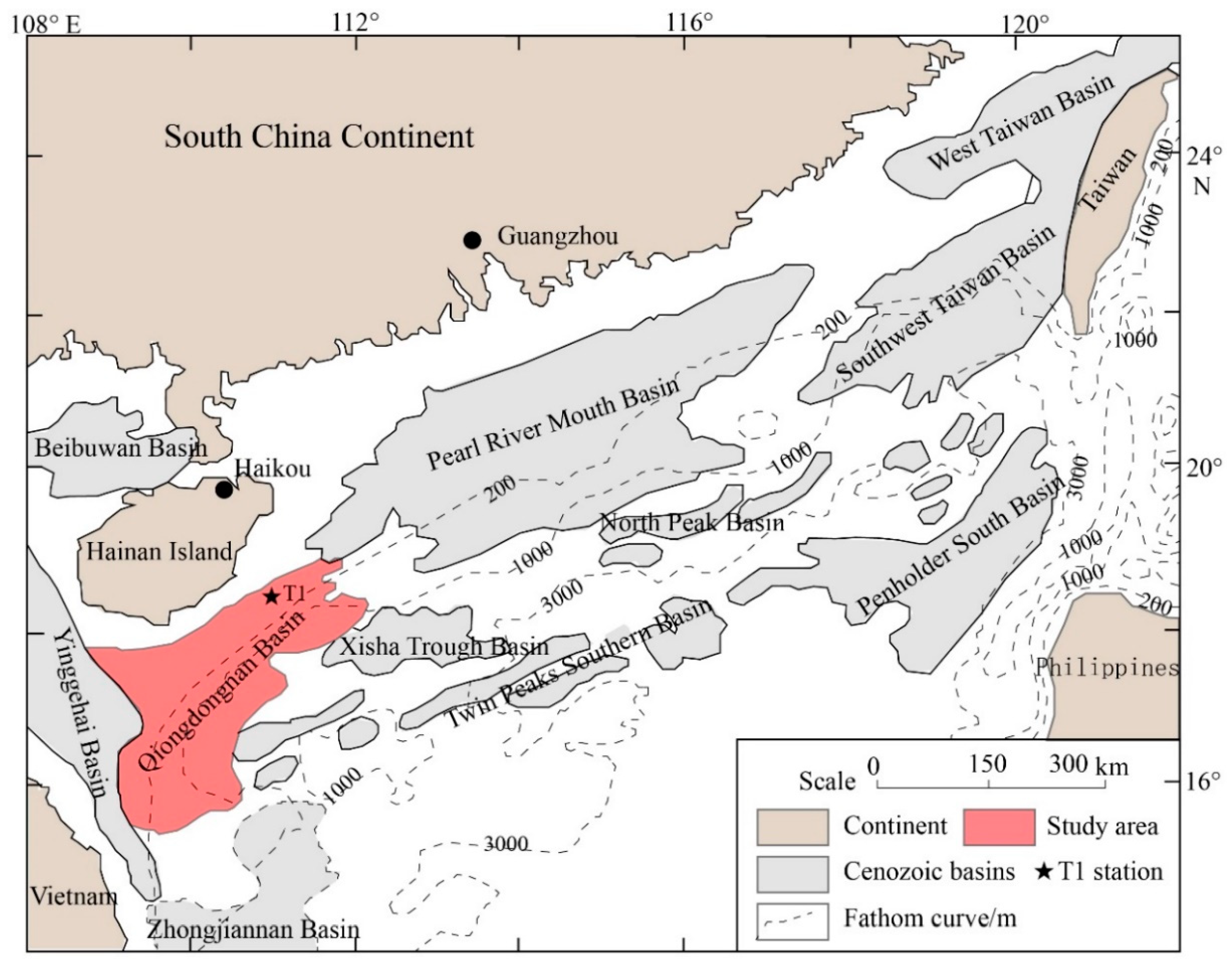
2. Geologic Site Description
3. Methods
3.1. Numerical Simulator
3.2. Biodegradation Kinetics and Parameters
4. Model Setup and Case Settings
4.1. Conceptual Model and Boundary Conditions
4.2. Physical Parameters Used in the Model
4.3. Initial Water Chemistry
4.4. Sedimentary Minerals
4.5. Case Settings
5. Results and Discussion
5.1. Analysis of the Forming Mechanism of the Barite Front
- Original depositional barite in sediments (providing a source of barium);
- AOM-SR process caused by anaerobic microbes (promoting the release of barium);
- Methane leakage flux (promotes AOM-SR and carries the barium).
