Detection of Trace Elements/Isotopes in Olympic Dam Copper Concentrates by nanoSIMS
Abstract
:1. Introduction
2. Background
3. Methods
4. Results and Discussion
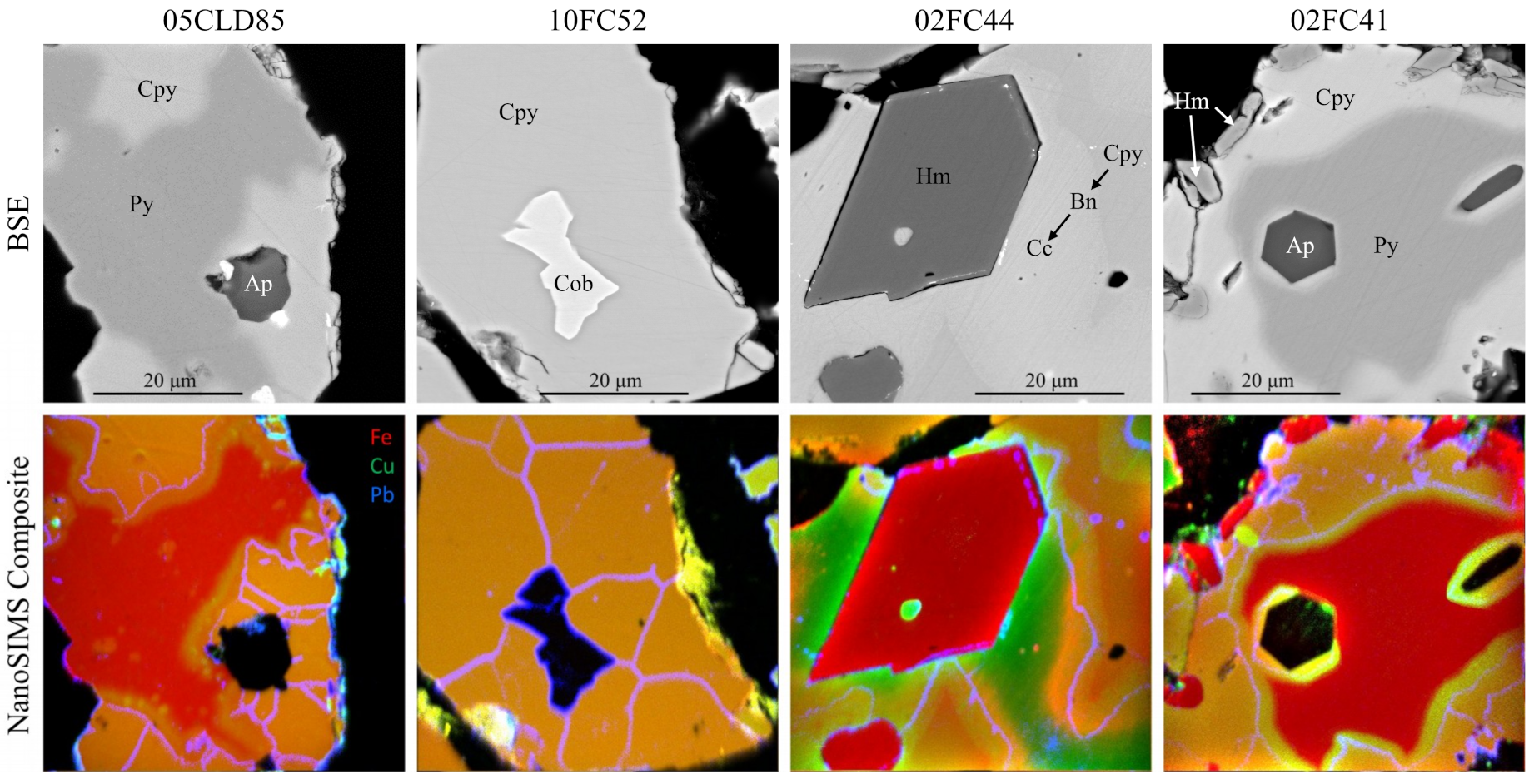
4.1. Precious Metals
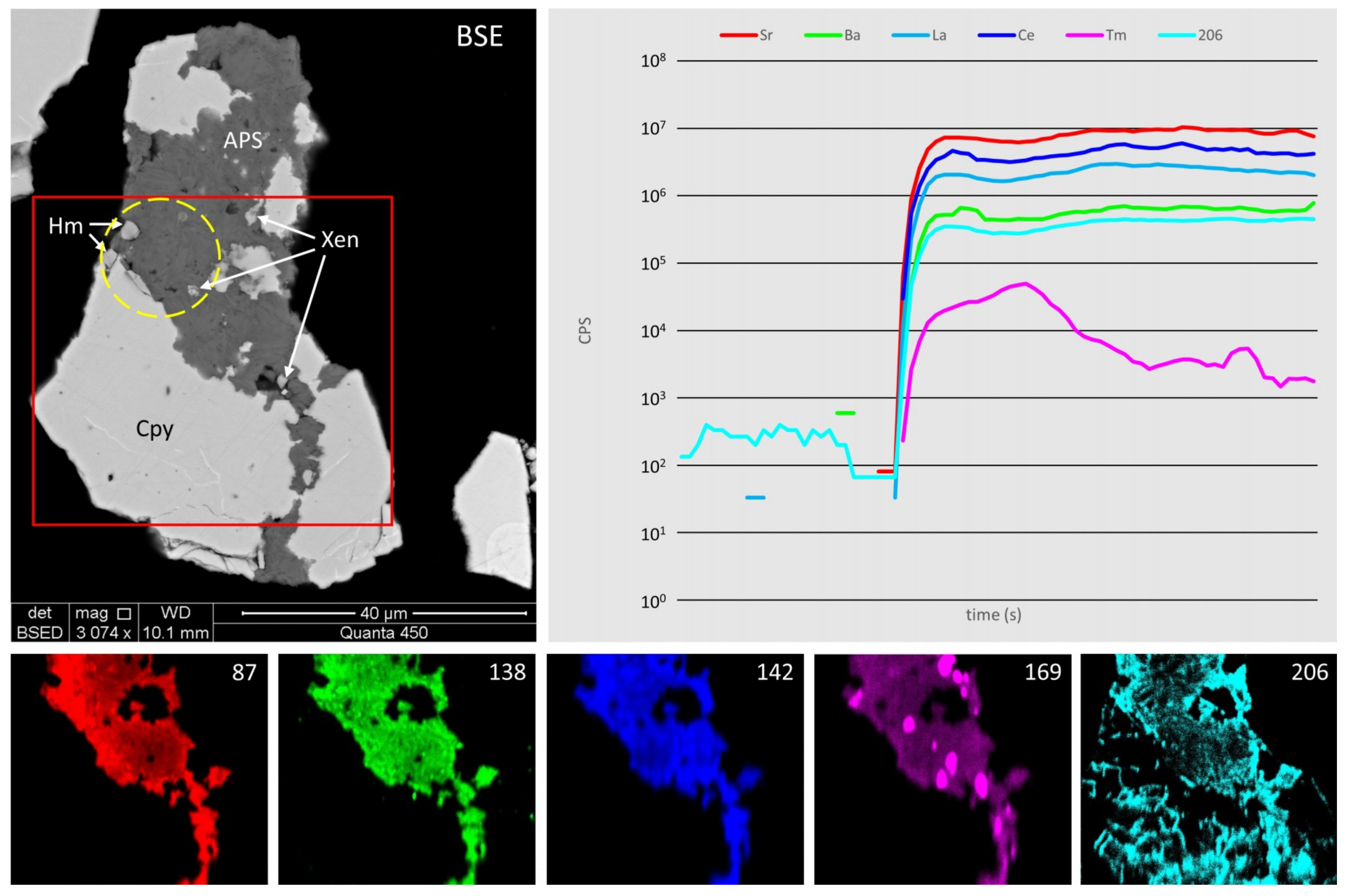
4.2. Rare Earth Elements
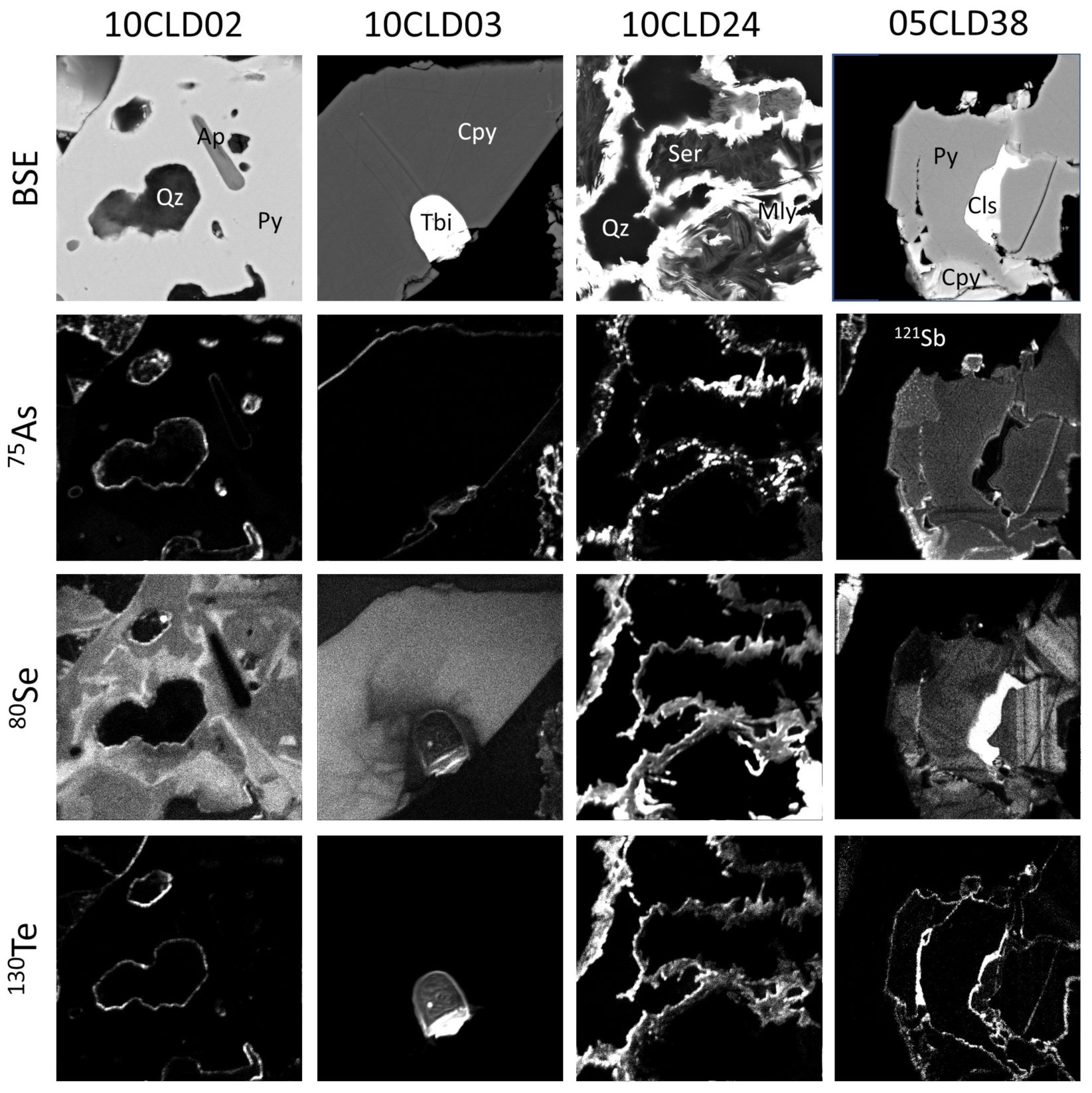
4.3. Penalty Elements
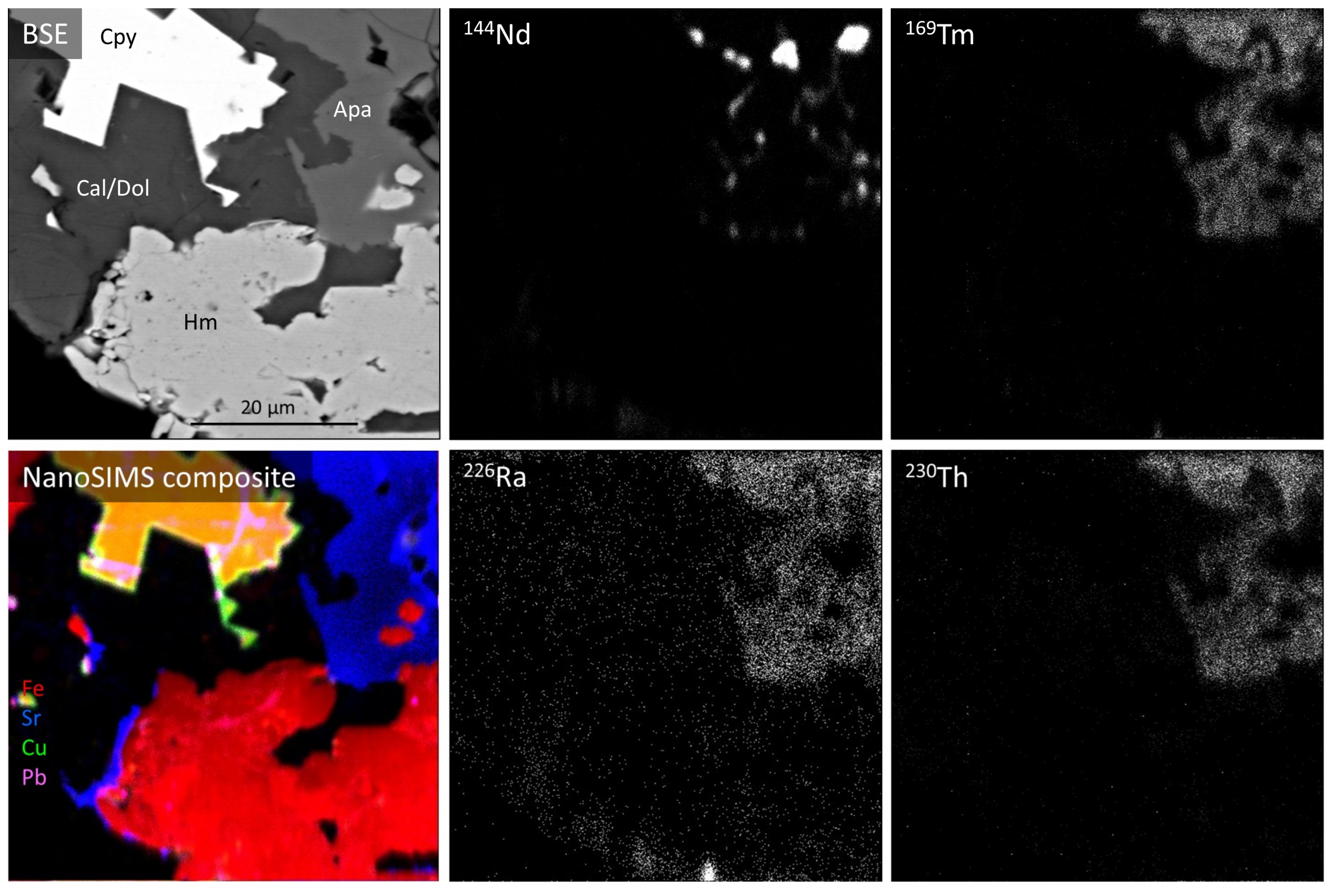
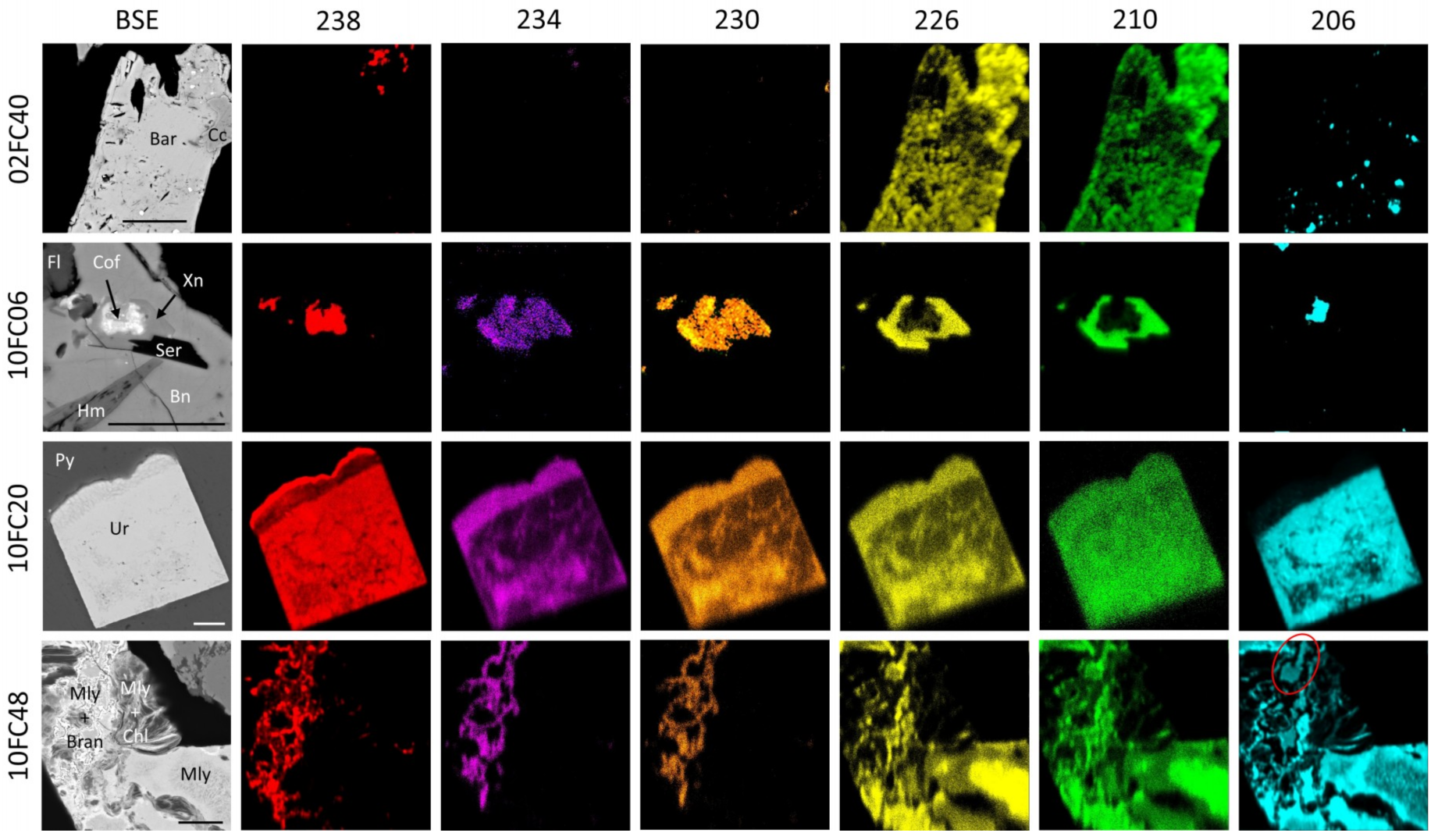
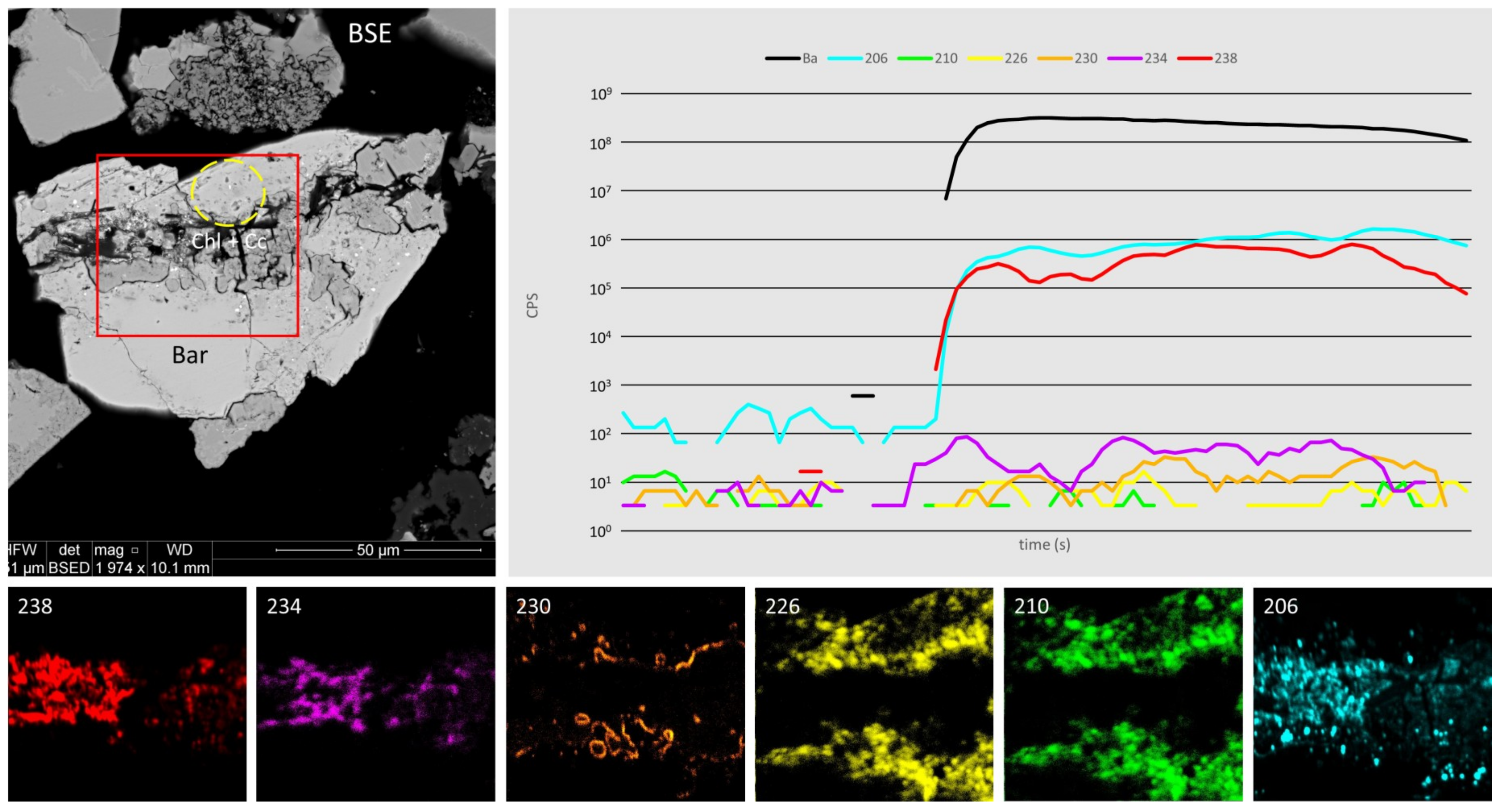
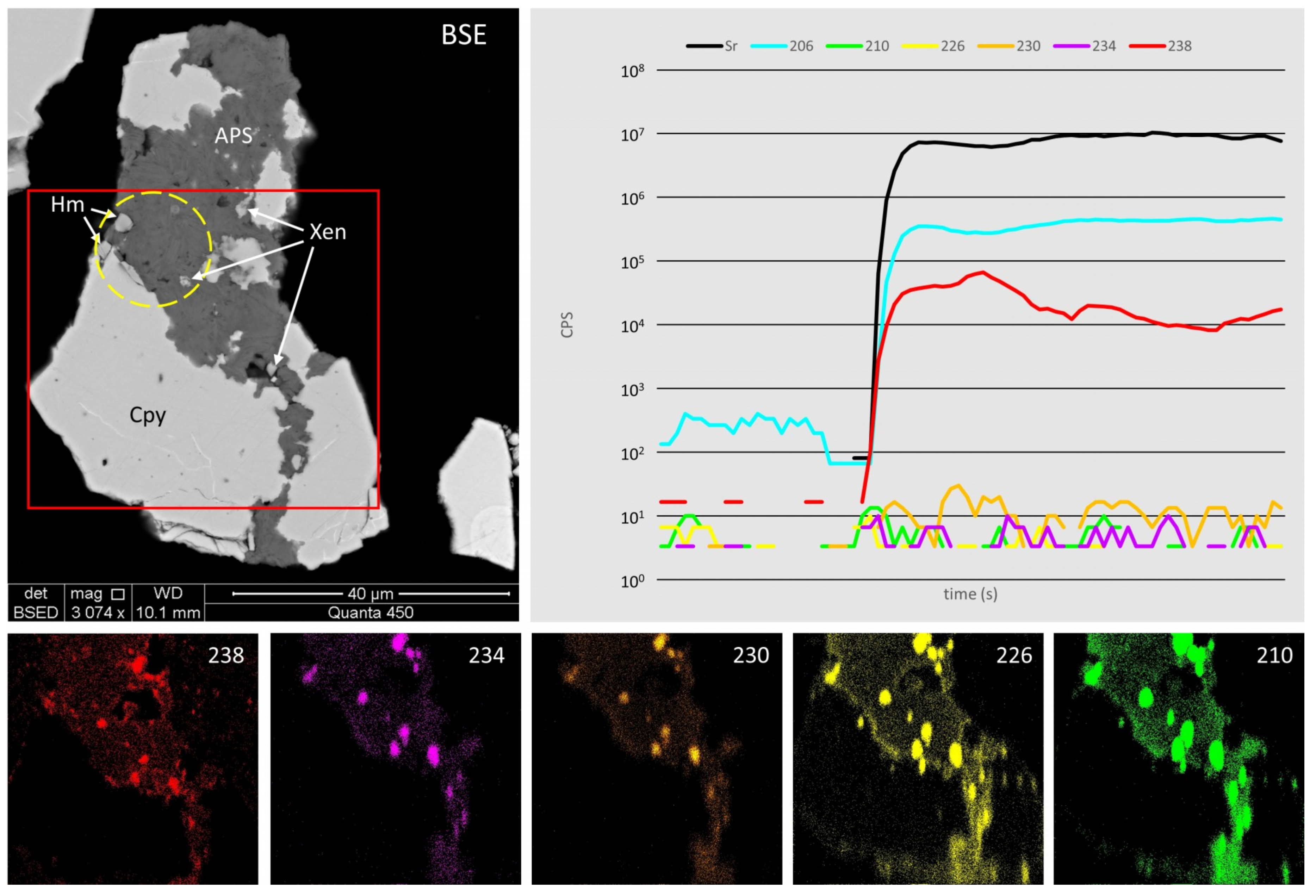
4.4. Radionuclide Distributions
4.5. Instrument Capabilities and Future Development
5. Conclusions
- (1)
- The combined sensitivity and resolution of the nanoSIMS analytical platform provides invaluable information regarding the deportment of trace elements and isotopes and the ultimate destination of components that have been remobilized or reconcentrated, both within the orebody and during mineral processing.
- (2)
- Precious metal extraction may benefit from identifying previously unknown host minerals.
- (3)
- Potentially economic elements may be monitored and archived for future extraction if commodity prices become favorable or game-changing processing methods are developed.
- (4)
- Deleterious “penalty” elements may be closely monitored and reduction/elimination techniques optimized based on new information, especially with respect to hitherto unrecognized host minerals.
- (5)
- Radionuclide daughters of the 238U decay chain may be located in situ within ores and concentrates and this information used to develop models of their behavior during copper ore processing that will be useful for optimization of extraction.
- (6)
- The nanoSIMS has proven a valuable tool in determining the spatial distribution of trace elements and isotopes in fine-grained copper ores. It complements observations and qualitative data obtained from other methods and offers unique insights into element/isotope distributions in cases where these are too low in absolute concentration and/or too fine to be resolved by other methods. NanoSIMS data can provide researchers with crucial evidence needed to answer questions of ore formation and ore processing.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lane, D.J.; Cook, N.J.; Grano, S.R.; Ehrig, K. Selective leaching of penalty elements from copper concentrates: A review. Miner. Eng. 2016, 98, 110–121. [Google Scholar] [CrossRef]
