Evidence for a Carbonatite-Influenced Source Assemblage for Intraplate Basalts from the Buckland Volcanic Province, Queensland, Australia
Abstract
:1. Introduction
2. Geological Setting
3. Analytical Methods
3.1. Whole-Rock Geochemistry
3.2. Olivine Analyses
4. Results
4.1. Whole-Rock Major and Minor Element Compositions
4.2. Whole-Rock Incompatible Elements
4.3. Olivine Chemistry
5. Discussion
5.1. Constraints on Igneous Processes in the Buckland Magma Source Region
5.2. Constraints on Source Mineralogy from Whole-Rock Geochemistry
5.3. Olivine/Melt Equilibrium in the Buckland Rocks
Characterising Olivine Xenocrysts
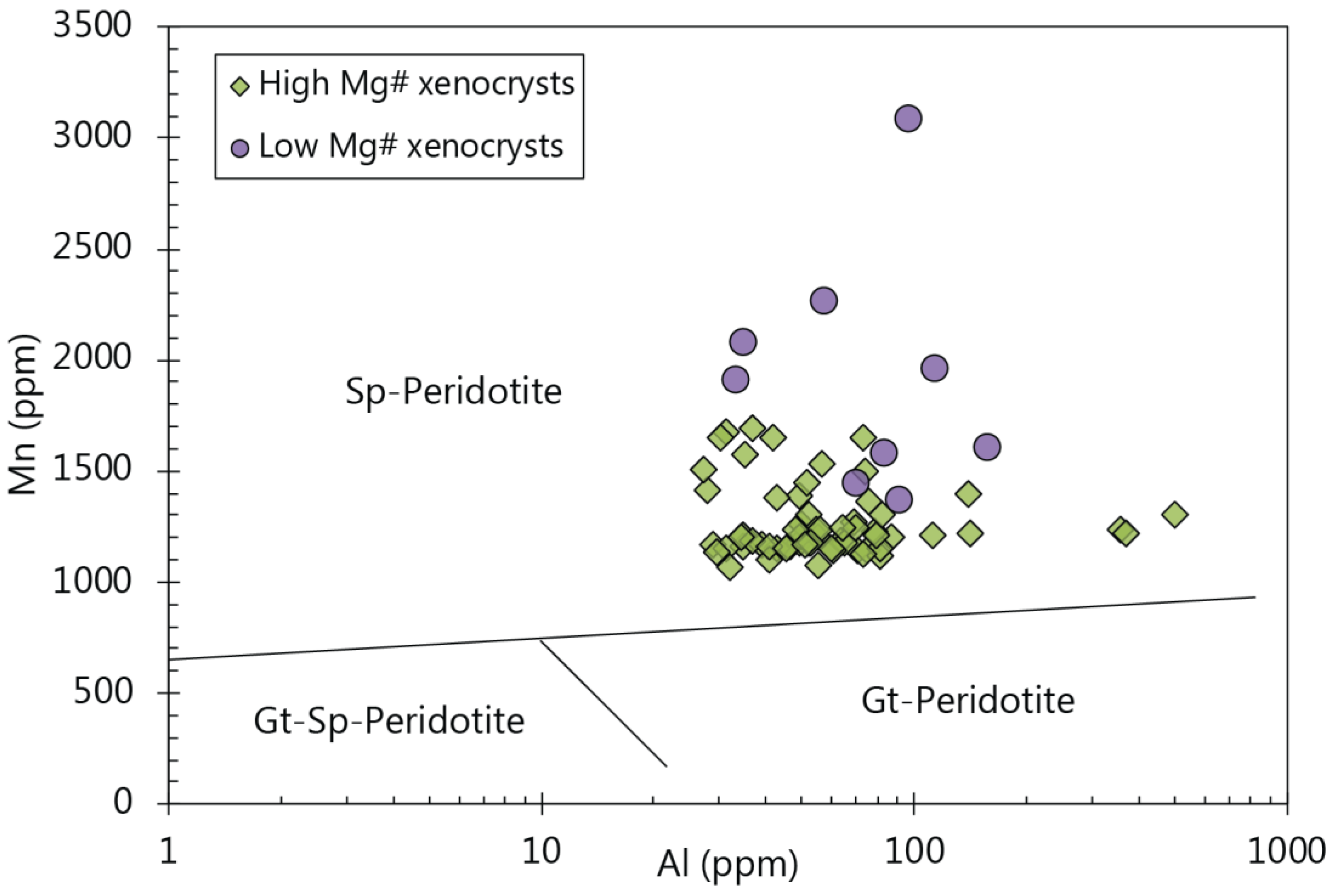
5.4. Constraints on Using Olivine Chemistry as a Source Assemblage Indicator