5.2. Impacts of Methane Leakage on the Distribution of Authigenic Minerals
5.3. Analysis of Forming Mechanism of Multi-Front Barite
- Cold seep areas;
- AOM-SR under the reaction of microorganisms;
- Periodical activities of cold seep.
6. Conclusions
- The formation of a barite front is relevant to the activities of cold seep, which leaks from the deep sediments carrying -bearing fluid. The up-moving reacts with sulfate, forming the barite front in the sulfate-rich zones;
- The location of the barite front is closely related to the methane leakage flux: the higher the leakage flux is, the shallower the location of the barite front. Additionally, multiple peaks appeared due to the periodical cold seep leakages, which provide us with records of cold seep activities;
- Based on the simulated results, a linear relationship with a negative correlation between the leakage flux of methane and the precipitation ratio of barite to calcite was regressed, which might serve as a workable guidance when searching for natural gas hydrate through geochemical characteristics.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Astrom, E.K.; Carroll, M.L.; Ambrose, W.G., Jr.; Carroll, J. Arctic Cold Seeps in Marine Methane Hydrate Environments: Impacts on Shelf Macrobenthic Community Structure Offshore Svalbard. Mar. Ecol. Process Ser. 2016, 552, 1–18. [Google Scholar] [CrossRef]
- Campbell, K.A.; Nelson, C.S.; Alfaro, A.C.; Boyd, S.; Greinert, J.; Nyman, S.; Grosjean, E.; Logan, G.A.; Gregory, M.R.; Cooke, S.; et al. Geological Imprint of Methane Seepage on the Seabed and Biota of the Convergent Hikurangi Margin, New Zealand: Box Core and Grab Carbonate Results. Mar. Geol. 2010, 272, 285–306. [Google Scholar] [CrossRef]
- Stevens, E.W.; Bailey, J.V.; Flood, B.E.; Jones, D.S.; Gilhooly, W.P.; Joye, S.B.; Teske, A.; Mason, O.U. Barite Encrustation of Benthic Sulfur-Oxidizing Bacteria at a Marine Cold Seep. Geobiology. 2015, 13, 588–603. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, K.; Huang, Y. Origin and Nature of Cold Seep in Northeastern Dongsha Area, South China Sea: Evidence from Chimney-Like Seep Carbonates. Chin. Sci. Bull. 2013, 58, 3689–3697. [Google Scholar] [CrossRef]
- Jakubowicz, M.; Dopieralska, J.; Belka, Z. Tracing the Composition and Origin of Fluids at an Ancient Hydrocarbon Seep (Hollard Mound, Middle Devonian, Morocco): A Nd, REE and Stable Isotope Study. Geochimica et Cosmochimica ACTA 2015, 156, 50–74. [Google Scholar] [CrossRef]
- Toki, T.; Higa, R.; Ijiri, A.; Tsunogai, U.; Ashi, J. Origin and Transport of Pore Fluids in the Nankai Accretionary Prism Inferred from Chemical and Isotopic Compositions of Pore Water at Cold Seep Sites Off Kumano. Earth Planets Space 2014, 66, 173. [Google Scholar] [CrossRef]
