Extracellular Vesicles as Next-Generation Biomarkers in Lung Cancer Patients: A Case Report on Adenocarcinoma and Squamous Cell Carcinoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Clinical Examination
2.3. Sampling
2.4. EVs Isolation
2.5. EVs Characterization
2.6. Statistical Analysis
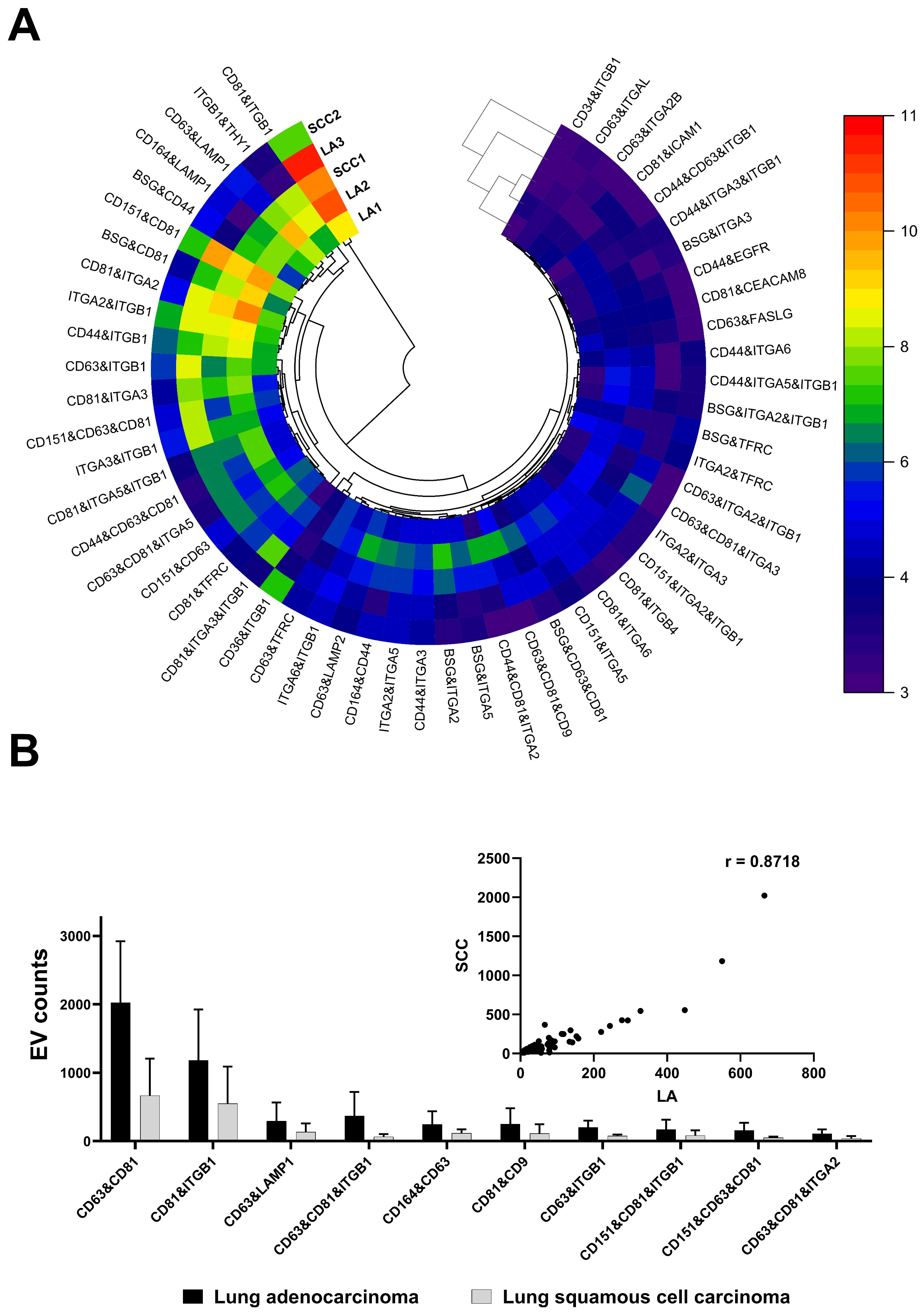
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, D.; Yan, J.; Shen, X.; Sun, Y.; Thulin, M.; Cai, Y.; Wik, L.; Shen, Q.; Oelrich, J.; Qian, X.; et al. Profiling surface proteins on individual exosomes using a proximity barcoding assay. Nat. Commun. 2019, 10, 3854. [Google Scholar] [CrossRef]
- Wang, X.; Tian, L.; Lu, J.; Ng, I.O.-L. Exosomes and cancer—Diagnostic and prognostic biomarkers and therapeutic vehicle. Oncogenesis 2022, 11, 54. [Google Scholar] [CrossRef]
- Moeinzadeh, L.; Razeghian-Jahromi, I.; Zarei-Behjani, Z.; Bagheri, Z.; Razmkhah, M. Composition, Biogenesis, and Role of Exosomes in Tumor Development. Stem Cells Int. 2022, 2022, 8392509. [Google Scholar] [CrossRef]
- Ferguson, S.; Yang, K.S.; Weissleder, R. Single extracellular vesicle analysis for early cancer detection. Trends Mol. Med. 2022, 28, 681–692. [Google Scholar] [CrossRef]
- Liu, S.-Y.; Liao, Y.; Hosseinifard, H.; Imani, S.; Wen, Q.; Biology, D. Diagnostic role of extracellular vesicles in cancer: A comprehensive systematic review and meta-analysis. Front. Cell. Dev. Biol. 2021, 9, 705791. [Google Scholar] [CrossRef]
- Bebelman, M.P.; Janssen, E.; Pegtel, D.M.; Crudden, C. The forces driving cancer extracellular vesicle secretion. Neoplasia 2021, 23, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Karhemo, P.R.; Hyvönen, M.; Laakkonen, P. Metastasis-associated cell surface oncoproteomics. Front. Pharmacol. 2012, 3, 192. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, R.; Xu, B.; Bhullar, R.P.; Chelikani, P. Chapter Twelve—Expression of G Protein-Coupled Receptors in Mammalian Cells. In Methods in Enzymology; Shukla, A.K., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 556, pp. 267–281. [Google Scholar]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef] [PubMed]
- Lam, K.C.K.; Lam, M.K.N.; Chim, C.S.; Chan, G.C.F.; Li, J.C.B. The functional role of surface molecules on extracellular vesicles in cancer, autoimmune diseases, and coagulopathy. J. Leukoc. Biol. 2020, 108, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Hallal, S.; Tűzesi, Á.; Grau, G.E.; Buckland, M.E.; Alexander, K.L. Understanding the extracellular vesicle surface for clinical molecular biology. J. Extracell. Vesicles 2022, 11, e12260. [Google Scholar] [CrossRef] [PubMed]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- WHO Classification of Tumours Editorial Board. Thoracic Tumours, 5th ed.; IARC: Lyon, France, 2021; Volume 5, Available online: https://publications.iarc.fr/Book-And-Report-Series/Who-Classification-Of-Tumours/Thoracic-Tumours-2021 (accessed on 28 January 2024 ).