5.5. Possible Source Indicators from Minor and Trace Elements in Olivine
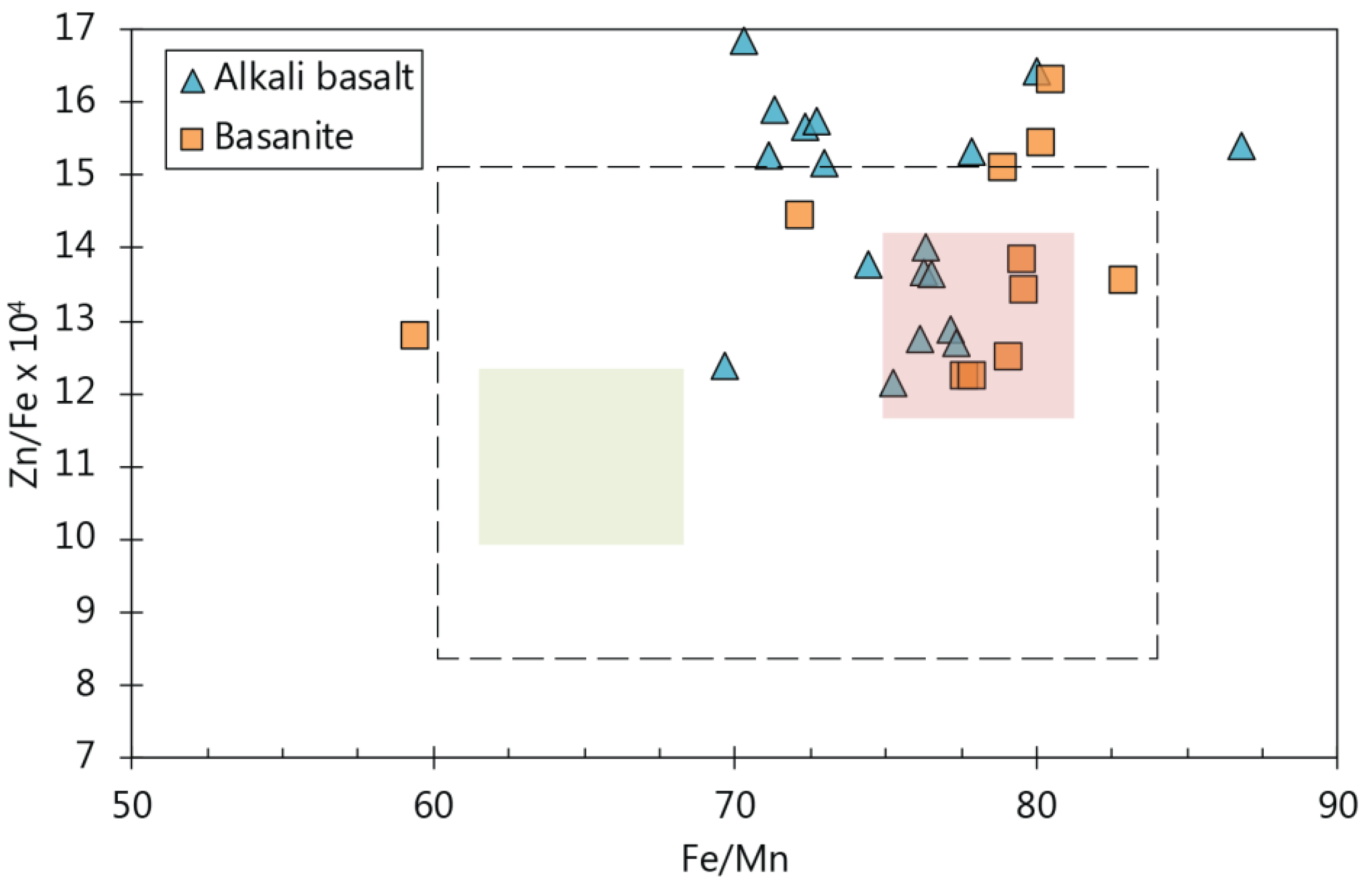
5.5.1. Indicators for a Pyroxene-Rich Source Assemblage
5.5.2. Possible Indicators for Clinopyroxene and Amphibole in the Source
5.5.3. Elevated P and Li Concentrations
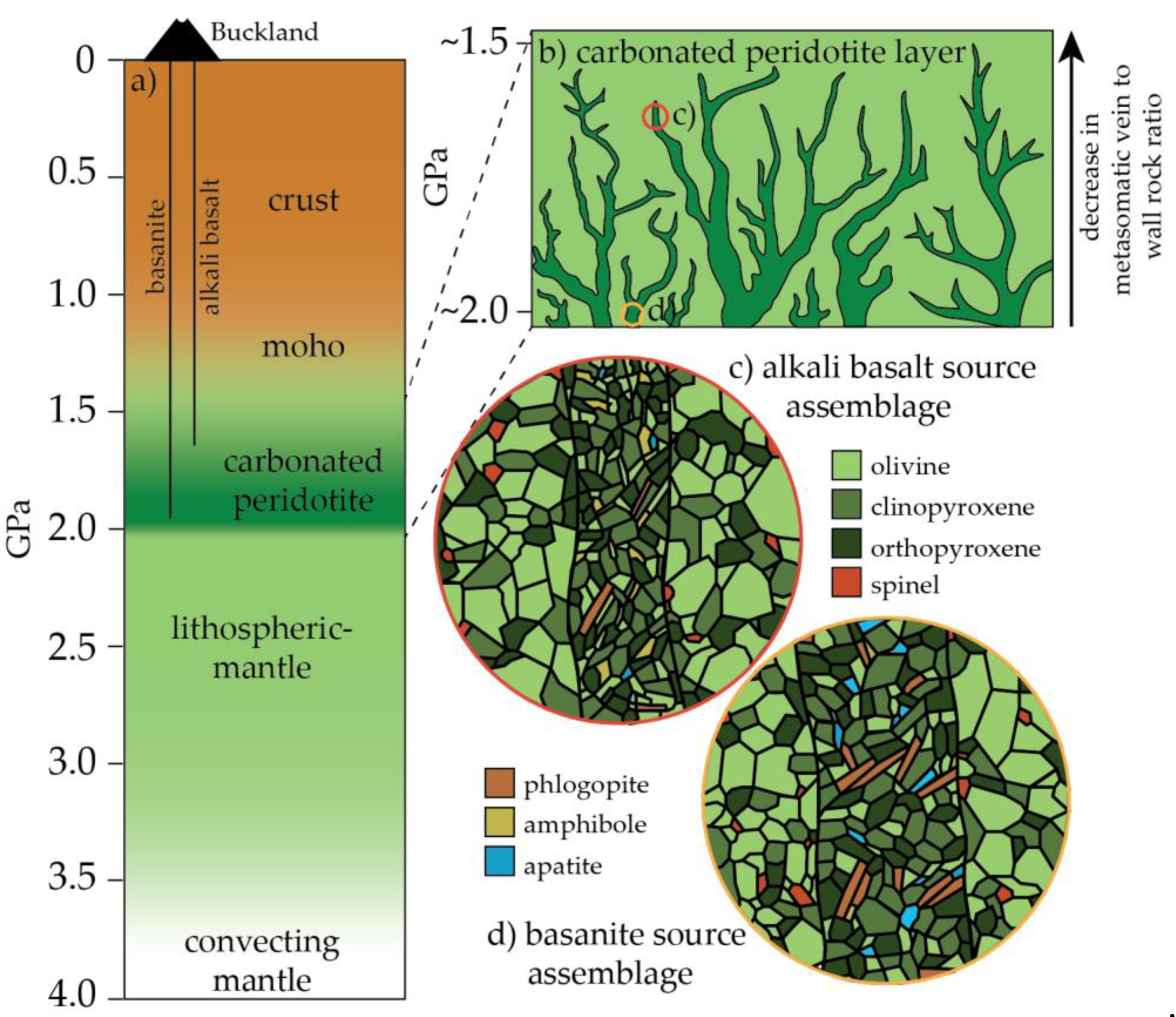
5.6. A Carbonated Peridotite Source Assemblage for the Buckland Volcanic Province
6. Conclusions
- Carbonate-rich melt sourced below the thicker lithosphere to the southwest of the Buckland volcanic province modified the Buckland source to locally produce olivine-websterite lithologies. This may be a widespread process in eastern Australia due to many regional variations in the topography of the lithosphere base.