- Liang, Q.; Hu, Y.; Feng, D.; Peckmann, J.; Chen, L.; Yang, S.; Liang, J.; Tao, J.; Chen, D. Authigenic Carbonates from Newly Discovered Active Cold Seeps on the Northwestern Slope of the South China Sea: Constraints on Fluid Sources, Formation Environments, and Seepage Dynamics. Deep-sea Res. Part I-Oceanogr. Res. Pap. 2017, 124, 31–41. [Google Scholar] [CrossRef]
- Makogon, Y.F. Natural Gas Hydrates. A Promising Source of Energy. J. Nat. Gas Sci. Eng. 2010, 2, 49–59. [Google Scholar] [CrossRef]
- Liu, X.; Xu, T.; Tian, H.L.; Wei, M.C.; Jin, G.R.; Liu, N. Numerical Modeling Study of Mineralization Induced by Methane Cold Seep at the Sea Bottom. Mar. Pet. Geol. 2016, 75, 14–28. [Google Scholar] [CrossRef]
- Orcutt, B.; Samarkin, V.; Boetius, A.; Joye, S. On the Relationship Between Methane Production and Oxidation by Anaerobic Methanotrophic Communities from Cold Seeps of the Gulf of Mexico. Environ. Microbiol. 2008, 10, 1108–1117. [Google Scholar] [CrossRef]
- Boetius, A.; Ravenschlag, K.; Schubert, C.J.; Rickert, D.; Widdel, F.; Gieseke, A.; Amann, R.; Jørgensen, B.B.; Witte, U.; Pfannkuche, O. A marine microbial consortium apparently mediating anaerobic oxidation of methane. Nature 2000, 407, 623. [Google Scholar] [CrossRef] [PubMed]
- Knittel, K.; Boetius, A. Anaerobic Oxidation of Methane: Progress with an Unknown Process. Annu. Rev. Microbiol. 2009, 63, 311–334. [Google Scholar] [CrossRef] [PubMed]
- Orphan, V.J.; House, C.H.; Hinrichs, K.U.; McKeegan, K.D.; DeLong, E.F. Multiple archaeal groups mediate methane oxidation in anoxic cold seep sediments. Proc. Natl. Acad. Sci. USA 2002, 99, 7663–7668. Available online: http://sims.ess.ucla.edu/PDF/Orphan_et_al_PNAS_2002.pdf (accessed on 10 February 2019). [CrossRef] [PubMed]
- Torres, M.E.; McManus, J.; Hammond, D.E.; de Angelis, M.A.; Heeschen, K.U.; Colbert, S.L.; Tryon, M.D.; Brown, K.M.; Suess, E. Fluid and Chemical Fluxes in and Out of Sediments Hosting Methane Hydrate Deposits on Hydrate Ridge, Or, I: Hydrological Provinces. Earth Planet. Sci. Lett. 2002, 201, 525–540. [Google Scholar] [CrossRef]
- Roberts, H.H.; Aharon, P. Hydrocarbon-Derived Carbonate Buildups of the Northern Gulf of Mexico Continental Slope: A Review of Submersible Investigations. Geo-Marine Lett. 1994, 14, 135–148. [Google Scholar] [CrossRef]
- Turner, S.C. Tracking Sulfur Diagenesis in Methane Rich Marine Sediments on the Cascadia Margin: Comparing Sulfur Isotopes of Bulk Sediment and Chromium Reducible Sulfur. Master’s Theses, University of New Hampshire, Durham, NH, USA, 2018. [Google Scholar]
- Torres, M.E.; Bohrmann, G.; Suess, E. Authigenic Barites and Fluxes of Barium Associated with Fluid Seeps in the Peru Subduction Zone. Earth Planet. Sci. Lett. 1996, 144, 469–481. [Google Scholar] [CrossRef]
- Greinert, J.; Bohrmann, G.; Suess, E. Gas Hydrate-Associated Carbonates and Methane-Venting at Hydrate Ridge: Classification, Distribution, and Origin of Authigenic Lithologies. Nat. Gas Hydrates Occur. Distrib. Detect. 2001, 124, 99–113. [Google Scholar]