- Ruzycka-Ayoush, M.; Nowicka, A.M.; Kowalczyk, A.; Gluchowska, A.; Targonska, A.; Mosieniak, G.; Sobczak, K.; Donten, M.; Grudzinski, I.P. Exosomes derived from lung cancer cells: Isolation, characterization, and stability studies. Eur. J. Pharm. Sci. Off. J. Eur. Fed. Pharm. Sci. 2023, 181, 106369. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Deng, W.; Yuan, H.; Zhu, D.; Zhao, R.; Zhang, P.; Xue, J.; Yuan, Z.; Zhang, T.; Jiang, Y.; et al. Single extracellular vesicle surface protein-based blood assay identifies potential biomarkers for detection and screening of five cancers. Mol. Oncol. 2024, 18, 743–761. [Google Scholar] [CrossRef]
- Funakoshi, T.; Tachibana, I.; Hoshida, Y.; Kimura, H.; Takeda, Y.; Kijima, T.; Nishino, K.; Goto, H.; Yoneda, T.; Kumagai, T.; et al. Expression of tetraspanins in human lung cancer cells: Frequent downregulation of CD9 and its contribution to cell motility in small cell lung cancer. Oncogene 2003, 22, 674–687. [Google Scholar] [CrossRef] [PubMed]
- Sadej, R.; Grudowska, A.; Turczyk, L.; Kordek, R.; Romanska, H.M. CD151 in cancer progression and metastasis: A complex scenario. Lab. Investig. 2014, 94, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Kumari, S.; Devi, G.t.; Badana, A.; Dasari, V.R.; Malla, R.R. CD151-A Striking Marker for Cancer Therapy. Biomark. Cancer 2015, 7, 7–11. [Google Scholar] [CrossRef]
- Valdembri, D.; Serini, G. The roles of integrins in cancer. Fac. Rev. 2021, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Su, C.Y.; Li, J.Q.; Zhang, L.L.; Wang, H.; Wang, F.H.; Tao, Y.W.; Wang, Y.Q.; Guo, Q.R.; Li, J.J.; Liu, Y.; et al. The Biological Functions and Clinical Applications of Integrins in Cancers. Front. Pharmacol. 2020, 11, 579068. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhao, Y.; Yuan, Z.; Qin, G. Research advances on structure and biological functions of integrins. SpringerPlus 2016, 5, 1094. [Google Scholar] [CrossRef]
- Blandin, A.F.; Renner, G.; Lehmann, M.; Lelong-Rebel, I.; Martin, S.; Dontenwill, M. β1 Integrins as Therapeutic Targets to Disrupt Hallmarks of Cancer. Front. Pharmacol. 2015, 6, 279. [Google Scholar] [CrossRef]
- Matsumoto, Y.; Kage, H.; Morota, M.; Zokumasu, K.; Ando, T.; Maemura, K.; Watanabe, K.; Kawakami, M.; Hinata, M.; Ushiku, T.; et al. Integrin alpha 2 is associated with tumor progression and postoperative recurrence in non-small cell lung cancer. Jpn. J. Clin. Oncol. 2023, 53, 63–73. [Google Scholar] [CrossRef]