- Amphibole and clinopyroxene reaction products caused by a carbonatite metasomatic agent reacting with mantle peridotite in the Buckland source region can be recognised in the chemistry of the olivine phenocrysts.
- A combination of olivine compositions and whole-rock geochemistry irons out many uncertainties in interpretations that can arise from using olivine compositions alone.
- High Mg# olivine xenocrysts are probably derived from xenoliths above the metasomatised horizon.
- Low Mg# olivine xenocrysts have many minor and trace element signatures that reflect mantle peridotite. They may be formed in an enriched domain where the minor and trace elements have had time to re-equilibrate between sub-solidus phases.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Herzberg, C. Identification of Source Lithology in the Hawaiian and Canary Islands: Implications for Origins. J. Petrol. 2011, 52, 113–146. [Google Scholar] [CrossRef]
- Yang, Z.-F.; Li, J.; Liang, W.-F.; Luo, Z.-H. On the chemical markers of pyroxenite contributions in continental basalts in Eastern China: Implications for source lithology and the origin of basalts. Earth Sci. Rev. 2016, 157, 18–31. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.-Q.; Zheng, F.; Zhao, Z.-F.; Zheng, Y.-F. Geochemical insights into the lithology of mantle sources for Cenozoic alkali basalts in West Qinling, China. Lithos 2018, 302–303, 86–98. [Google Scholar] [CrossRef]
- Heinonen, J.S.; Fusswinkel, T. High Ni and low Mn/Fe in olivine phenocrysts of the Karoo meimechites do not reflect pyroxenitic mantle sources. Chem. Geol. 2017, 467, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Herzberg, C.; Vidito, C.; Starkey, N.A. Nickel–cobalt contents of olivine record origins of mantle peridotite and related rocks. Am. Mineral. 2016, 101, 1952–1966. [Google Scholar] [CrossRef]
- Howarth, G.H.; Harris, C. Discriminating between pyroxenite and peridotite sources for continental flood basalts (CFB) in southern Africa using olivine chemistry. Earth Planet. Sci. Lett. 2017, 475, 143–151. [Google Scholar] [CrossRef]
- Lambart, S.; Laporte, D.; Provost, A.; Schiano, P. Fate of Pyroxenite-derived Melts in the Peridotitic Mantle: Thermodynamic and Experimental Constraints. J. Petrol. 2012, 53, 451–476. [Google Scholar] [CrossRef]
- le Roex, A.; Class, C. Metasomatic enrichment of Proterozoic mantle south of the Kaapvaal Craton, South Africa: origin of sinusoidal REE patterns in clinopyroxene and garnet. Contrib. Mineral. Petrol. 2016, 171, 14. [Google Scholar] [CrossRef]
- Green, D.H. The origin of basaltic and nephelinitic magmas. Trans. Leic. Philos. Lit. Soc. 1970, 64, 28–54. [Google Scholar]
- Frey, F.A.; Green, D.H. The mineralogy, geochemistry and origin of Iherzolite inclusions in Victorian basanites. Geochim. Cosmochim. Acta 1974, 38, 1023–1059. [Google Scholar] [CrossRef]
- Frey, F.A.; Green, D.H.; Roy, S.D. Integrated Models of Basalt Petrogenesis: A Study of Quartz Tholeiites to Olivine Melilitites from South Eastern Australia Utilizing Geochemical and Experimental Petrological Data. J. Petrol. 1978, 19, 463–513. [Google Scholar] [CrossRef]
- Brey, G. Origin of olivine melilitites—Chemical and experimental constraints. J. Volcanol. Geotherm. Res. 1978, 3, 61–88. [Google Scholar] [CrossRef]
- Foley, S. Vein-plus-wall-rock melting mechanisms in the lithosphere and the origin of potassic alkaline magmas. Lithos 1992, 28, 435–453. [Google Scholar] [CrossRef]
- Wass, S.Y.; Henderson, P.; Elliott, C.J.; Bailey, D.K.; Tarney, J.; Dunham Kingsley, C. Chemical heterogeneity and metasomatism in the upper mantle: evidence from rare earth and other elements in apatite-rich xenoliths in basaltic rocks from eastern Australia. Philos. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1980, 297, 333–346. [Google Scholar] [CrossRef]
- O’Reilly, S.Y.; Griffin, W.L. Apatite in the mantle: implications for metasomatic processes and high heat production in Phanerozoic mantle. Lithos 2000, 53, 217–232. [Google Scholar] [CrossRef]
- Foley, S.; Musselwhite, D.; Van der Laan, S. Melt compositions from ultramafic vein assemblages in the lithospheric mantle: a comparison of cratonic and non-cratonic settings. In Proceedings of the VIIth International Kimberlite Conference, Cape Town, South Africa, 11–17 April 1998; pp. 238–246. [Google Scholar]