- Yang, T.; Jiang, S.Y.; Ge, L.; Yang, J.H.; Wu, N.Y.; Zhang, G.X.; Liu, J.; Chen, D.H. Geochemistry of pore waters from HQ-1PC of the Qiongdongnan Basin, Northern South China Sea, and its implications for gas hydrate exploration. Sci. China Earth Sci. 2013, 56, 329–338. [Google Scholar] [CrossRef]
- Dickens, G.R. Sulfate Profiles and Barium Fronts in Sediment on the Blake Ridge: Present and Past Methane Fluxes through a Large Gas Hydrate Reservoir. Geochimica et Cosmochimica ACTA 2001, 65, 529–543. [Google Scholar] [CrossRef]
- Suess, E. Marine Cold Seeps: Background and Recent Advances. In Hydrocarbons, Oils and Lipids: Diversity, Origin, Chemistry and Fate. Handbook of Hydrocarbon and Lipid Microbiology; Springer: Cham, Switzerland, 2018; pp. 1–21. [Google Scholar]
- Griffith, E.M.; Paytan, A.; Wortmann, U.G.; Eisenhauer, A.; Scher, H.D. Combining metal and nonmetal isotopic measurements in barite to identify mode of formation. Chem. Geol. 2018, 500, 148–158. [Google Scholar] [CrossRef]
- Gingele, F.X.; Zabel, M.; Kasten, S.; Bonn, W.J.; Nürnberg, C.C. Biogenic Barium as a Proxy for Paleoproductivity: Methods and Limitations of Application. In Use of Proxies in Paleoceanography; Springer: Berlin, Germany, 1999. [Google Scholar]
- Riedinger, N.; Kasten, S.; GrÖger, J.; Franke, C.; Pfeifer, K. Active and buried authigenic barite fronts in sediments from the Eastern Cape Basin. Earth Planet. Sci. Lett. 2006, 241, 876–887. [Google Scholar] [CrossRef]
- Feng, D.; Roberts, H.H. Geochemical Characteristics of the Barite Deposits at Cold Seeps from the Northern Gulf of Mexico Continental Slope. Earth Planet. Sci. Lett. 2011, 309, 89–99. [Google Scholar] [CrossRef]
- Jiang, H.; Dong, H.; Ji, S.; Ye, Y.; Wu, N. Microbial Diversity in the Deep Marine Sediments from the Qiongdongnan Basin in South China Sea. Geomicrobiol. J. 2007, 24, 505–517. [Google Scholar] [CrossRef]
- Wu, S.; Yuan, S.; Zhang, G.; Ma, Y.; Mi, L.; Xu, N. Seismic Characteristics of a Reef Carbonate Reservoir and Implications for Hydrocarbon Exploration in Deepwater of the Qiongdongnan Basin, Northern South China Sea. Mar. Pet. Geol. 2009, 26, 817–823. [Google Scholar] [CrossRef]
- Wu, N.Y.; Ye, Y.; Wu, D.D.; Liu, J.; Zhang, P.P.; Jiang, H.C.; Dong, H.L.; Zhang, X.; Zhang, X.H.; Lei, Z.S. Geochemical characteristics of sediments from southeast Hainan Basin, South China Sea and micro-methane-seep activity. Geol. Res. South China Sea 2007, 00, 40–47. (In Chinese) [Google Scholar]
- Zhong, Z.H.; Wang, L.S.; Li, X.X.; Xia, B.; Sun, Z.; Zhang, M.Q.; Wu, G.G. The Paleogene Basin-filling evolution of Qiongdongnan Basin and its relation with seafloor spreading of the South China Sea. Mar. Geol. Quat. Geol. 2004, 24, 29–36. [Google Scholar]