- Brown, R.J.; Milton, M.J. Analytical techniques for trace element analysis: An overview. TrAC Trends Anal. Chem. 2005, 24, 266–274. [Google Scholar] [CrossRef]
- Kilburn, M.R.; Wacey, D. Nanoscale secondary ion mass spectrometry (NanoSIMS) as an analytical tool in the geosciences. In Principles and Practice of Analytical Techniques in Geosciences; Royal Society of Chemistry: London, UK, 2015; pp. 1–34. [Google Scholar]
- Kilburn, M.R.; Clode, P.L. Elemental and isotopic imaging of biological samples using NanoSIMS. In Electron Microscopy; Humana Press: Totowa, NJ, USA, 2014; pp. 733–755. [Google Scholar]
- Gao, D.; Huang, X.; Tao, Y. A critical review of NanoSIMS in analysis of microbial metabolic activities at single-cell level. Crit. Rev. Biotechnol. 2016, 36, 884–890. [Google Scholar] [CrossRef]
- Hoppe, P. NanoSIMS: A new tool in cosmochemistry. Appl. Surf. Sci. 2006, 252, 7102–7106. [Google Scholar] [CrossRef]
- Valle, N.; Drillet, J.; Perlade, A.; Migeon, H.N. Application of SIMS nano-analysis to the development of new metallurgical solutions. Appl. Surf. Sci. 2008, 255, 1569–1571. [Google Scholar] [CrossRef]
- Yardley, S.S.; Moore, K.L.; Ni, N.; Wei, J.F.; Lyon, S.; Preuss, M.; Lozano-Perez, S.; Grovenor, C.R. An investigation of the oxidation behaviour of zirconium alloys using isotopic tracers and high resolution SIMS. J. Nucl. Mater. 2013, 443, 436–443. [Google Scholar] [CrossRef]
- Barker, S.L.L.; Hickey, K.A.; Cline, J.S.; Dipple, G.M.; Kilburn, M.; Vaughan, J.R.; Longo, A.A. Uncloaking invisible gold: Use of nanoSIMS to evaluate gold, trace elements, and sulfur isotopes in pyrite from Carlin-type gold deposits. Econ. Geol. 2009, 104, 897–904. [Google Scholar] [CrossRef]