- Pilet, S.; Baker, M.B.; Stolper, E.M. Metasomatized Lithosphere and the Origin of Alkaline Lavas. Science 2008, 320, 916. [Google Scholar] [CrossRef]
- Adam, J. The Geochemistry and Experimental Petrology of Sodic Alkaline Basalts from Oatlands, Tasmania. J. Petrol. 1990, 31, 1201–1223. [Google Scholar] [CrossRef]
- Pilet, S.; Baker, M.B.; Müntener, O.; Stolper, E.M. Monte Carlo Simulations of Metasomatic Enrichment in the Lithosphere and Implications for the Source of Alkaline Basalts. J. Petrol. 2011, 52, 1415–1442. [Google Scholar] [CrossRef] [Green Version]
- Pilet, S.; Ulmer, P.; Villiger, S. Liquid line of descent of a basanitic liquid at 1.5 GPa: constraints on the formation of metasomatic veins. Contrib. Mineral. Petrol. 2010, 159, 621–643. [Google Scholar] [CrossRef]
- Sobolev, A.V.; Hofmann, A.W.; Sobolev, S.V.; Nikogosian, I.K. An olivine-free mantle source of Hawaiian shield basalts. Nature 2005, 434, 590–597. [Google Scholar] [CrossRef]
- Sobolev, A.V.; Hofmann, A.W.; Kuzmin, D.V.; Yaxley, G.M.; Arndt, N.T.; Chung, S.-L.; Danyushevsky, L.V.; Elliott, T.; Frey, F.A.; Garcia, M.O.; et al. The Amount of Recycled Crust in Sources of Mantle-Derived Melts. Science 2007, 316, 412. [Google Scholar] [CrossRef]
- Foley, S.F.; Prelevic, D.; Rehfeldt, T.; Jacob, D.E. Minor and trace elements in olivines as probes into early igneous and mantle melting processes. Earth Planet. Sci. Lett. 2013, 363, 181–191. [Google Scholar] [CrossRef]
- Matzen, A.K.; Baker, M.B.; Beckett, J.R.; Stolper, E.M. The Temperature and Pressure Dependence of Nickel Partitioning between Olivine and Silicate Melt. J. Petrol. 2013, 54, 2521–2545. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.W. Intraplate Volcanism in Eastern Australia and New Zealand; Cambridge University Press: Cambridge, UK, 1989; p. 408. [Google Scholar]
- Kesson, S.E. The primary geochemistry of the monaro alkaline volcanics, southeastern Australia—Evidence for upper mantle heterogeneity. Contrib. Mineral. Petrol. 1973, 42, 93–108. [Google Scholar] [CrossRef]
- Zhang, M.; O’Reilly, S.Y. Multiple sources for basaltic rocks from Dubbo, eastern Australia: geochemical evidence for plume—Lithospheric mantle interaction. Chem. Geol. 1997, 136, 33–54. [Google Scholar] [CrossRef]
- Griffin, W.L.; O’Reilly, S.Y. The lower crust in eastern Australia: xenolith evidence. Geol. Soc. Lond. Spec. Publ. 1986, 24, 363. [Google Scholar] [CrossRef]
- O’Reilly, S.Y.; Griffin, W.L. Eastern Australia—4000 kilometres of mantle samples. In Mantle Xenoliths; Nixon, P.H., Ed.; Wiley: Chichester, UK, 1987; pp. 267–280. [Google Scholar]
- O’Reilly, S.Y.; Griffin, W.L. Mantle metasomatism beneath western Victoria, Australia: I. Metasomatic processes in Cr-diopside lherzolites. Geochim. Cosmochim. Acta 1988, 52, 433–447. [Google Scholar] [CrossRef]
- O’Reilly, S.Y. Volatile-rich mantle beneath eastern Australia. In Mantle Xenoliths; Nixon, P.H., Ed.; Wiley: Chichester, UK, 1987; pp. 661–670. [Google Scholar]
- Yaxley, G.M.; Green, D.H.; Kamenetsky, V. Carbonatite Metasomatism in the Southeastern Australian Lithosphere. J. Petrol. 1998, 39, 1917–1930. [Google Scholar] [CrossRef]
- Griffin, W.L.; Sutherland, F.L.; Hollis, J.D. Geothermal profile and crust-mantle transition beneath east-central Queensland: Volcanology, xenolith petrology and seismic data. J. Volcanol. Geotherm. Res. 1987, 31, 177–203. [Google Scholar] [CrossRef]
- O’Reilly, S.Y.; Zhang, M. Geochemical characteristics of lava-field basalts from eastern Australia and inferred sources: Connections with the subcontinental lithospheric mantle? Contrib. Mineral. Petrol. 1995, 121, 148. [Google Scholar] [CrossRef]
- Yaxley, G.M.; Crawford, A.J.; Green, D.H. Evidence for carbonatite metasomatism in spinel peridotite xenoliths from western Victoria, Australia. Earth Planet. Sci. Lett. 1991, 107, 305–317. [Google Scholar] [CrossRef]
- Zhang, M.; Stephenson, P.J.; O’Reilly, S.Y.; McCulloch, M.T.; Norman, M. Petrogenesis and Geodynamic Implications of Late Cenozoic Basalts in North Queensland, Australia: Trace-element and Sr–Nd–Pb Isotope Evidence. J. Petrol. 2001, 42, 685–719. [Google Scholar] [CrossRef]
