Characterization of Cross-Species Transmission of Drosophila melanogaster Nora Virus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fly Husbandry and Dechorionation
2.2. Infection of Individuals from Different Drosophila Species
2.3. Infection of M. domestica and G. mellonella
2.4. Infection of G. sigillatus
2.5. Infection of T. molitor
2.6. Collection of Native Invertebrates
2.7. Commercially Available D. melanogaster Stocks
2.8. RNA Extraction and RT-PCR Analysis of Nora Virus
3. Results
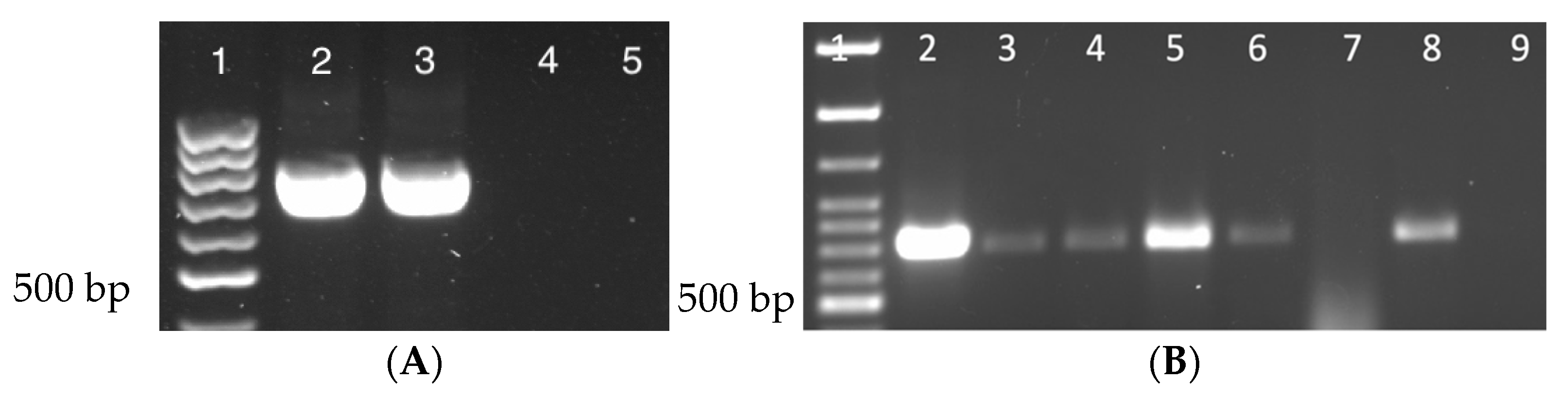
3.1. Validation of Nora virus Infection Using RT-PCR
3.2. Nora Virus Is Transmitted across Drosophila Species
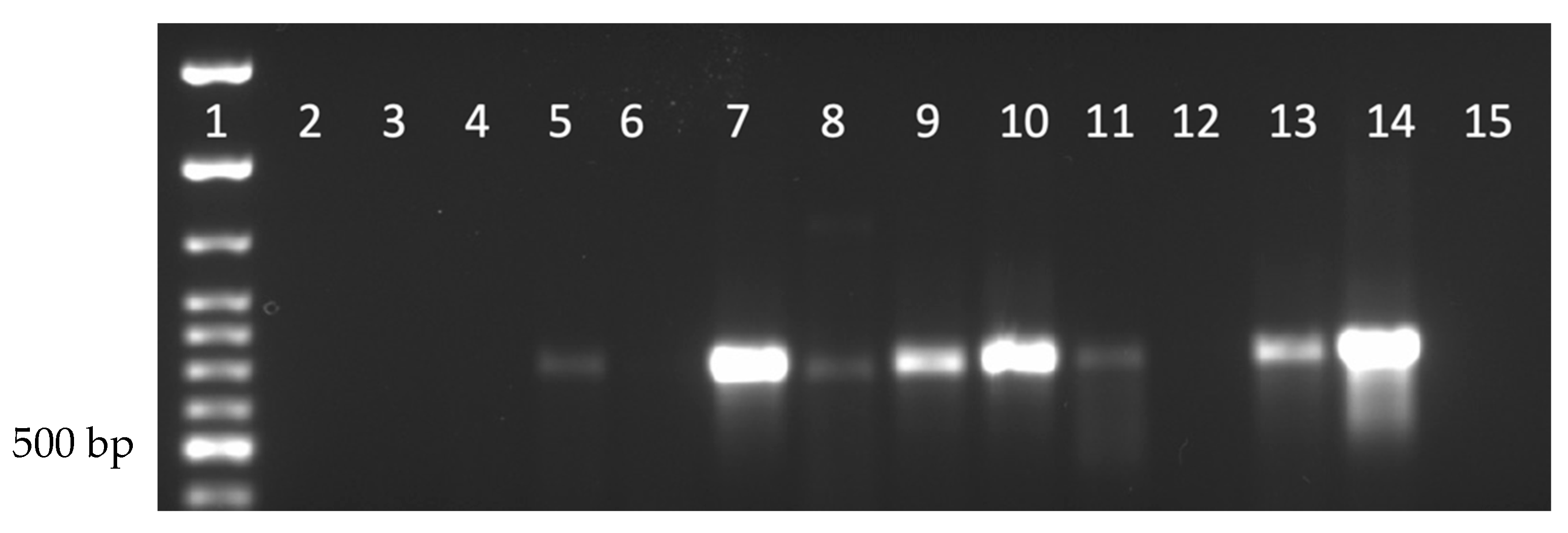
3.3. Nora Virus Is Transmitted to Individuals of Select Laboratory Reared Insect Species
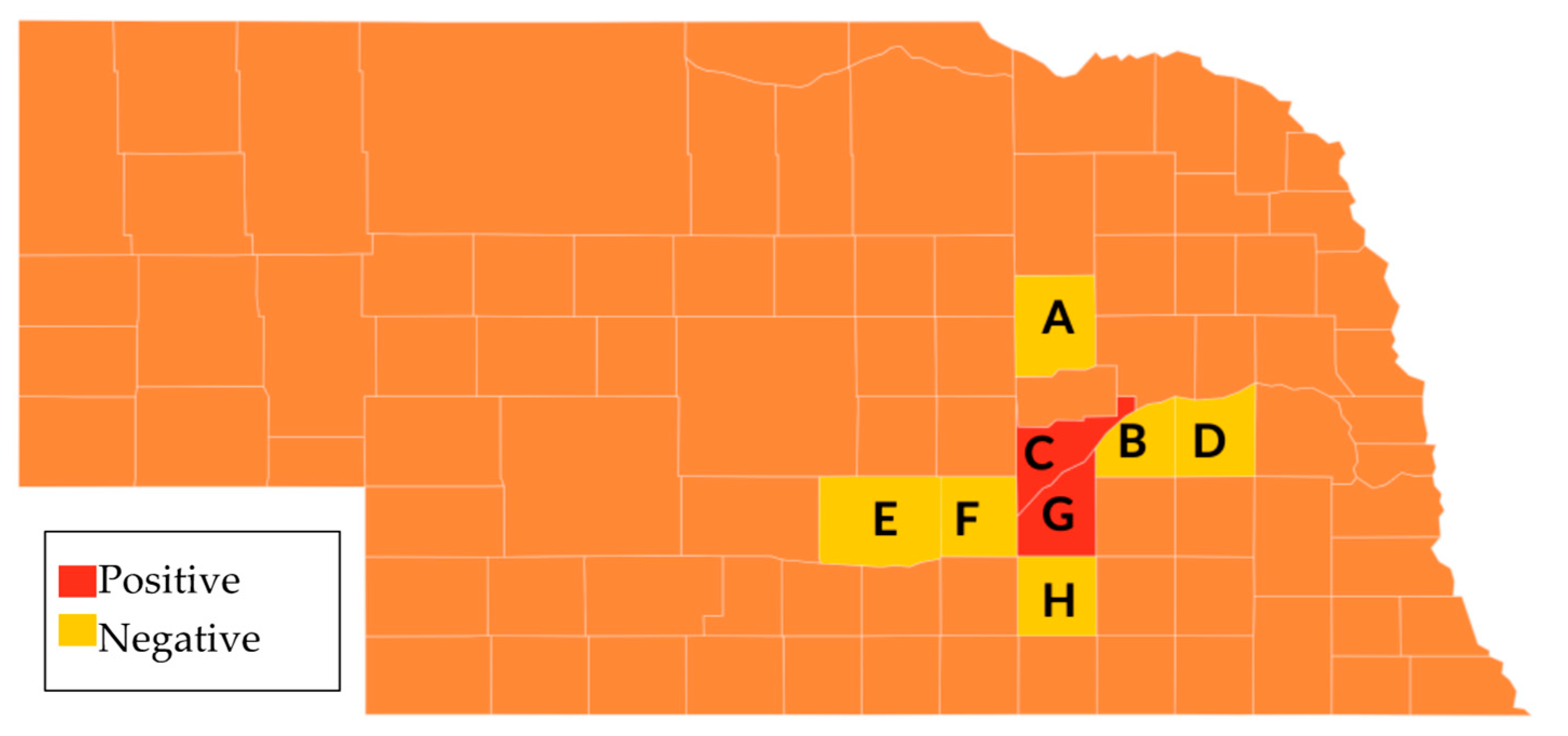
3.4. Nora Virus Is Present in Individuals of Native Nebraska Invertebrate Species
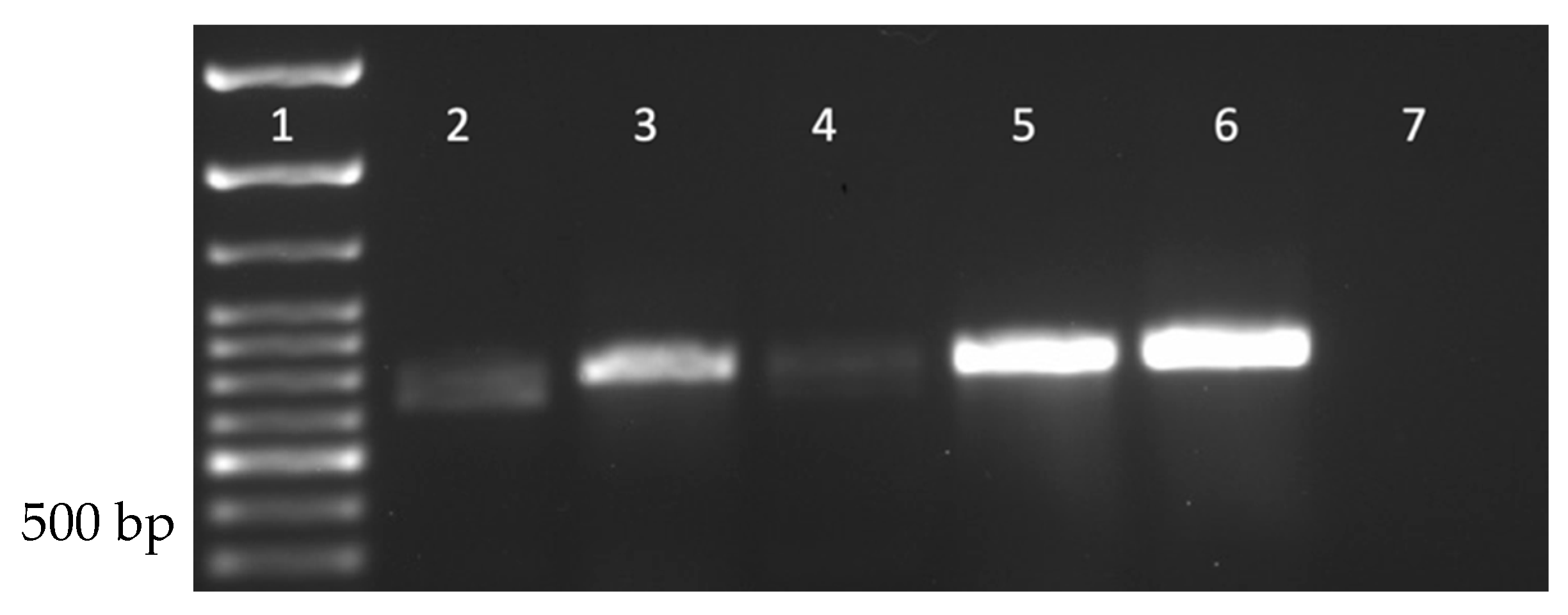
3.5. Nora Virus Is Present in Commercially Available Drosophila Stocks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Habayeb, M.S.; Ekengren, S.K.; Hultmark, D. Nora virus, a present virus in Drosophila, defines a new picorna-like virus family. J. Gen. Virol. 2006, 87, 3045–3051. [Google Scholar] [CrossRef]
- van Mierlo, J.T.; Bronkhorst, A.W.; Overheul, G.J.; Sadanandan, S.A.; Ekström, J.-O.; Heestermans, M.; Hultmark, D.; Antoniewski, C.; van Rij, R.P. Convergent evolution of argonaute-2 slicer antagonism in two distinct insect RNA viruses. PLoS Pathog. 2012, 8, e1002872. [Google Scholar] [CrossRef] [PubMed]
