Eight Typical Aroma Compounds of ‘Panguxiang’ Pear during Development and Storage Identified via Metabolomic Profiling
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Experimental Design
2.2. Metabolite Extraction and LC-MS
2.3. Bioinformatics Analyses of Metabolome and Transcriptome
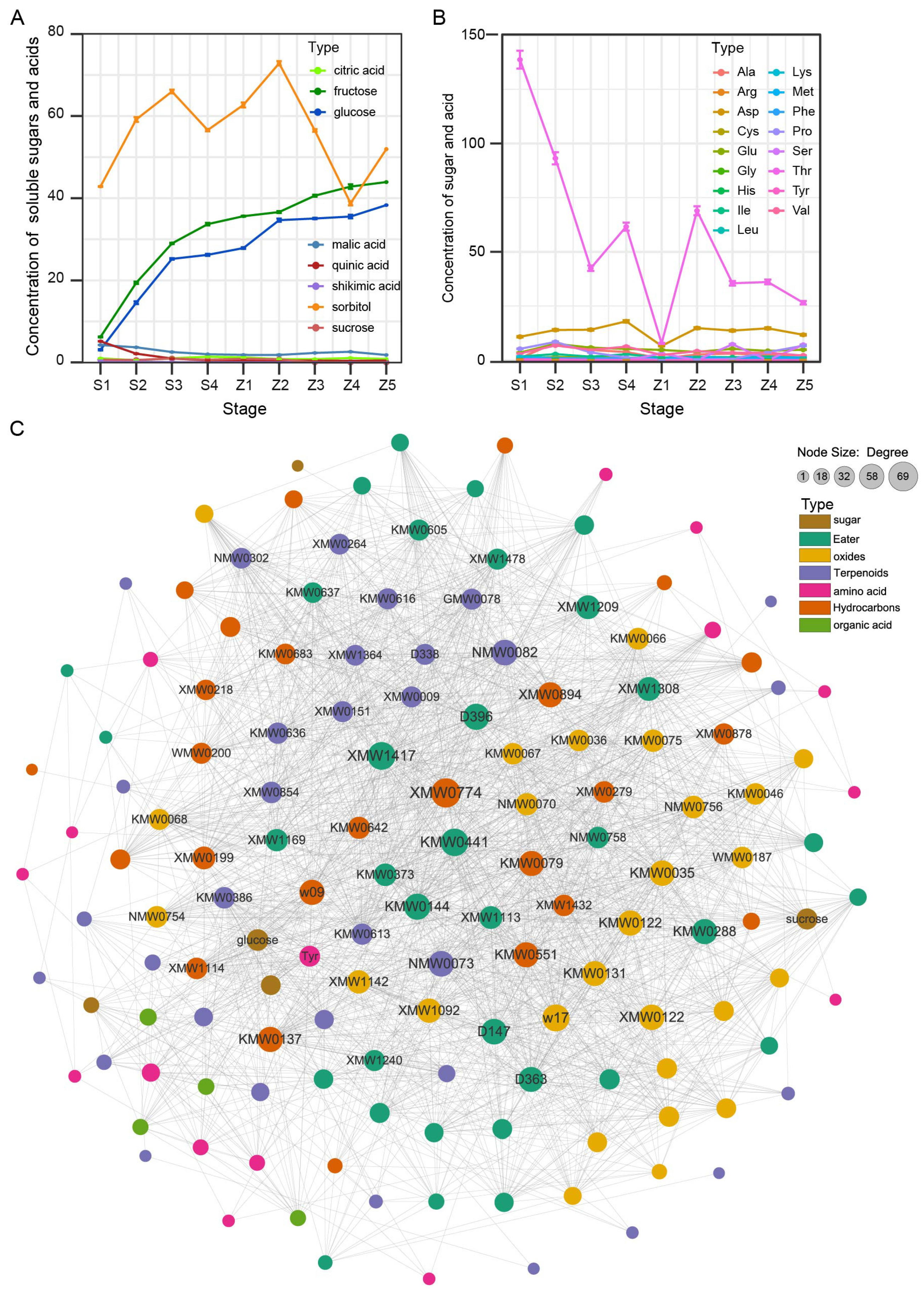
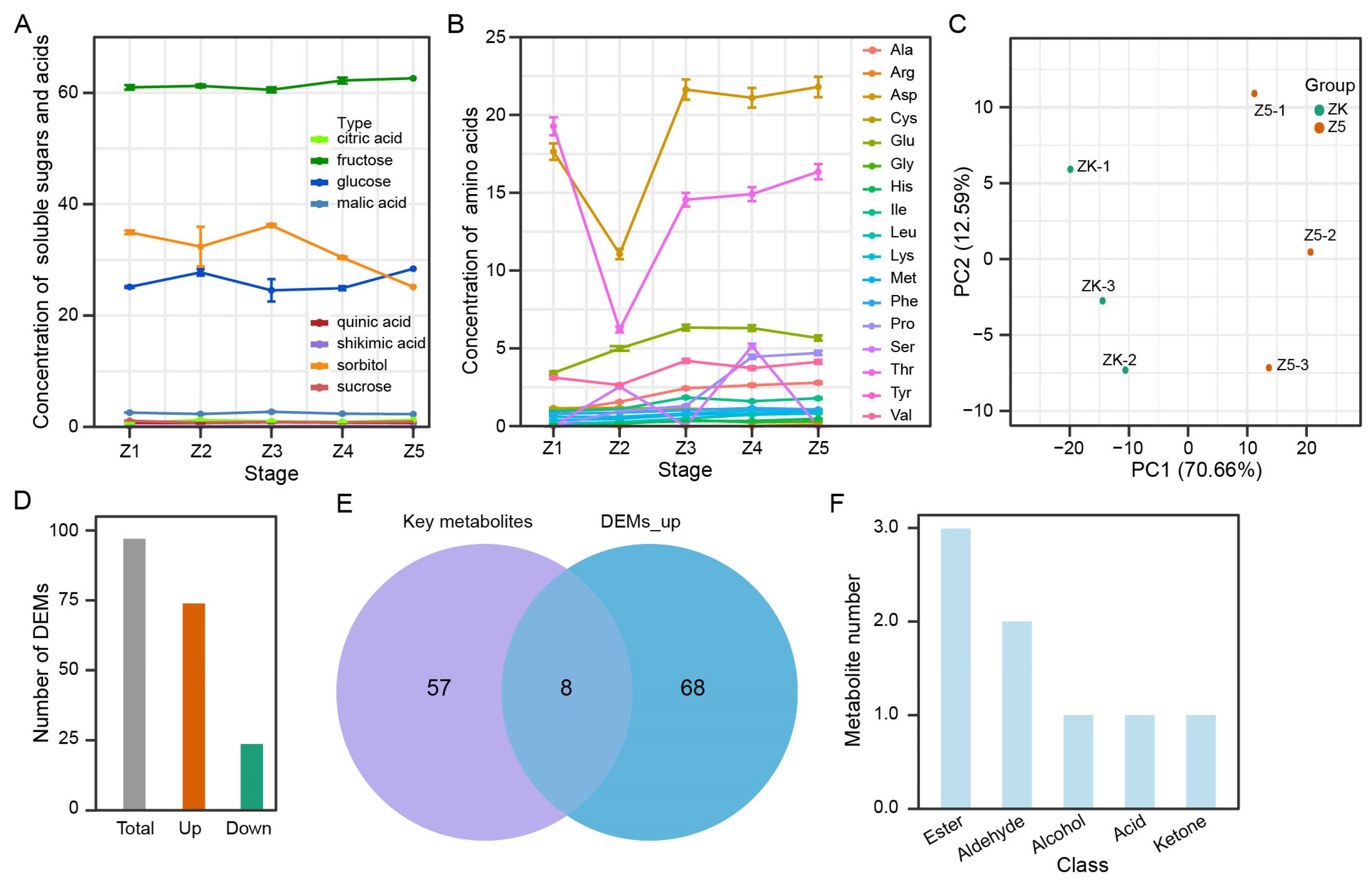
2.4. Soluble Sugar, Organic Acid and Amino Acid Contents in Pear
2.5. Statistical Analysis
3. Results
3.1. Changes of Metabolome in Pear during Development and Storage
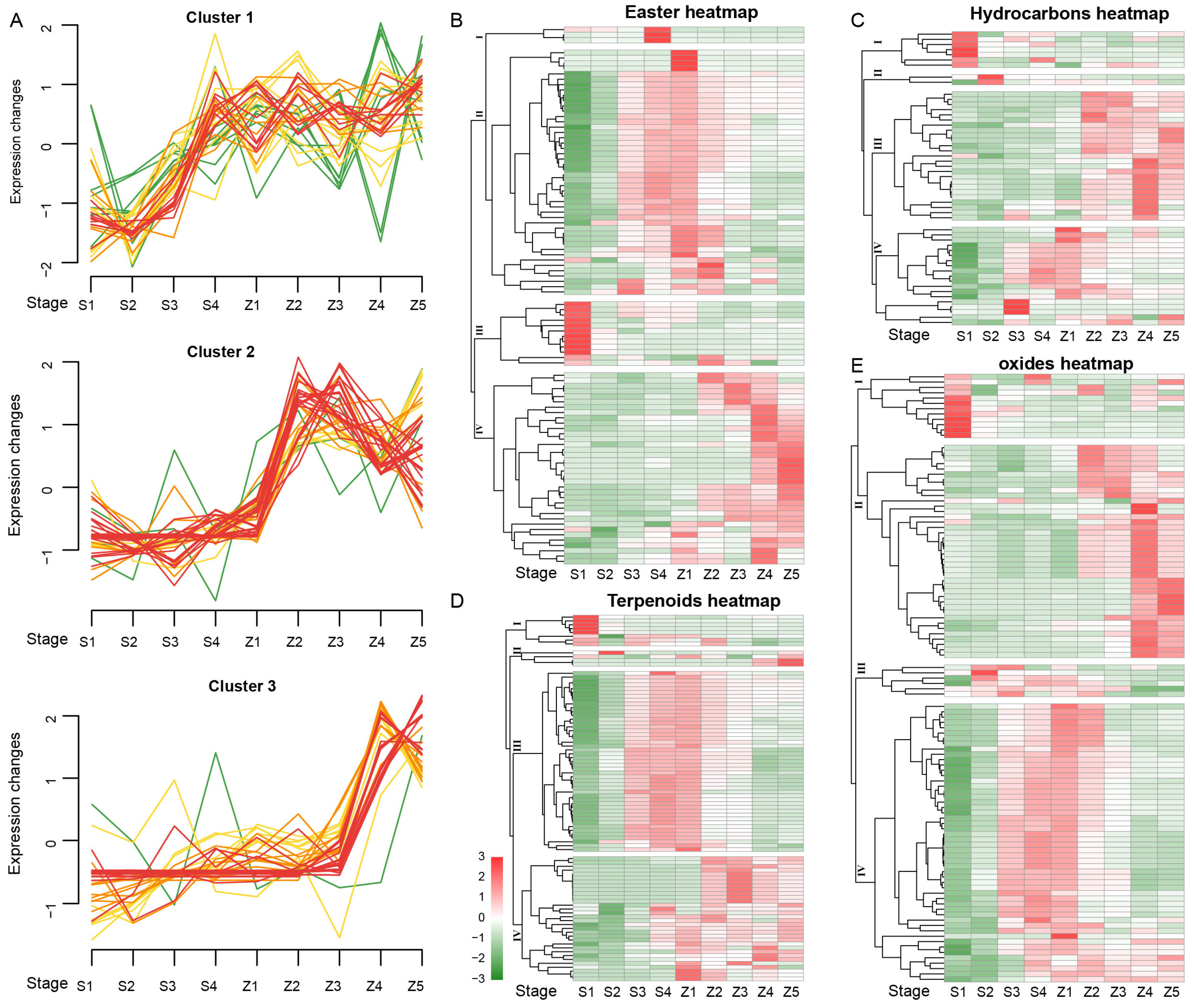
3.2. Classification and Trend of Metabolites in Pear during Development and Storage
3.3. Screening and Determination of Key Aroma Metabolites
3.4. Identification of Key Aroma Generation Substrates
3.5. Eight Characteristic Aroma Substances of the ‘Panguxiang’ Compared to the ‘Korla’
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Forster, B. Wild Crop Relatives: Genomic and Breeding Resources. Cereals. Edited by C. Kole. Heidelberg, Dordrecht, London, New York: Springer (2011), pp. 497, £153.00. ISBN 978-3-642-14227-7. Exp. Agric. 2011, 47, 736. [Google Scholar] [CrossRef]
- Jansma, S.Y.; Sergeeva, L.I.; Tikunov, Y.M.; Kohlen, W.; Ligterink, W.; Rieu, I. Low Salicylic Acid Level Improves Pollen Development Under Long-Term Mild Heat Conditions in Tomato. Front Plant Sci. 2022, 13, 828743. [Google Scholar] [CrossRef] [PubMed]
- Villatoro, C.; Altisent, R.; Echeverría, G.; Graell, J.; López, M.L.; Lara, I. Changes in Biosynthesis of Aroma Volatile Compounds during on-tree Maturation of ‘Pink Lady®’ Apples. Postharvest Biol. Technol. 2008, 47, 286–295. [Google Scholar] [CrossRef]