- Li, Y.; Sun, C.; Tan, Y.; Zhang, H.; Li, Y.; Zou, H. ITGB1 enhances the Radioresistance of human Non-small Cell Lung Cancer Cells by modulating the DNA damage response and YAP1-induced Epithelial-mesenchymal Transition. Int. J. Biol. Sci. 2021, 17, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Human Protein Atlas. Available online: https://www.proteinatlas.org/ (accessed on 2 February 2024).
- Ranjbar, M.A.; Jamshidi, M. Overexpression of Lysosome-Associated Membrane Protein 1 in Oral Squamous Cell Carcinoma and its Correlation with Tumor Differentiation and Metastasis. Iran. J. Otorhinolaryngol. 2022, 34, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.L.; Huang, A.F.; Huang, S.M.; Ho, C.L.; Chang, Y.L.; Chan, J.Y. CD164 promotes lung tumor-initiating cells with stem cell activity and determines tumor growth and drug resistance via Akt/mTOR signaling. Oncotarget 2017, 8, 54115–54135. [Google Scholar] [CrossRef] [PubMed]


| Patient | WHO Classification | Histopathologic Grade | Pathologic Stage Classification (pTNM) |
|---|---|---|---|
| H22/518 F/75 | Squamous cell carcinoma | G3 | pT3, N0, Mx, PL0, V0/L1, R0 |
| H22/987 F/78 | Adenocarcinoma | G1 | pT2b, N0, Mx, PL0, L0/V0, R0 |
| H22/3994 M/64 | Squamous cell carcinoma | G2 | pT2a, pN0, Mx PL1, L0/V0, R0 |
| H22/9303 F/62 | Adenocarcinoma | G2 | pT2a, pN0, Mx PL1, L0/V0, R0 |
| H22/3486 F/63 | Adenocarcinoma | G2 | pT1b, N0, Mx PL1, L0/V0, R0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruzycka-Ayoush, M.; Prochorec-Sobieszek, M.; Cieszanowski, A.; Glogowski, M.; Szumera-Cieckiewicz, A.; Podgorska, J.; Targonska, A.; Sobczak, K.; Mosieniak, G.; Grudzinski, I.P. Extracellular Vesicles as Next-Generation Biomarkers in Lung Cancer Patients: A Case Report on Adenocarcinoma and Squamous Cell Carcinoma. Life 2024, 14, 408. https://doi.org/10.3390/life14030408
Ruzycka-Ayoush M, Prochorec-Sobieszek M, Cieszanowski A, Glogowski M, Szumera-Cieckiewicz A, Podgorska J, Targonska A, Sobczak K, Mosieniak G, Grudzinski IP. Extracellular Vesicles as Next-Generation Biomarkers in Lung Cancer Patients: A Case Report on Adenocarcinoma and Squamous Cell Carcinoma. Life. 2024; 14(3):408. https://doi.org/10.3390/life14030408
Chicago/Turabian StyleRuzycka-Ayoush, Monika, Monika Prochorec-Sobieszek, Andrzej Cieszanowski, Maciej Glogowski, Anna Szumera-Cieckiewicz, Joanna Podgorska, Alicja Targonska, Kamil Sobczak, Grazyna Mosieniak, and Ireneusz P. Grudzinski. 2024. "Extracellular Vesicles as Next-Generation Biomarkers in Lung Cancer Patients: A Case Report on Adenocarcinoma and Squamous Cell Carcinoma" Life 14, no. 3: 408. https://doi.org/10.3390/life14030408