- Ekström, J.-O.; Habayeb, M.S.; Srivastava, V.; Kieselbach, T.; Wingsle, G.; Hultmark, D. Drosophila Nora virus capsid proteins differ from those of other picorna-like viruses. Virus Res. 2011, 160, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Licking-Murray, K.D.; Carlson, D.J.; Sowle, R.; Carlson, K.A. In vitro assembly and evaluation of Nora virus VLPs. Acta Virol. 2021, 65, 381–389. [Google Scholar] [CrossRef]
- Sadanandan, S.A.; Ekström, J.-O.; Jonna, V.R.; Hofer, A.; Hultmark, D. VP3 is crucial for the stability of Nora virus virions. Virus Res. 2016, 223, 20–27. [Google Scholar] [CrossRef] [Green Version]
- Laurinmäki, P.; Shakeel, S.; Ekström, J.-O.; Mohammadi, P.; Hultmark, D.; Butcher, S.J. Structure of Nora virus at 2.7 Å resolution and implications of receptor binding, capsid stability and taxonomy. Sci. Rep. 2020, 10, 19675. [Google Scholar] [CrossRef]
- Oliveira, D.C.S.G.; Hunter, W.B.; Ng, J.; Desjardins, C.A.; Dang, P.M.; Werren, J.H. Data mining cDNAs reveals three new single stranded RNA viruses in Nasonia (Hymenoptera: Pteromalidae). Insect Mol. Biol. 2010, 19, 99–107. [Google Scholar] [CrossRef] [Green Version]
- Torres, L.; Almazán, C.; Ayllón, N.; Galindo, R.C.; Rosario-Cruz, R.; Quiroz-Romero, H.; Gortazar, C.; De La Fuente, J. Identification of microorgnisms in partially fed female horn flies, Haematobia irritans. Parasitol. Res. 2012, 111, 1391–1395. [Google Scholar] [CrossRef]
- Jakubowska, A.K.; D’Angiolo, M.; González-Martínez, R.M.; Millán-Leiva, A.; Carballo, A.; Murillo, R.; Caballero, P.; Herrero, S. Simultaneous occurrence of covert infections with small RNA viruses in the lepidopteran Spodoptera exigua. J. Invertebr. Pathol. 2014, 121, 56–63. [Google Scholar] [CrossRef]