- Schwab, W.; Davidovich-Rikanati, R.; Lewinsohn, E. Biosynthesis of Plant-derived Flavor Compounds. Plant J. 2008, 54, 712–732. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.L.; Yan, S.; Feng, Z.; Xiao, L.; Hu, X.S. Changes in the Volatile Compounds and Chemical and Physical Properties of Yali pear (Pyrus bertschneideri Reld.) during Storage. Food Chem. 2006, 97, 248–255. [Google Scholar] [CrossRef]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Ibdah, M.; Meir, A.; Yosef, E.; Zamir, D.; Tadmor, Y. Not just Colors—Carotenoid Degradation as a Link between Pigmentation and Aroma in Tomato and Watermelon Fruit. Trends Food Sci. Technol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Lalel, H.J.D.; Singh, Z.; Tan, S.C. Aroma Volatiles Production during Fruit Ripening of ‘Kensington Pride’ mango. Postharvest Biol. Technol. 2003, 27, 323–336. [Google Scholar] [CrossRef]
- Obando-Ulloa, J.M.; Moreno, E.; García-Mas, J.; Nicolai, B.; Lammertyn, J.; Monforte, A.J.; Fernández-Trujillo, J.P. Climacteric or Non-climacteric Behavior in Melon Fruit: 1. Aroma Volatiles. Postharvest Biol. Technol. 2008, 49, 27–37. [Google Scholar] [CrossRef]
- Van de Poel, B.; Vandendriessche, T.; Hertog, M.L.A.T.M.; Nicolai, B.M.; Geeraerd, A. Detached Ripening of Non-climacteric Strawberry Impairs Aroma Profile and Fruit Quality. Postharvest Biol. Technol. 2014, 95, 70–80. [Google Scholar] [CrossRef]
- Wang, Y.; Yang, C.; Li, S.; Yang, L.; Wang, Y.; Zhao, J.; Jiang, Q. Volatile Characteristics of 50 Peaches and Nectarines Evaluated by HP–SPME with GC–MS. Food Chem. 2009, 116, 356–364. [Google Scholar] [CrossRef]
- Maul, F.; Sargent, S.A.; Sims, C.A.; Baldwin, E.A.; Balaban, M.O.; Huber, D.J. Tomato Flavor and Aroma Quality as Affected by Storage Temperature. Food Sci. Technol. 2000, 65, 1228–1237. [Google Scholar] [CrossRef]