- Fougerouse, D.; Micklethwaite, S.; Halfpenny, A.; Reddy, S.M.; Cliff, J.B.; Martin, L.A.J.; Kilburn, M.; Guagliardo, P.; Ulrich, S. The golden ark: Arsenopyrite crystal plasticity and the retention of gold through high strain and metamorphism. Terra Nova 2016, 28, 181–187. [Google Scholar] [CrossRef]
- Fougerouse, D.; Micklethwaite, S.; Tomkins, A.G.; Mei, Y.; Kilburn, M.; Guagliardo, P.; Fisher, L.A.; Halfpenny, A.; Gee, M.; Paterson, D.; et al. Gold remobilization and formation of high-grade ore shoots driven by dissolution-reprecipitation replacement and Ni substitution into auriferous arsenopyrite. Geochim. Cosmochim. Acta 2016, 178, 143–159. [Google Scholar] [CrossRef]
- Didier, A.; Bosse, V.; Bouloton, J.; Mostefaoui, S.; Viala, M.; Paquette, J.L.; Devidal, J.L.; Duhamel, R. NanoSIMS mapping and LA-ICP-MS chemical and U-Th-Pb data in monazite from a xenolith enclosed in andesite (Central Slovakia Volcanic Field). Contrib. Mineral. Petrol. 2016, 170, 45. [Google Scholar] [CrossRef]
- Zhang, J.; Lin, Y.; Yang, W.; Shen, W.; Hao, J.; Hu, S.; Cao, M. Improved precision and spatial resolution of sulfur isotope analysis using NanoSIMS. J. Anal. At. Spectrom. 2014, 29, 1934–1943. [Google Scholar] [CrossRef]
- Hauri, E.H.; Papineau, D.; Wang, J.; Hillion, F. High-precision analysis of multiple sulfur isotopes using NanoSIMS. Chem. Geol. 2016, 420, 148–161. [Google Scholar] [CrossRef] [Green Version]
- Ito, M.; Messenger, S. Isotopic imaging of refractory inclusions in meteorites with the NanoSIMS 50L. Appl. Surf. Sci. 2008, 255, 1446–1450. [Google Scholar] [CrossRef]
- Peteranderl, R.; Lechene, C. Measure of carbon and nitrogen stable isotope ratios in cultured cells. J. Am. Soc. Mass Spectrom. 2004, 15, 478–485. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; Lin, Y.T.; Zhang, J.C.; Hao, J.L.; Shen, W.J.; Hu, S. Precise micrometre-sized Pb-Pb and U-Pb dating with NanoSIMS. J. Anal. At. Spectrom. 2012, 27, 479–487. [Google Scholar] [CrossRef]
- Skirrow, R.G.; Bastrakov, E.; Davidson, G.J.; Raymond, O.L.; Heithersay, P. The Geological Framework, Distribution and Controls of Fe-oxide Cu-Au Mineralisation in the Gawler Craton, South Australia. Part II—Alteration and Mineralisation. In Hydrothermal Iron Oxide Copper-Gold & Related Deposits: A Global Perspective; Porter, T.M., Ed.; PGC Publishing: Adelaide, Australia, 2002; pp. 33–47. [Google Scholar]