- Li, T.; Guan, R.; Wu, Y.; Chen, S.; Yuan, G.; Miao, X.; Li, H. The novel Agrotis ipsilon Nora virus confers deleterious effects to the fitness of Spodoptera frugiperda (Lepidoptera: Noctuidae). Front. Microbiol. 2021, 12, 727202. [Google Scholar] [CrossRef]
- Remnant, E.J.; Shi, M.; Buchmann, G.; Blacquiere, T.; Holmes, E.C.; Beekman, M.; Ashe, A. A diverse range of novel RNA viruses in geographically distinct honey bee populations. J. Virol. 2017, 91, e00158-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Xu, P.; Yuan, H.; Graham, R.I.; Wilson, K.; Wu, K. Discovery and characterization of a novel picorna-like RNA virus in the cotton bollworm Helocoverpa armigera. J. Invertebr. Pathol. 2019, 160, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, A.; Towery, L.; McCown, A.; Carlson, K.A. Impaired geotaxis as a novel phenotype of Nora virus infection of Drosophila melanogaster. Scientifica 2020, 2020, 1804510. [Google Scholar] [CrossRef]
- Cordes, E.J.; Licking-Murray, K.D.; Carlson, K.A. Differential gene expression related to Nora virus infection of Drosophila melanogaster. Virus Res. 2013, 175, 95–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lopez, W.; Page, A.M.; Carlson, D.J.; Ericson, B.L.; Cserhati, M.F.; Guda, C.G.; Carlson, K.A. Analysis of immune-related genes during Nora virus infection of Drosophila melanogaster using next generation sequencing. AIMS Microbiol. 2018, 4, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Habayeb, M.S.; Cantera, R.; Casanova, G.; Ekström, J.-O.; Albright, S.; Hultmark, D. The Drosophila Nora virus is an enteric virus, transmitted via feces. J. Invertebr. Pathol. 2009, 101, 29–33. [Google Scholar] [CrossRef]