- El Hadi, M.A.; Zhang, F.J.; Wu, F.F.; Zhou, C.H.; Tao, J. Advances in Fruit Aroma Volatile Research. Molecules 2013, 18, 8200–8229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, G.; Zhang, X.; Yan, Z.; Cheng, Y.; Li, H.; Xu, G. Monitoring Volatile Organic Compounds in Different Pear Cultivars during Storage Using HS-SPME with GC-MS. Foods 2022, 11, 3778. [Google Scholar] [CrossRef] [PubMed]
- Cheong, K.W.; Tan, C.P.; Mirhosseini, H.; Hamid, N.S.A.; Osman, A.; Basri, M. Equilibrium Headspace Analysis of Volatile Flavor Compounds Extracted from Soursop (Annona muricata) using Solid-phase Microextraction. Food Res. Int. 2010, 43, 1267–1276. [Google Scholar] [CrossRef]
- Zhang, C.; Hao, Y.J. Advances in Genomic, Transcriptomic, and Metabolomic Analyses of Fruit Quality in Fruit Crops. Hortic. Plant J. 2020, 6, 361–371. [Google Scholar] [CrossRef]
- Bood, K.G.; Zabetakis, I. The Biosynthesis of Strawberry Flavor (II): Biosynthetic and Molecular Biology Studies. J. Food Sci. 2002, 67, 2–8. [Google Scholar] [CrossRef]
- Li, M.; Dunwell, J.M.; Zhang, H.; Wei, S.; Li, Y.; Wu, J.; Zhang, S. Network Analysis Reveals the Co-expression of Sugar and Aroma Genes in the Chinese White Pear (Pyrus bretschneideri). Gene 2018, 677, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Kohl, M.; Wiese, S.; Warscheid, B. Cytoscape: Software for Visualization and Analysis of Biological Networks. Data Min. Proteom. 2011, 696, 291–303. [Google Scholar]