- Skirrow, R.G.; Bastrakov, E.N.; Barovich, K.; Fraser, G.L.; Creaser, R.A.; Fanning, C.M.; Raymond, O.L.; Davidson, G.J. Timing of iron oxide Cu-Au-(U) hydrothermal activity and Nd isotope constraints on metal sources in the Gawler craton, South Australia. Econ. Geol. 2007, 102, 1441–1470. [Google Scholar] [CrossRef]
- Ehrig, K.; McPhie, J.; Kamenetsky, V.S. Geology and Mineralogical Zonation of the Olympic Dam Iron Oxide Cu-U-Au-Ag Deposit, South Australia. In Geology and Genesis of Major Copper Deposits and Districts of the World, a Tribute to Richard Sillitoe; Hedenquist, J.W., Harris, M., Camus, F., Eds.; Society of Economic Geologists Special Publication 16: Littleton, CO, USA, 2012; pp. 237–267. [Google Scholar]
- Ehrig, K.; Kamenetsky, V.; McPhie, J.; Cook, N.J.; Ciobanu, C.L. Olympic Dam iron-oxide Cu-U-Au-Ag deposit. In Australian Ore Deposits; Phillips, G.N., Ed.; AusIMM: Melbourne, Australian, 2017; pp. 601–610. [Google Scholar]
- Roberts, D.E.; Hudson, G.R.T. The Olympic Dam copper-uranium-gold deposit, Roxby Downs, South Australia. Econ. Geol. 1983, 78, 799–822. [Google Scholar] [CrossRef]
- Reeve, J.S.; Cross, K.C.; Smith, R.N.; Oreskes, N. Olympic Dam copper-uranium-gold-silver deposit. In Geology of the Mineral Deposits of Australia and Papua New Guinea; Hughes, F.E., Ed.; AusIMM: Melbourne, Australian, 1990; pp. 1009–1035. [Google Scholar]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Ciobanu, C.L.; Pring, A. Uraninite from the Olympic Dam IOCG-U-Ag deposit: Linking textural and compositional variation to temporal evolution. Am. Mineral. 2016, 101, 1295–1320. [Google Scholar] [CrossRef]
- Macmillan, E.; Ciobanu, C.L.; Ehrig, K.; Cook, N.J.; Pring, A. Chemical zoning and lattice distortion in uraninite from Olympic Dam, South Australia. Am. Mineral. 2016, 101, 2351–2354. [Google Scholar] [CrossRef] [Green Version]