- Jezovit, J.A.; Levine, J.D.; Schneider, J. Phylogeny, environment and sexual communication across the Drosophila genus. J. Exp. Biol. 2017, 220, 42–52. [Google Scholar] [CrossRef] [Green Version]
- Gryllodes sigillatus Draft Assessment Report. Available online: https://www.awe.gov.au/sites/default/files/env/consultations/03754e31-19da-4746-ab08-c2bf575da692/files/gryllodes-sigillatus-draft-assessment-report.pdf (accessed on 21 June 2022).
- Moore, N.F.; Kearns, A.; Pullin, J.S. Characterization of Cricket paralysis virus-induced polypeptides in Drosophila cells. J. Virol. 1980, 33, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Constantino, M.; Christian, P.; Marina, C.F.; Williams, T. A comparison of techniques for detecting Invertebrate iridescent virus 6. J. Virol. Meth. 2001, 98, 109–118. [Google Scholar] [CrossRef]
- Gencer, D.; Yesilyurt, A.; Gullu, M.; Nalcacioglu, R. Insecticidal activities of wild type and recombinant invertebrate iridescent viruses on five common pests. Turk. Entomol. Derg. 2020, 44, 365–373. [Google Scholar] [CrossRef]
- Duffield, K.R.; Hunt, J.; Sadd, B.M.; Sakaluk, S.K.; Oppert, B.; Rosario, K.; Behle, R.W.; Ramirez, J.L. Active and covert infections of Cricket iridovirus and Acheta domesticus densovirus in reared Gryllodes sigillatus crickets. Front. Microbiol. 2021, 12, 780796. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Cherry, S. Viruses and antiviral immunity in Drosophila. Dev. Comp. Immunol. 2014, 42, 67–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munn, B.; Ericson, B.; Carlson, D.J.; Carlson, K.A. Detecting the presence of Nora virus in Drosophila utilizing single fly RT-PCR. Bioscene J. Coll. Biol. Teach. 2015, 41, 40–44. [Google Scholar]