- Li, G.P.; Jia, H.J.; Li, J.H.; Wang, Q.; Zhang, M.J.; Teng, Y.W. Emission of Volatile Esters and Transcription of Ethylene- and Aroma-related Genes during Ripening of ‘Pingxiangli’ Pear Fruit (Pyrus ussuriensis Maxim.). Sci. Hortic. 2014, 170, 17–23. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhang, J.; Wang, Z.; Qi, K.; Li, H.; Tian, R.; Wu, X.; Qiao, X.; Zhang, S.; et al. Comparative Analysis of Volatile Aromatic Compounds from a Wide Range of Pear (Pyrus L.) Germplasm Resources Based on HS-SPME with GC-MS. Food Chem. 2023, 418, 135963. [Google Scholar] [CrossRef]
- Yi, X.K.; Liu, G.F.; Rana, M.M.; Zhu, L.W.; Jiang, S.L.; Huang, Y.F.; Wei, S. Volatile Profiling of Two Pear Genotypes with Different Potential for White Pear Aroma Improvement. Sci. Hortic. 2016, 206, 221–228. [Google Scholar] [CrossRef]
- Rudell, D.R.; Mattinson, D.S.; Mattheis, J.P.; Wyllie, S.G.; Fellman, J.K. Investigations of Aroma Volatile Biosynthesis under Anoxic Conditions and in Different Tissues of “Redchief Delicious” Apple Fruit (Malus domestica Borkh.). J. Agric. Food Chem. 2002, 50, 2627–2632. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Gao, W.; Song, M.; Li, M.; He, D.; Wang, Z. Integrated Metabolome and Transcriptome Analysis of Fruit Flavor and Carotenoids Biosynthesis Differences Between Mature-Green and Tree-Ripe of cv. “Golden Phoenix” Mangoes (Mangifera indica L.). Front. Plant Sci. 2022, 13, 816492. [Google Scholar] [CrossRef] [PubMed]
- Shi, F.; Zhou, X.; Zhou, Q.; Tan, Z.; Yao, M.-M.; Wei, B.-D.; Ji, S.-J. Transcriptome Analyses Provide New Possible Mechanisms of Aroma Ester Weakening of ‘Nanguo’ Pear after Cold Storage. Sci. Hortic. 2018, 237, 247–256. [Google Scholar] [CrossRef]

| Class | Number | Class | Number | Class | Number |
|---|---|---|---|---|---|
| Acid | 7 | Ester | 97 | Nitrogen compounds | 4 |
| Alcohol | 34 | Halogenated hydrocarbons | 2 | Phenol | 5 |
| Aldehyde | 29 | Heterocyclic compound | 66 | Sulfur compounds | 3 |