- Yang, T.; Jiang, S.; Ge, L.; Yang, J.; Wu, N.; Zhang, G.; Liu, J. Geochemical Characteristics of Pore Water in Shallow Sediments from Shenhu Area of South China Sea and their Significance for Gas Hydrate Occurrence. Chin. Sci. Bull. 2010, 55, 752–760. [Google Scholar] [CrossRef]
- Chen, D.F.; Li, X.X.; Xia, B. Distribution of gas hydrate stable zones and resource prediction in the Qiongdongnan Basin of the South China Sea. Chin. J. Geophys. 2004, 47, 483–489. [Google Scholar] [CrossRef]
- Wu, B.H.; Zhang, G.X.; Zhu, Y.H.; Lu, Z.Q.; Chen, B.Y. Process of gas hydrate investigation in China offshore. Earth Sci. Front. 2003, 10, 177–189. [Google Scholar]
- He, J.X.; Su, P.B.; Lu, Z.Q.; Zhang, W.; Liu, Z.J.; Li, X.T. Prediction of Gas Sources of Natural Gas Hydrate in the Qiongdongnan Basin, Northern South China Sea, and its Nigration, Accumulation and Reservoir Formation Pattern. Nat. Gas Ind. 2015, 35, 19–29. [Google Scholar]
- Xu, T.; Spycher, N.; Sonnenthal, E.; Zhang, G.; Zheng, L.; Pruess, K. Toughreact Version 2.0: A Simulator for Subsurface Reactive Transport Under Non-Isothermal Multiphase Flow Conditions. Comput. Geosci. 2011, 37, 763–774. [Google Scholar] [CrossRef]
- Pruess, K. Tough2: A General-Purpose Numerical Simulator for Multiphase Fluid and Heat Flow. NasaSti/recon Technical Report N. Available online: https://cloudfront.escholarship.org/dist/prd/content/qt0wx8q119/qt0wx8q119.pdf (accessed on 7 February 2019).
- Pruess, K.; Oldenburg, C.M.; Moridis, G.J. Tough2 User’s Guide Version 2. Office of Scientific & Technical Information Technical Reports. 1999. Available online: http://citeseerx.ist.psu.edu/viewdoc/summary?doi=10.1.1.396.8810 (accessed on 7 February 2019).
- Dale, A.W.; Regnier, P.; Knab, N.J.; Jorgensen, B.B.; van Cappellen, P. Anaerobic Oxidation of Methane (AOM) in Marine Sediments from the Skagerrak (Denmark): II. Reaction-Transport Modeling. Geochimica et Cosmochimica ACTA 2008, 72, 2880–2894. [Google Scholar] [CrossRef]
- Dale, A.W.; van Cappellen, P.; Aguilera, D.R.; Regnier, P. Methane efflux from marine sediments in passive and active margins: Estimations from bioenergetic reaction–transport simulations. Earth Planet. Sci. Lett. 2008, 265, 329–344. [Google Scholar] [CrossRef]
- Xu, T.; Bei, K.; Tian, H.; Cao, Y. Laboratory Experiment and Numerical Simulation on Authigenic Mineral Formation Induced by Seabed Methane Seeps. Mar. Pet. Geol. 2017, 88, 950–960. [Google Scholar] [CrossRef]
- Wu, D.D.; Ye, Y.; Wu, N.Y.; Jiang, H.C.; Ji, S.S.; Dong, H.L.; Liu, J. Early diagenesis records and chemical composition abnormalities in pore water for methane-seep in sediments from the southern Qiongdongnan Basin. Acta Oceanol. Sin. 2009, 31, 86–96. [Google Scholar]
- Liu, L.; Wu, N. Simulation of Advective Methane Flux and Aom in Shenhu Area, the Northern South China Sea. Environ. Earth Sci. 2014, 71, 697–707. [Google Scholar] [CrossRef]