- Wellman, P.; McDougall, I. Cainozoic igneous activity in eastern Australia. Tectonophysics 1974, 23, 49–65. [Google Scholar] [CrossRef]
- Davies, D.R.; Rawlinson, N.; Iaffaldano, G.; Campbell, I.H. Lithospheric controls on magma composition along Earth’s longest continental hotspot track. Nature 2015, 525, 511–514. [Google Scholar] [CrossRef] [PubMed]
- Davies, D.R.; Rawlinson, N. On the origin of recent intraplate volcanism in Australia. Geology 2014, 42, 1031–1034. [Google Scholar] [CrossRef] [Green Version]
- Demidjuk, Z.; Turner, S.; Sandiford, M.; George, R.; Foden, J.; Etheridge, M. U-series isotope and geodynamic constraints on mantle melting processes beneath the Newer Volcanic Province in South Australia. Earth Planet. Sci. Lett. 2007, 261, 517–533. [Google Scholar] [CrossRef]
- Jessop, K. Intrusions, Dykes, Plugs and Flows of the Buckland Tertiary Basalt Province, Central Queensland. Master’s Thesis, Macquarie University, North Ryde, Australia, July 2012. [Google Scholar]
- Waltenburg, K. Geochronology of Weathered Basaltic Flows at Carnavon Gorge. Bachelor’s Thesis, University of Queensland, Brisbane, Australia, November 2006. [Google Scholar]
- Crossingham, T.J.; Ubide, T.; Vasconcelos, P.M.; Knesel, K.M.; Mallmann, G. Temporal constraints on magma generation and differentiation in a continental volcano: Buckland, eastern Australia. Lithos 2018, 302–303, 341–358. [Google Scholar] [CrossRef]
- Kennett, B.L.N.; Salmon, M.; Saygin, E.; Group, A.W. AusMoho: The variation of Moho depth in Australia. Geophys. J. Int. 2011, 187, 946–958. [Google Scholar] [CrossRef]
- Meeuws, F.J.E.; Holford, S.P.; Foden, J.D.; Schofield, N. Distribution, chronology and causes of Cretaceous—Cenozoic magmatism along the magma-poor rifted southern Australian margin: Links between mantle melting and basin formation. Mar. Pet. Geol. 2016, 73, 271–298. [Google Scholar] [CrossRef]
- Skae, A. The Petrology of the Buckland Volcanic Province, Central Queensland, Australia. Ph.D. Thesis, University of Oxford, Oxford, UK, 1998. [Google Scholar]
- Jochum, K.P.; Weis, U.; Stoll, B.; Kuzmin, D.; Yang, Q.; Raczek, I.; Jacob, D.E.; Stracke, A.; Birbaum, K.; Frick, D.A.; et al. Determination of Reference Values for NIST SRM 610–617 Glasses Following ISO Guidelines. Geostand. Geoanal. Res. 2011, 35, 397–429. [Google Scholar] [CrossRef]
- Jochum, K.P.; Willbold, M.; Raczek, I.; Stoll, B.; Herwig, K. Chemical Characterisation of the USGS Reference Glasses GSA-1G, GSC-1G, GSD-1G, GSE-1G, BCR-2G, BHVO-2G and BIR-1G Using EPMA, ID-TIMS, ID-ICP-MS and LA-ICP-MS. Geostand. Geoanal. Res. 2007, 29, 285–302. [Google Scholar] [CrossRef]
- Batanova, V.G.; Sobolev, A.V.; Kuzmin, D.V. Trace element analysis of olivine: High precision analytical method for JEOL JXA-8230 electron probe microanalyser. Chem. Geol. 2015, 419, 149–157. [Google Scholar] [CrossRef]
- Griffin, W.L.; Powell, W.; Pearson, N.J.; O’Reilly, S.Y. Glitter: Data reduction software for Laser Ablation ICP-MS. In Laser Ablation-ICP-MS in the Earth Sciences: Current and Outstanding Issues; Sylvester, P., Ed.; Mineralogical Association of Canada: Ottawa, ON, Canada, 2008; Volume 40, pp. 308–311. [Google Scholar]
- Bussweiler, Y.; Giuliani, A.; Greig, A.; Kjarsgaard, B.A.; Petts, D.; Jackson, S.E.; Barrett, N.; Luo, Y.; Pearson, D.G. Trace element analysis of high-Mg olivine by LA-ICP-MS—Characterization of natural olivine standards for matrix-matched calibration and application to mantle peridotites. Chem. Geol. 2019, 524, 136–157. [Google Scholar] [CrossRef]
- De Hoog, J.C.M.; Gall, L.; Cornell, D.H. Trace-element geochemistry of mantle olivine and application to mantle petrogenesis and geothermobarometry. Chem. Geol. 2010, 270, 196–215. [Google Scholar] [CrossRef] [Green Version]
- Foley, S.F.; Jacob, D.E.; O’Neill, H.S.C. Trace element variations in olivine phenocrysts from Ugandan potassic rocks as clues to the chemical characteristics of parental magmas. Contrib. Mineral. Petrol. 2011, 162, 1–20. [Google Scholar] [CrossRef]