- Macmillan, E.; Ciobanu, C.L.; Ehrig, K.; Cook, N.J.; Pring, A. Replacement of uraninite by bornite via coupled dissolution-reprecipitation: Evidence from texture and microstructure. Can. Mineral. 2016, 54, 1369–1383. [Google Scholar] [CrossRef]
- Macmillan, E.; Cook, N.J.; Ehrig, K.; Pring, A. Chemical and textural interpretation of late-stage coffinite and brannerite from the Olympic Dam IOCG-Ag-U deposit. Mineral. Mag. 2017, 81, 1323–1366. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Cook, N.J.; Ehrig, K. Ore minerals down to the nanoscale: Cu-(Fe)-sulphides from the iron oxide copper gold deposit at Olympic Dam, South Australia. Ore Geol. Rev. 2017, 81, 1218–1235. [Google Scholar] [CrossRef]
- Schmandt, D.S.; Cook, N.J.; Ehrig, K.; Gilbert, S.; Wade, B.P.; Rollog, M.; Ciobanu, C.L.; Kamenetsky, V.S. Uptake of trace elements by baryte during copper ore processing: A case study from Olympic Dam, South Australia. Miner. Eng. 2019, 135, 83–94. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Wade, B.P.; Cook, N.J.; Schmidt Mumm, A.; Giles, D. Uranium-bearing hematite from the Olympic Dam Cu-U-Au deposit, South Australia: A geochemical tracer and reconnaissance Pb-Pb geochronometer. Precambrina Res. 2013, 238, 129–147. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Slattery, A.; Verdugo-Ihl, M.R.; Courtney-Davies, L.; Gao, W. Advances and opportunities in ore mineralogy. Minerals 2017, 7, 233. [Google Scholar] [CrossRef]
- Verdugo-Ihl, M.R.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Courtney-Davies, L.; Gilbert, S. Textures and U-W-Sn-Mo signatures in hematite from the Cu-U-Au-Ag orebody at Olympic Dam, South Australia: Defining the archetype for IOCG deposits. Ore Geol. Rev. 2017, 91, 173–195. [Google Scholar] [CrossRef]
- Schmandt, D.S.; Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Wade, B.P.; Gilbert, S.; Kamenetsky, V.S. Rare Earth Element Fluorocarbonate Minerals from the Olympic Dam Cu-U-Au-Ag Deposit, South Australia. Minerals 2017, 7, 202. [Google Scholar] [CrossRef]