- Wang, J.; Guo, C.; Hou, Z.; Fu, Y.; Yan, J. Distributions and Vertical Variation Patterns of Sound Speed of Surface Sediments in South China Sea. J. Asian Earth Sci. 2014, 89, 46–53. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhu, W.; Mi, L.; Zhang, G.; Hu, S.; He, L. “Uniform Geothermal Gradient” and Heat Flow in the Qiongdongnan and Pearl River Mouth Basins of the South China Sea. Mar. Pet. Geol. 2009, 26, 1152–1162. [Google Scholar] [CrossRef]
- Wang, H.B.; Liang, J.; Gong, Y.H.; Huang, Y.Y.; Liu, X.W.; Sha, Z.B. Estimation of the Heat Flow in the Northern of the South China Sea Based on the Seismic Data of Gas Hydrate. Geoscience 2005, 19, 67–73. (In Chinese) [Google Scholar]
- Wu, L.S.; Yang, S.X.; Liang, J.Q.; Su, X.; Yang, T.; Zhang, X.; Cheng, S.H.; Lu, H.F. Geochemical characteristics of sediments at Site HQ-48PC in Qiongdongnan area, the north of the South China Sea, and their implication for gas hydrates. Geoscience 2010, 24, 534–544. (In Chinese) [Google Scholar]
- Mao, S.D.; Duan, Z.H.; Zhang, D.H.; Shi, L.L.; Chen, Y.L.; Li, J. Thermodynamic modeling of binary CH4–H2O fluid inclusions. Geochimica et Cosmochimica ACTA 2011, 75, 5892–5902. [Google Scholar] [CrossRef]
- Reed, M.H. Calculation of Multicomponent Chemical Equilibria and Reaction Processes in Systems Involving Minerals, Gases and an Aqueous Phase. Geochimica et Cosmochimica ACTA 1982, 46, 513–528. [Google Scholar] [CrossRef]
- Steefel, C.I.; Lasaga, A.C. A Coupled Model for Transport of Multiple Chemical Species and Kinetic Precipitation/Dissolution Reactions with Application to Reactive Flow in Single Phase Hydrothermal Systems. Amer. J. Sci. 1994, 294, 529–592. [Google Scholar] [CrossRef]
- Yeh, G.T.; Tripathi, V.S. A Model for Simulating Transport of Reactive Multispecies Components: Model Development and Demonstration. Water Resour. Res. 1991, 27, 3075–3094. [Google Scholar] [CrossRef]
- Xu, T.; Sonnenthal, E.; Spycher, N.; Pruess, K. Toughreact—A Simulation Program for Non-Isothermal Multiphase Reactive Geochemical Transport in Variably Saturated Geologic Media: Applications to Geothermal Injectivity and CO2 Geological Sequestration. Comput. Geosci. 2006, 32, 145–165. [Google Scholar] [CrossRef]
- Lasaga, A.C.; Soler, J.M.; Ganor, J.; Burch, T.E.; Nagy, K.L. Chemical Weathering Rate Laws and Global Geochemical Cycles. Geochimica et Cosmochimica ACTA 1994, 58, 2361–2386. [Google Scholar] [CrossRef]
- Palandri, J.L.; Kharaka, Y.K. A Compilation of Rate Parameters of Water mineral Interaction Kinetics for Application to Geochemical Modeling; Open-File Report 2004-1068; Geological Survey: Menlo Park, CA, USA, 2004. [CrossRef]








| Parameters | Meaning | Value | Unit |
|---|---|---|---|
| Half-saturated constant of methane | 1.5 × 10−3 | mol/L | |
| Half-saturated constant of sulfate | 1.0 × 10−3 | mol/L | |