- Sun, S.; McDonough, W.F. Chemical and isotopic systematics of oceanic basalts: Implications for mantle composition and processes. Geol. Soc. Lond. Spec. Publ. 1989, 42, 313. [Google Scholar] [CrossRef]
- Ewart, A. Fractionation, assimilation and source melting: A petrogenetic overview. In Intraplate Volcanism in Eastern Australia and New Zealand; Johnson, R.W., Ed.; Cambridge University Press: Cambridge, UK, 1989; pp. 324–333. [Google Scholar]
- Green, D.H. The origin of basaltic and nephelinitic magmas in the earth’s mantle. Tectonophysics 1969, 7, 409–422. [Google Scholar] [CrossRef]
- Dupuy, C.; Liotard, J.M.; Dostal, J. Zr/Hf fractionation in intraplate basaltic rocks: Carbonate metasomatism in the mantle source. Geochim. Cosmochim. Acta 1992, 56, 2417–2423. [Google Scholar] [CrossRef]
- Sweeney, R.J.; Prozesky, V.; Przybylowicz, W. Selected trace and minor element partitioning between peridotite minerals and carbonatite melts at 18–46 kb pressure. Geochim. Cosmochim. Acta 1995, 59, 3671–3683. [Google Scholar] [CrossRef]
- Nelson, D.R.; Chivas, A.R.; Chappell, B.W.; McCulloch, M.T. Geochemical and isotopic systematics in carbonatites and implications for the evolution of ocean-island sources. Geochim. Cosmochim. Acta 1988, 52, 1–17. [Google Scholar] [CrossRef]
- Blundy, J.; Dalton, J. Experimental comparison of trace element partitioning between clinopyroxene and melt in carbonate and silicate systems, and implications for mantle metasomatism. Contrib. Mineral. Petrol. 2000, 139, 356–371. [Google Scholar] [CrossRef]
- Foley, S.F.; Yaxley, G.M.; Rosenthal, A.; Buhre, S.; Kiseeva, E.S.; Rapp, R.P.; Jacob, D.E. The composition of near-solidus melts of peridotite in the presence of CO2 and H2O between 40 and 60 kbar. Lithos 2009, 112, 274–283. [Google Scholar] [CrossRef]
- Coltorti, M.; Bonadiman, C.; Hinton, R.W.; Siena, F.; Upton, B.G.J. Carbonatite Metasomatism of the Oceanic Upper Mantle: Evidence from Clinopyroxenes and Glasses in Ultramafic Xenoliths of Grande Comore, Indian Ocean. J. Petrol. 1999, 40, 133–165. [Google Scholar] [CrossRef]
- Rudnick, R.L.; McDonough, W.F.; Chappell, B.W. Carbonatite metasomatism in the northern Tanzanian mantle: Petrographic and geochemical characteristics. Earth Planet. Sci. Lett. 1993, 114, 463–475. [Google Scholar] [CrossRef]
- Baker, M.B.; Wyllie, P.J. High-pressure apatite solubility in carbonate-rich liquids: Implications for mantle metasomatism. Geochim. Cosmochim. Acta 1992, 56, 3409–3422. [Google Scholar] [CrossRef]
- Palme, H.; O’Neill, H.S.C. Cosmochemical Estimates of Mantle Composition. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Oxford, UK, 2014; pp. 1–39. [Google Scholar]
- Adam, J.; Green, T. Trace element partitioning between mica- and amphibole-bearing garnet lherzolite and hydrous basanitic melt: 2. Tasmanian Cainozoic basalts and the origins of intraplate basaltic magmas. Contrib. Mineral. Petrol. 2011, 161, 883–899. [Google Scholar] [CrossRef]
- Gee, L.L.; Sack, R.O. Experimental Petrology of Melilite Nephelinites. J. Petrol. 1988, 29, 1233–1255. [Google Scholar] [CrossRef]
- Bussweiler, Y.; Brey, G.P.; Pearson, D.G.; Stachel, T.; Stern, R.A.; Hardman, M.F.; Kjarsgaard, B.A.; Jackson, S.E. The aluminum-in-olivine thermometer for mantle peridotites—Experimental versus empirical calibration and potential applications. Lithos 2017, 272–273, 301–314. [Google Scholar] [CrossRef]
- Taura, H.; Yurimoto, H.; Kurita, K.; Sueno, S. Pressure dependence on partition coefficients for trace elements between olivine and the coexisting melts. Phys. Chem. Miner. 1998, 25, 469–484. [Google Scholar] [CrossRef]
- Förster, M.W.; Prelević, D.; Schmück, H.R.; Buhre, S.; Marschall, H.R.; Mertz-Kraus, R.; Jacob, D.E. Melting phlogopite-rich MARID: Lamproites and the role of alkalis in olivine-liquid Ni-partitioning. Chem. Geol. 2018, 476, 429–440. [Google Scholar] [CrossRef]
- Veter, M.; Foley, S.F.; Mertz-Kraus, R.; Groschopf, N. Trace elements in olivine of ultramafic lamprophyres controlled by phlogopite-rich mineral assemblages in the mantle source. Lithos 2017, 292–293, 81–95. [Google Scholar] [CrossRef]
- Herzberg, C.; Cabral, R.A.; Jackson, M.G.; Vidito, C.; Day, J.M.D.; Hauri, E.H. Phantom Archean crust in Mangaia hotspot lavas and the meaning of heterogeneous mantle. Earth Planet. Sci. Lett. 2014, 396, 97–106. [Google Scholar] [CrossRef]