- Schmandt, D.S.; Cook, N.J.; Ciobanu, C.L.; Ehrig, K.; Wade, B.P.; Gilbert, S.; Kametetsky, V.S. Rare earth element phosphate minerals from the Olympic Dam Cu-U-Au-Ag deposit, South Australia: Recognizing temporal-spatial controls on REE mineralogy in an evolved IOCG systems. Can. Mineral. 2019, 57, 3–24. [Google Scholar] [CrossRef]
- Krneta, S.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Kontonikas-Charos, A. Rare Earth Element Behaviour in Apatite from the Olympic Dam Cu-U-Au-Ag Deposit, South Australia. Minerals 2017, 7, 135. [Google Scholar] [CrossRef]
- Schmandt, D.S. Mineralogical Distribution of Radionuclides in Copper-Uranium Ores, Olympic Dam, South Australia. Ph.D. Thesis, The University of Adelaide, Adelaide, Australia, 2019; 371p. [Google Scholar]
- Owen, N.D.; Cook, N.J.; Rollog, M.; Ehrig, K.J.; Schmandt, D.S.; Ram, R.; Brugger, J.; Ciobanu, C.L.; Wade, B.; Guagliardo, P. REE-, Sr- Ca-aluminum-phosphate-sulfate minerals of the alunite supergroup and their role as hosts for radionuclides. Am. Mineral. 2019. in review. [Google Scholar]
- BHP Annual Report 2018. Section 6. 2018, p. 259. Available online: www.bhp.com (accessed on 25 March 2019).
- BHP Billiton Olympic Dam Expansion Supplementary Environmental Impact Statement. Chapter 26. 2011, pp. 689–758. Available online: www.bhp.com (accessed on 25 May 2019).
- Rollog, M.; Cook, N.J.; Guagliardo, P.; Ehrig, K.; Kilburn, M. In Situ spatial distribution mapping of radionuclides in minerals by nanoSIMS. Geochem. Explor. Environ. Anal. 2019. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The ImageJ ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [Green Version]
- Poczatek, C.; Kaufman, Z.; Lechene, C. OpenMIMS ImageJ Plugin Guide; Harvard Medical School: Boston, MA, USA, 2009. [Google Scholar]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 1988. [Google Scholar]
- Mironov, A.G.; Karmanov, N.S.; Mironov, A.A.; Khodyreva, E.V. Gold-brannerite nuggets in placers of the Ozernoe ore cluster (Buryatia). Russ. Geol. Geophys. 2008, 49, 743–748. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; Danyushevsky, L.V.; Gilbert, S. Minor elements in bornite and associated Cu-(Fe)-sulfides: A LA-ICPMS study. Geochim. Cosmochim. Acta 2011, 73, 4761–4791. [Google Scholar] [CrossRef]