| Amine | 4 | Hydrocarbons | 34 | Terpenoids | 91 |
| Aromatics | 19 | Ketone | 39 |
| Index | Formula | Compounds | Class I | Degree | cpd_ID | kegg_map |
|---|---|---|---|---|---|---|
| XMW1234 | C5H8N2O3 | (2E)-2-(Acetylhydrazono)propanoic acid | Acid | 32 | - | - |
| D456 | C12H24O | 8-Dodecen-1-ol, (Z)- | Alcohol | 34 | - | - |
| KMW0570 | C12H22O | 2-Dodecenal, (E)- | Aldehyde | 34 | - | - |
| KMW0475 | C10H16O | (2E,4Z)-2,4-Decadienal | Aldehyde | 30 | - | - |
| KMW0373 | C9H10O2 | Benzoic acid, ethyl ester | Ester | 43 | - | - |
| NMW0203 | C12H22O4 | Diisopropyl adipate | Ester | 34 | C14523 | - |
| KMW0580 | C12H20O2 | 2,4-Decadienoic acid, ethyl ester, (E,Z)- | Ester | 32 | C08486 | - |
| NMW0070 | C9H8O2 | 1,2-Propanedione, 1-phenyl- | Ketone | 42 | C17268 | ko00360, ko01100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Ma, C.; Li, S.; Wang, H.; Fang, L.; Feng, J.; Wang, Y.; Li, Z.; Cai, Q.; Geng, X.; et al. Eight Typical Aroma Compounds of ‘Panguxiang’ Pear during Development and Storage Identified via Metabolomic Profiling. Life 2023, 13, 1504. https://doi.org/10.3390/life13071504
Li H, Ma C, Li S, Wang H, Fang L, Feng J, Wang Y, Li Z, Cai Q, Geng X, et al. Eight Typical Aroma Compounds of ‘Panguxiang’ Pear during Development and Storage Identified via Metabolomic Profiling. Life. 2023; 13(7):1504. https://doi.org/10.3390/life13071504
Chicago/Turabian StyleLi, Huiyun, Chaowang Ma, Shunfu Li, Huimin Wang, Lisha Fang, Jian Feng, Yanmei Wang, Zhi Li, Qifei Cai, Xiaodong Geng, and et al. 2023. "Eight Typical Aroma Compounds of ‘Panguxiang’ Pear during Development and Storage Identified via Metabolomic Profiling" Life 13, no. 7: 1504. https://doi.org/10.3390/life13071504