- Herzberg, C.; Asimow, P.D. PRIMELT3 MEGA.XLSM software for primary magma calculation: Peridotite primary magma MgO contents from the liquidus to the solidus. Geochem. Geophys. Geosyst. 2015, 16, 563–578. [Google Scholar] [CrossRef] [Green Version]
- Herzberg, C.; Asimow, P.D.; Ionov, D.A.; Vidito, C.; Jackson, M.G.; Geist, D. Nickel and helium evidence for melt above the core–mantle boundary. Nature 2013, 493, 393. [Google Scholar] [CrossRef] [PubMed]
- Gavrilenko, M.; Herzberg, C.; Vidito, C.; Carr, M.J.; Tenner, T.; Ozerov, A. A Calcium-in-Olivine Geohygrometer and its Application to Subduction Zone Magmatism. J. Petrol. 2016, 57, 1811–1832. [Google Scholar] [CrossRef]
- Davis, F.A.; Hirschmann, M.M. The effects of K2O on the compositions of near-solidus melts of garnet peridotite at 3 GPa and the origin of basalts from enriched mantle. Contrib. Mineral. Petrol. 2013, 166, 1029–1046. [Google Scholar] [CrossRef]
- Smith, D.; Griffin, W.L.; Ryan, C.G. Compositional evolution of high-temperature sheared lherzolite PHN 1611. Geochim. Cosmochim. Acta 1993, 57, 605–613. [Google Scholar] [CrossRef]
- McDonough, W.F.; Sun, S.S. The composition of the Earth. Chem. Geol. 1995, 120, 223–253. [Google Scholar] [CrossRef]
- Wilkinson, J.F.G. Ultramafic inclusions and high pressure megacrysts from a nephelinite sill, Nandewar Mountains, north-eastern New South Wales, and their bearing on the origin of certain ultramafic inclusions in alkaline volcanic rocks. Contrib. Mineral. Petrol. 1975, 51, 235–262. [Google Scholar] [CrossRef]
- Tiepolo, M.; Oberti, R.; Zanetti, A.; Vannucci, R.; Foley, S.F. Trace-Element Partitioning Between Amphibole and Silicate Melt. Rev. Mineral. Geochem. 2007, 67, 417–452. [Google Scholar] [CrossRef]
- Cherniak, D.J.; Liang, Y. Titanium diffusion in olivine. Geochim. Cosmochim. Acta 2014, 147, 43–57. [Google Scholar] [CrossRef]
- Jollands, M.C.; Hermann, J.; O’Neill, H.S.C.; Spandler, C.; Padrón-Navarta, J.A. Diffusion of Ti and some Divalent Cations in Olivine as a Function of Temperature, Oxygen Fugacity, Chemical Potentials and Crystal Orientation. J. Petrol. 2016, 57, 1983–2010. [Google Scholar] [CrossRef] [Green Version]
- Paster, T.P.; Schauwecker, D.S.; Haskin, L.A. The behavior of some trace elements during solidification of the Skaergaard layered series. Geochim. Cosmochim. Acta 1974, 38, 1549–1577. [Google Scholar] [CrossRef]
- Klemme, S.; van der Laan, S.R.; Foley, S.F.; Günther, D. Experimentally determined trace and minor element partitioning between clinopyroxene and carbonatite melt under upper mantle conditions. Earth Planet. Sci. Lett. 1995, 133, 439–448. [Google Scholar] [CrossRef]
- Gervasoni, F.; Klemme, S.; Rohrbach, A.; Grützner, T.; Berndt, J. Experimental constraints on mantle metasomatism caused by silicate and carbonate melts. Lithos 2017, 282–283, 173–186. [Google Scholar] [CrossRef]
- Foley, S.F.; Barth, M.G.; Jenner, G.A. Rutile/melt partition coefficients for trace elements and an assessment of the influence of rutile on the trace element characteristics of subduction zone magmas. Geochim. Cosmochim. Acta 2000, 64, 933–938. [Google Scholar] [CrossRef] [Green Version]
- Klemme, S.; Günther, D.; Hametner, K.; Prowatke, S.; Zack, T. The partitioning of trace elements between ilmenite, ulvospinel, armalcolite and silicate melts with implications for the early differentiation of the moon. Chem. Geol. 2006, 234, 251–263. [Google Scholar] [CrossRef]
- Dohmen, R.; Kasemann, S.A.; Coogan, L.; Chakraborty, S. Diffusion of Li in olivine. Part I: Experimental observations and a multi species diffusion model. Geochim. Cosmochim. Acta 2010, 74, 274–292. [Google Scholar] [CrossRef]
- Ammannati, E.; Jacob, D.E.; Avanzinelli, R.; Foley, S.F.; Conticelli, S. Low Ni olivine in silica-undersaturated ultrapotassic igneous rocks as evidence for carbonate metasomatism in the mantle. Earth Planet. Sci. Lett. 2016, 444, 64–74. [Google Scholar] [CrossRef]
- Jaques, A.L.; Foley, S.F. Insights into the petrogenesis of the West Kimberley lamproites from trace elements in olivine. Mineral. Petrol. 2018, 112, 519–537. [Google Scholar] [CrossRef]