- Wasserstein, B. Precision lattice measurements of galena. Am. Mineral. 1951, 36, 102–115. [Google Scholar]
- Evans, N.J.; Byrne, J.P.; Keegan, J.T.; Dotter, L.E. Determination of uranium and thorium in zircon, apatite, and fluorite: Application to laser (U-Th)/He thermochronology. J. Anal. Chem. 2005, 60, 1159–1165. [Google Scholar] [CrossRef]
- Dill, H.G. The geology of aluminum phosphates and sulfates of the alunite group minerals: A review. Earth-Sci. Rev. 2001, 53, 35–93. [Google Scholar] [CrossRef]
- Palache, C.; Berman, H.; Frondel, C.; Dana, E.S. The System of Mineralogy of James Dwight Dana and Edward Salisbury Dana, Yale University, 1837–1892: Halides, Nitrates, Borates, Carbonates, Sulfates, Phosphates, Arsenates, Tungstates, Molybdates, etc; John Wiley and Sons: Hoboken, NJ, USA, 1951. [Google Scholar]
- Åmli, R. Mineralogy and-Rare Earth Geochemistry of Apatite and Xenotime from the Gloserheia Granite Pegmatite, Froland, Southern Norway. Am. Mineral. 1975, 60, 607–620. [Google Scholar]
- Ciobanu, C.L.; Cook, N.J.; Kelson, C.R.; Guerin, R.; Kalleske, N.; Danyushevsky, L. Trace element heterogeneity in molybdenite fingerprints stages of mineralization. Chem. Geol. 2013, 347, 175–189. [Google Scholar] [CrossRef]
- Owen, N.D.; Ciobanu, C.L.; Cook, N.J.; Slattery, A.; Basak, A. Nanoscale study of clausthalite-bearing symplectites in Cu-Au-(U) ores: Implications for ore genesis. Minerals 2018, 8, 67. [Google Scholar] [CrossRef]
- Dmitrijeva, M.; Ehrig, K.J.; Ciobanu, C.L.; Cook, N.J.; Verdugo-Ihl, M.R.; Metcalfe, A.V. Defining IOCG signatures through compositional data analysis: A case study of lithogeochemical zoning from the Olympic Dam deposit, South Australia. Ore Geol. Rev. 2019, 105, 86–101. [Google Scholar] [CrossRef]
- Doerner, H.A.; Hoskins, W.M. Co-precipitation of radium and barium sulphates. J. Am. Chem. Soc. 1925, 47, 662–715. [Google Scholar] [CrossRef]
- Itzkovitch, I.J.; Ritcey, G.M. Removal of Radionuclides from Process Streams—A Survey; CANMET Report 79-21; Energy, Mines, and Resources Canada: Ottawa, ON, Canada, 1979; 171p.