| Maximum reaction rate of AOM | 1.62 × 10−9 | mol/L/s | |
| Minimum reaction concentration of methane | 1.0 × 10−9 | mol/L | |
| Minimum reaction concentration of sulfate | 6.0 × 10−9 | mol/L |
| Parameters | Value | Parameters | Value |
|---|---|---|---|
| Rock density | 2600 kg/m3 | Top pressure | 151 bar |
| Pore water density | 1025 kg/m3 | Top temperature | 3.3 °C |
| Porosity | 0.613 | Geothermal gradient in sediment | 3.76 °C/100 m |
| Permeability | 10−15 m2 | Sediment heat conductivity | 1.7 W·m−1·K−1 |
| Components | Sea Water Components (Top Boundary Water) (mol/L) | Sedimentary Water (mol/L) | Bottom Boundary Water (mol/L) |
|---|---|---|---|
| Ca2+ | 1.06 × 10−2 | 1.06 × 10−2 | 1.06 × 10−2 |
| Mg2+ | 5.31 × 10−2 | 5.31 × 10−2 | 5.31 × 10−2 |
| Na+ | 4.96 × 10−1 | 4.96 × 10−1 | 4.96 × 10−1 |
| K+ | 1.23 × 10−2 | 1.23 × 10−2 | 1.23 × 10−2 |
| Ba2+ | 6.00 × 10−7 | 6.00 × 10−7 | 6.00 × 10−7 |
| Fe2+ | 5.14 × 10−4 | 5.14 × 10−4 | 5.14 × 10−4 |
| SO42- | 2.82 × 10−2 | 1.00 × 10−6 | 1.00 × 10−6 |
| HCO3- | 2.00 × 10−3 | 2.00 × 10−3 | 2.00 × 10−3 |
| Cl- | 5.53 × 10−1 | 5.53 × 10−1 | 5.53 × 10−1 |
| CH4(aq) | 1.00 × 10−6 | 1.00 × 10−6 | 1.54 × 10−1 |
| Minerals | Volume Fraction (%) | Saturation Index (SI) | Minerals | Volume Fraction (%) | Saturation Index (SI) |
|---|---|---|---|---|---|
| Quartz | 15.3 | −1.98948 | Barite | 22.3 | −6.46652 |
| Illite | 25.6 | 9.26616 | Chlorite | 13.1 | −0.88747 |
| Kaolinite | 13.5 | 7.10012 | Albite | 2.3 | −0.75465 |
| Calcite | 7.9 | −6.83590 |
| Schemes | Methane Leakage Flux/mmol·cm−2·a−1 | Corresponding Saturated Flow/kg·s−1 |
|---|---|---|
| Base-case 1 | 5.95 × 10−3 (according to reports) | 1.2232 × 10−8 |
| Base-case 2 | 0 (no methane flux) | 1.2232 × 10−8 |
| Case 1 | 2.975 × 10−3 (0.5 times to Base-case 1) | 6.1160 × 10−9 |
| Case 2 | 2.975 × 10−2 (5 times to Base-case 1) | 6.1160 × 10−8 |
| Case 3 | 5.95 × 10−2 (10 times to Base-case 1) | 1.2232 × 10−7 |
| Case 4 | 1.19 × 10−1 (20 times to Base-case 1) | 2.4464 × 10−7 |
| Case 5 | 4.46 × 10−3 (0.75 times to Base-case 1) | 9.1700 × 10−9 |
| Case 6 | 4.46 × 10−2 (7.5 times to Base-case 1) | 9.1700 × 10−8 |
| Case 7 | 8.93 × 10−2 (15 times to Base-case 1) | 1.8300 × 10−7 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, T.; Shang, S.; Tian, H.; Bei, K.; Cao, Y. Numerical Simulation on Authigenic Barite Formation in Marine Sediments. Minerals 2019, 9, 98. https://doi.org/10.3390/min9020098
Xu T, Shang S, Tian H, Bei K, Cao Y. Numerical Simulation on Authigenic Barite Formation in Marine Sediments. Minerals. 2019; 9(2):98. https://doi.org/10.3390/min9020098
Chicago/Turabian StyleXu, Tianfu, Songhua Shang, Hailong Tian, Keqi Bei, and Yuqing Cao. 2019. "Numerical Simulation on Authigenic Barite Formation in Marine Sediments" Minerals 9, no. 2: 98. https://doi.org/10.3390/min9020098