- Deddouche, S.; Matt, N.; Budd, A.; Mueller, S.; Kemp, C.; Galiana-Arnoux, D.; Dostert, C.; Antoniewski, C.; Homan, J.A.; Imler, J.-L. The DExD/H-box helicase Dicer-2 mediates the induction of antiviral activity in Drosophila. Nat. Immunol. 2008, 9, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Humann, J.A.; Imler, J.-L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 2005, 6, 946–953. [Google Scholar] [CrossRef]
- Kumar, N.; Sharma, S.; Barua, S.; Tripathi, B.N.; Rouse, B.T. Virological and immunological outcomes of coinfections. Clin. Microbiol. Rev. 2018, 31, e00111–e00117. [Google Scholar] [CrossRef]
| Scientific Name | Common Name | County | Result |
|---|---|---|---|
| Camponotus | Carpenter ant | Hamilton | Negative |
| Anax junius | Green darner | Merrick | Negative |
| Harmonia axyridis | Asian lady beetle | Merrick | Negative |
| Mantodea | Mantis | Merrick | Negative |
| Polistes fuscatus | Northern paper wasp | Butler | Negative |
| Helcystogramma badia | N/A | Merrick | Negative |
| Leucoma salicis | White satin moth | Merrick | Negative |
| Acheta domesticus | House cricket | Merrick | Negative |
| Apis mellifera | Western honey bee | Merrick | Negative |
| Spilosoma virginica | Yellow woolly bear | Merrick | Positive |
| Musca domestica | House fly | Merrick | Negative |
| Melanoplus femurrubrum | Red-legged grasshopper | Merrick | Negative |
| Phoberia atomaris | Common oak moth | Merrick | Negative |
| Hyles lineata | White-lined sphinx | Merrick | Negative |
| Ceratomia amyntor | Elm sphinx | Merrick | Negative |
| Pterophoridae | Plume moth | Merrick | Negative |
| Diplopoda | Millipede | Merrick | Positive |
| Boisea trivittata | Boxelder bug | Merrick | Negative |