- Smith, A.L. Radioactive-scale formation. J. Pet. Technol. 1987, 39, 697–706. [Google Scholar] [CrossRef]
- Putnis, A. Mineral replacement reactions. Rev. Mineral. Geochem. 2009, 70, 87–124. [Google Scholar] [CrossRef]
- Cook, N.J.; Ciobanu, C.L.; George, L.; Ehrig, K. Trace element analysis of minerals in magmatic-hydrothermal ores by laser ablation inductively-coupled plasma mass spectrometry: Approaches and opportunities. Minerals 2016, 6, 111. [Google Scholar] [CrossRef]
- Kontonikas-Charos, A.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Krneta, S.; Kamenetsky, V.S. Feldspar evolution in the Roxby Downs Granite, host to Fe-oxide Cu-Au-(U) mineralisation at Olympic Dam, South Australia. Ore Geol. Rev. 2017, 80, 838–859. [Google Scholar] [CrossRef]
- Courtney-Davies, L.; Tapster, S.R.; Ciobanu, C.L.; Cook, N.J.; Verdugo-Ihl, M.R.; Ehrig, K.J.; Kennedy, A.K.; Gilbert, S.E.; Condon, D.J.; Wade, B.P. A multi-technique evaluation of hydrothermal hematite U-Pb isotope systematics: Implications for ore deposit geochronology. Chem. Geol. 2019, 513, 54–72. [Google Scholar] [CrossRef]
- Miller, M.K. Atom Probe Tomography: Analysis at the Atomic Level; Springer Science & Business Media: New York, NY, USA, 2012; 239p. [Google Scholar]
- Ayache, J.; Beaunier, L.; Boumendil, J.; Ehret, G.; Laub, D. Sample Preparation Handbook for Transmission Electron Microscopy: Techniques; Springer Science & Business Media: New York, NY, USA, 2010; Volume 2. [Google Scholar]
- Ciobanu, C.L.; Kontonikas-Charos, A.; Slattery, A.; Cook, N.J.; Ehrig, K.; Wade, B.P. Short-range stacking disorder in mixed-layer compounds: A HAADF STEM study of bastnäsite-parisite intergrowths. Minerals 2017, 7, 227. [Google Scholar] [CrossRef]
- Kontonikas-Charos, A.; Ciobanu, C.L.; Cook, N.J.; Ehrig, K.; Ismail, R.; Krneta, S.; Basak, A. Feldspar mineralogy and rare earth element (re)mobilization in iron-oxide copper gold systems from South Australia: A nanoscale study. Mineral. Mag. 2018, 82, S173–S197. [Google Scholar] [CrossRef]
- Ciobanu, C.L.; Verdugo-Ihl, M.R.; Slattery, A.; Cook, N.J.; Ehrig, K.; Courtney-Davies, L.; Wade, B.P. Silician magnetite: Si-Fe-nanoprecipitates and other mineral inclusions in magnetite from the Olympic Dam deposit, South Australia. Minerals 2019, 9, 311. [Google Scholar] [CrossRef]
- Slodzian, G.; Hillion, F.; Stadermann, F.J.; Horreard, F. Oxygen isotopic measurements on the CAMECA NanoSIMS 50. Appl. Surf. Sci. 2003, 203, 798–801. [Google Scholar] [CrossRef]
- Wilson, R.G.; Novak, S.W. Systematics of secondary-ion-mass spectrometry relative sensitivity factors versus electron affinity and ionization potential for a variety of matrices determined from implanted standards of more than 70 elements. J. Appl. Phys. 1991, 69, 466–474. [Google Scholar] [CrossRef]







| Collection Date | ||||
|---|---|---|---|---|
| Sample Type | 08/2015 | 11/2016 | 12/2016 | 12/2017 |
| Flotation concentrate (FC) | 13 | 43 | 16 | |
| Acid-leached concentrate (CLD) | 51 | 31 | 44 | |
| Drill core samples | 7 | |||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rollog, M.; Cook, N.J.; Guagliardo, P.; Ehrig, K.; Ciobanu, C.L.; Kilburn, M. Detection of Trace Elements/Isotopes in Olympic Dam Copper Concentrates by nanoSIMS. Minerals 2019, 9, 336. https://doi.org/10.3390/min9060336
Rollog M, Cook NJ, Guagliardo P, Ehrig K, Ciobanu CL, Kilburn M. Detection of Trace Elements/Isotopes in Olympic Dam Copper Concentrates by nanoSIMS. Minerals. 2019; 9(6):336. https://doi.org/10.3390/min9060336
Chicago/Turabian StyleRollog, Mark, Nigel J. Cook, Paul Guagliardo, Kathy Ehrig, Cristiana L. Ciobanu, and Matt Kilburn. 2019. "Detection of Trace Elements/Isotopes in Olympic Dam Copper Concentrates by nanoSIMS" Minerals 9, no. 6: 336. https://doi.org/10.3390/min9060336