- Seitz, H.-M.; Woodland, A.B. The distribution of lithium in peridotitic and pyroxenitic mantle lithologies—An indicator of magmatic and metasomatic processes. Chem. Geol. 2000, 166, 47–64. [Google Scholar] [CrossRef]
- Foley, S.F. Rejuvenation and erosion of the cratonic lithosphere. Nat. Geosci. 2008, 1, 503. [Google Scholar] [CrossRef]
- Kelemen, P.B.; Hart, S.R.; Bernstein, S. Silica enrichment in the continental upper mantle via melt/rock reaction. Earth Planet. Sci. Lett. 1998, 164, 387–406. [Google Scholar] [CrossRef]







| Element | Ca | Cr | Si | Ni | Al | Mg | Fe | Mn |
|---|---|---|---|---|---|---|---|---|
| WDS Channel | 1 | 1 | 2 | 3 | 4 | 4 | 5 | 5 |
| WDS Crystal | TAP | TAP | TAP | TAP | TAP | TAP | TAP | TAP |
| Line | Kα | Kα | Kα | Kα | Kα | Kα | Kα | Kα |
| Standard | CaSiO3 | Cr2O3 | SCO 1 | Ni | Al2O3 | SCO 1 | Fe2O3 | Mn garnet |
| Measuring time (s) | 60 | 60 | 60 | 120 | 80 | 40 | 40 | 80 |
| Detection limit (ppm) | 40 | 50 | 60 | 54 | 30 | 90 | 120 | 80 |
| Element/Oxide | Alkali Basalt Phenocrysts | Basanite Phenocrysts | Low Mg# Xenocrysts | High Mg# Xenocrysts |
|---|---|---|---|---|
| SiO2 (wt.%) | 39.37 | 39.00 | 40.19 | 40.94 |
| Al2O3 | 0.05 | 0.08 | 0.01 | 0.02 |
| FeOtot | 15.21 | 17.44 | 12.86 | 9.47 |
| MnO | 0.21 | 0.22 | 0.18 | 0.14 |
| MgO | 43.15 | 41.64 | 46.09 | 48.76 |
| CaO | 0.21 | 0.16 | 0.05 | 0.04 |
| Cr2O3 | 0.03 | 0.02 | 0.00 | 0.00 |
| NiO | 0.29 | 0.23 | 0.36 | 0.39 |
| Total | 98.53 | 98.80 | 99.56 | 99.76 |
| Mg# | 83.49 | 80.97 | 86.63 | 90.18 |
| Li (ppm) | 4.84 | 3.30 | 3.05 | 2.14 |
| B | 5.86 | 4.76 | 3.80 | 5.67 |
| Na | 94 | 145 | 57 | 41 |
| Al | 225 | 265 | 64 | 64 |
| P | 148 | 129 | 40 | 51 |
| Ca | 1530 | 1058 | 244 | 266 |
| Sc | 5.40 | 4.22 | 2.45 | 2.74 |
| Ti | 74 | 95 | 16.9 | 14.2 |
| V | 8.68 | 7.56 | 2.37 | 2.40 |
| Cr | 257 | 105 | 45.4 | 40.5 |
| Mn | 1790 | 1865 | 1244 | 1188 |
| Co | 185 | 198 | 152 | 156 |
| Ni | 2211 | 1796 | 3012 | 3190 |
| Cu | 1.39 | 1.06 | 1.00 | 1.49 |
| Zn | 186 | 188 | 111 | 54 |
| Ga | 0.268 | 0.221 | 0.071 | 0.043 |
| Sr | 0.051 | 0.078 | 0.264 | 0.022 |
| Y | 0.156 | 0.118 | 0.028 | 0.019 |
| Zr | 0.097 | 0.097 | <0.0050 | <0.0028 |
| Nb | <0.0095 | 0.005 | 0.008 | <0.0010 |
| Ba | <0.0073 | <0.0069 | <0.0114 | 0.059 |
| La | <0.0001 | 0.003 | <0.0016 | <0.0017 |
| Ce | <0.0009 | 0.006 | <0.0012 | <0.0012 |
| Gd | <0.0067 | <0.0040 | <0.0091 | <0.0055 |
| Yb | 0.069s | 0.026 | <0.0080 | 0.024 |
| (Zn/Fe) × 104 | 15.7 | 13.8 | 11.3 | 7.3 |
| Fe/Mn | 73 | 80 | 71 | 68 |
| V/Sc | 1.6 | 1.8 | 1 | 0.9 |
| Ti/V | 8.5 | 12.6 | 7.1 | 5.9 |
| Ti/Sc | 13.7 | 22.5 | 6.9 | 5.2 |
| Alkali Basalts | Basanites | |
|---|---|---|
| 0.35 | 81.3–84.5 | 81.3–83.6 |
| 0.30 | 83.6–86.4 | 83.5–85.6 |
| 0.27 | 85.9–88.4 | 85.9–87.7 |
| 0.17 | 90.0–91.8 | 89.9–91.3 |
| Element | Mineral/Melt Partition Coefficients | ||||
|---|---|---|---|---|---|
| Amphibole | Apatite | Clinopyroxene | Orthopyroxene | Olivine | |
| P | 0.019–0.03 | 0.018–0.02 | 0.007 | 0.038–0.05 | |
| Sc | 1.62–35 | 0.22 | 1.40 | 0.64 | 0.23 |
| Ti | 0.37–18 | 0.363 | 0.10 | 0.016 | |
| V | 1.49–22 | 1.3 | 0.80 | 0.09 | |
| Cr | 3.56–53 | 3.8 | 5.9 | 0.96 | |
| Mn | 0.13 | 1.0 | 0.84 | 0.89 | |
| Fe | 0.80 | 0.75 | 1.09 | ||
| Co | 1.88–2.2 | <0.03 | 1.4 | 1.6 | 3.0 |
| Ni | 18–32 | 2.35 | 2.83 | 10.3 | |
| Cu | 1.1–1.8 | 0.28 | 0.42 | 0.22 | 0.50 |
| Zn | 0.33–0.41 | <0.25 | 0.47 | 0.67 | 1.1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shea, J.J.; Foley, S.F. Evidence for a Carbonatite-Influenced Source Assemblage for Intraplate Basalts from the Buckland Volcanic Province, Queensland, Australia. Minerals 2019, 9, 546. https://doi.org/10.3390/min9090546
Shea JJ, Foley SF. Evidence for a Carbonatite-Influenced Source Assemblage for Intraplate Basalts from the Buckland Volcanic Province, Queensland, Australia. Minerals. 2019; 9(9):546. https://doi.org/10.3390/min9090546
Chicago/Turabian StyleShea, Joshua J., and Stephen F. Foley. 2019. "Evidence for a Carbonatite-Influenced Source Assemblage for Intraplate Basalts from the Buckland Volcanic Province, Queensland, Australia" Minerals 9, no. 9: 546. https://doi.org/10.3390/min9090546