| Teleogryllus commodus | Black field cricket | Boone | Negative |
| Dermaptera | Earwig | Merrick | Negative |
| Chilopoda | Centipede | Merrick | Negative |
| Badumna longinqua | Grey house spider | Polk | Negative |
| Odontotaenius disjunctus | Horned passalus beetle | Hamilton | Positive |
| Scudderia furcata | Fork-tailed bush katydid | Hall | Negative |
| Pholidoptera griseoaptera | Dark bush-cricket | Merrick | Negative |
| Tenodera aridifolia sinensis | Chinese mantis | Merrick | Negative |
| Spodoptera ornithogalli | Yellow-striped armyworm | Merrick | Negative |
| Haematopis grataria | Chickweed geometer | Merrick | Negative |
| Armadillidiidae | Pill bug | Merrick | Negative |
| Badumna insignis | Black house spider | Buffalo | Negative |
| Brachypnoea | Leaf beetle | Merrick | Negative |
| Manduca | Hawkmoth | Merrick | Negative |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buhlke, E.G.; Hobbs, A.M.; Rajput, S.; Rokusek, B.; Carlson, D.J.; Gillan, C.; Carlson, K.A. Characterization of Cross-Species Transmission of Drosophila melanogaster Nora Virus. Life 2022, 12, 1913. https://doi.org/10.3390/life12111913
Buhlke EG, Hobbs AM, Rajput S, Rokusek B, Carlson DJ, Gillan C, Carlson KA. Characterization of Cross-Species Transmission of Drosophila melanogaster Nora Virus. Life. 2022; 12(11):1913. https://doi.org/10.3390/life12111913
Chicago/Turabian StyleBuhlke, Ella G., Alexis M. Hobbs, Sunanda Rajput, Blase Rokusek, Darby J. Carlson, Chelle Gillan, and Kimberly A. Carlson. 2022. "Characterization of Cross-Species Transmission of Drosophila melanogaster Nora Virus" Life 12, no. 11: 1913. https://doi.org/10.3390/life12111913







