Chrono-Endocrinology in Clinical Practice: A Journey from Pathophysiological to Therapeutic Aspects
Abstract
:1. Introduction
2. General Aspects of Biological Rhythms
2.1. Rhythm Parameters
2.2. Molecular Circadian Machinery
2.3. Synchronization Schedule and Chronotype
3. Endocrine Chronophysiology and Chronopathology
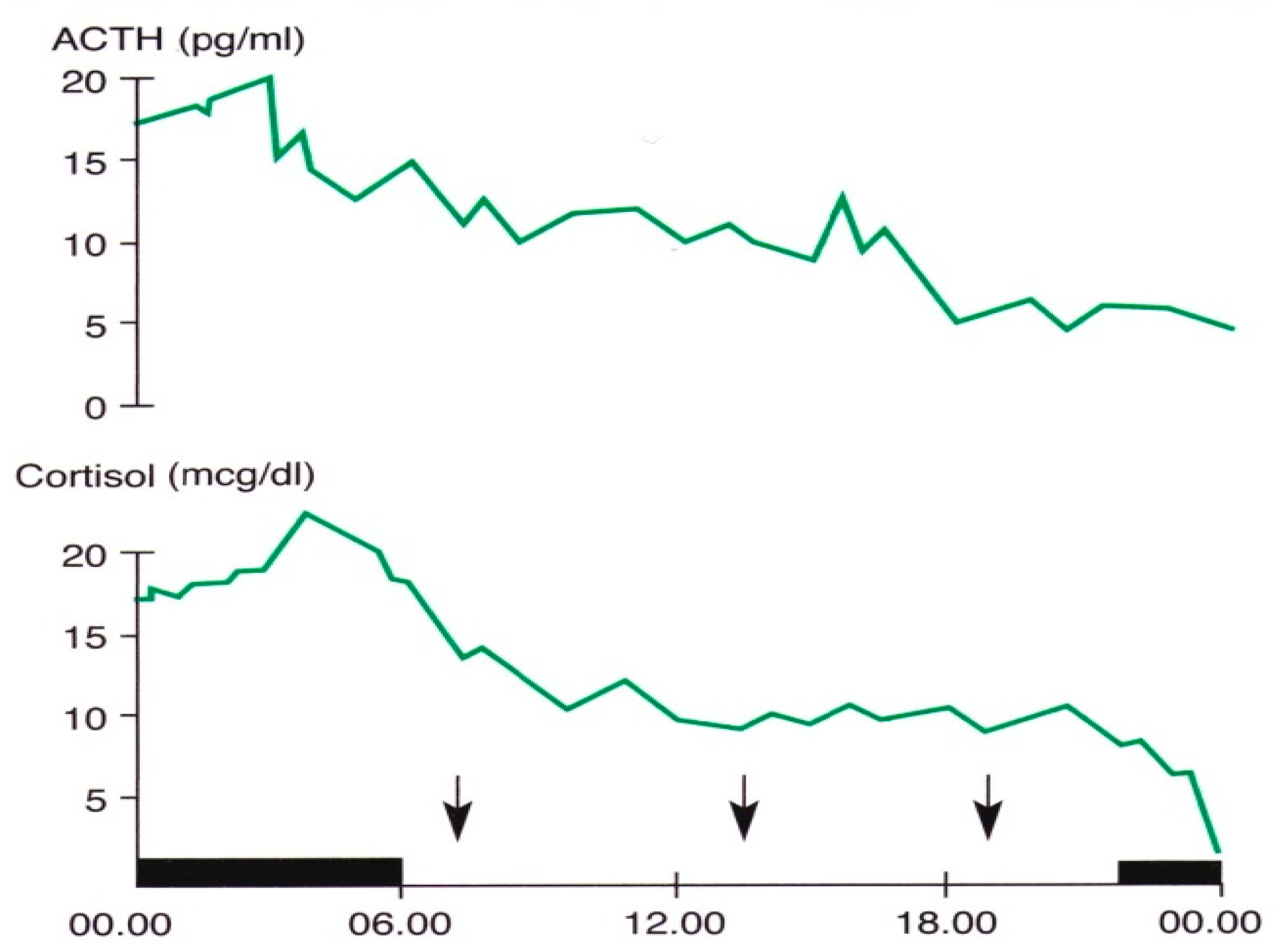
3.1. Chrono-Organization of the Hypothalamic–Pituitary–Adrenal Axis
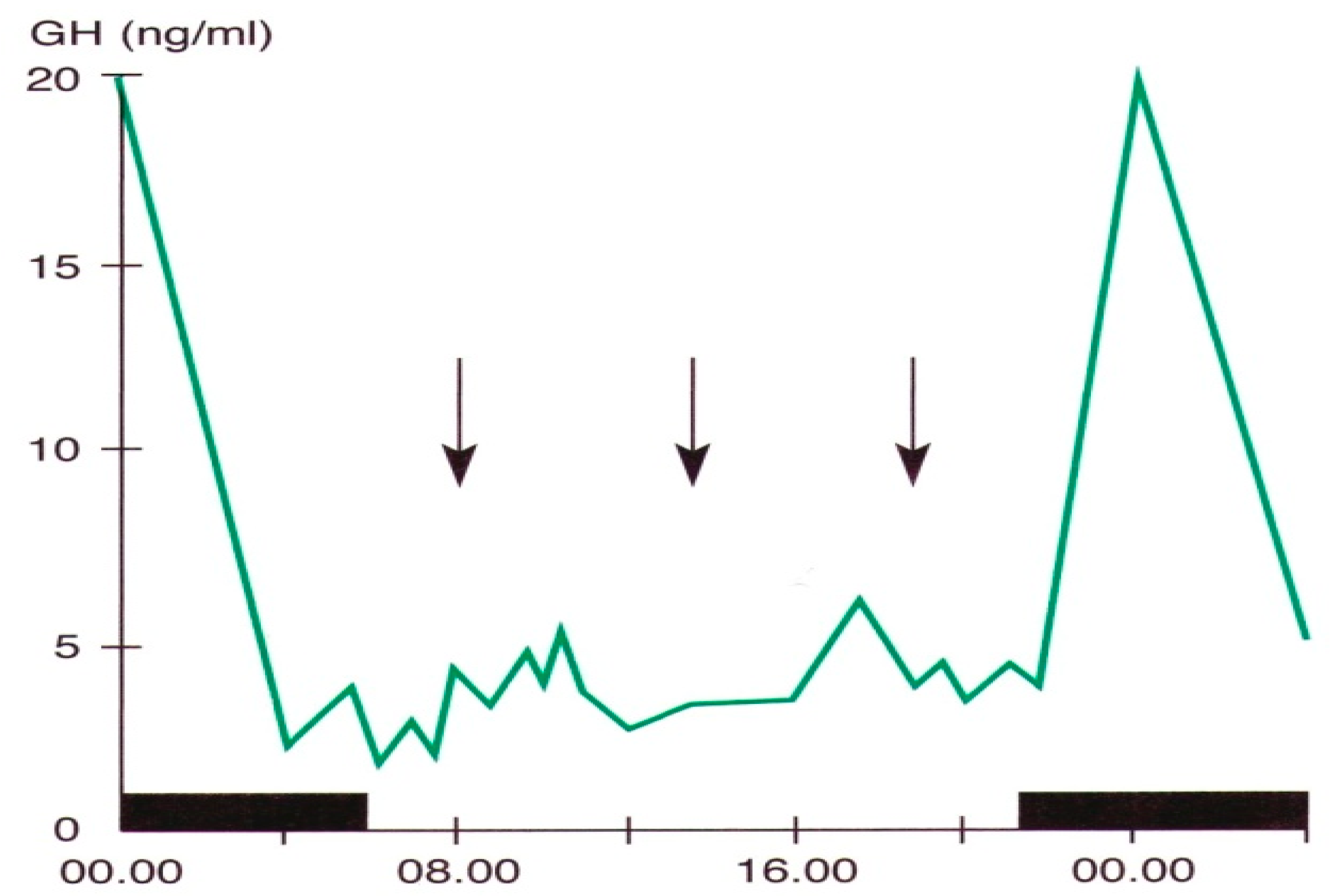
3.2. Growth Hormone
3.3. Prolactin
3.4. Hypothalamic–Pituitary–Thyroid Axis
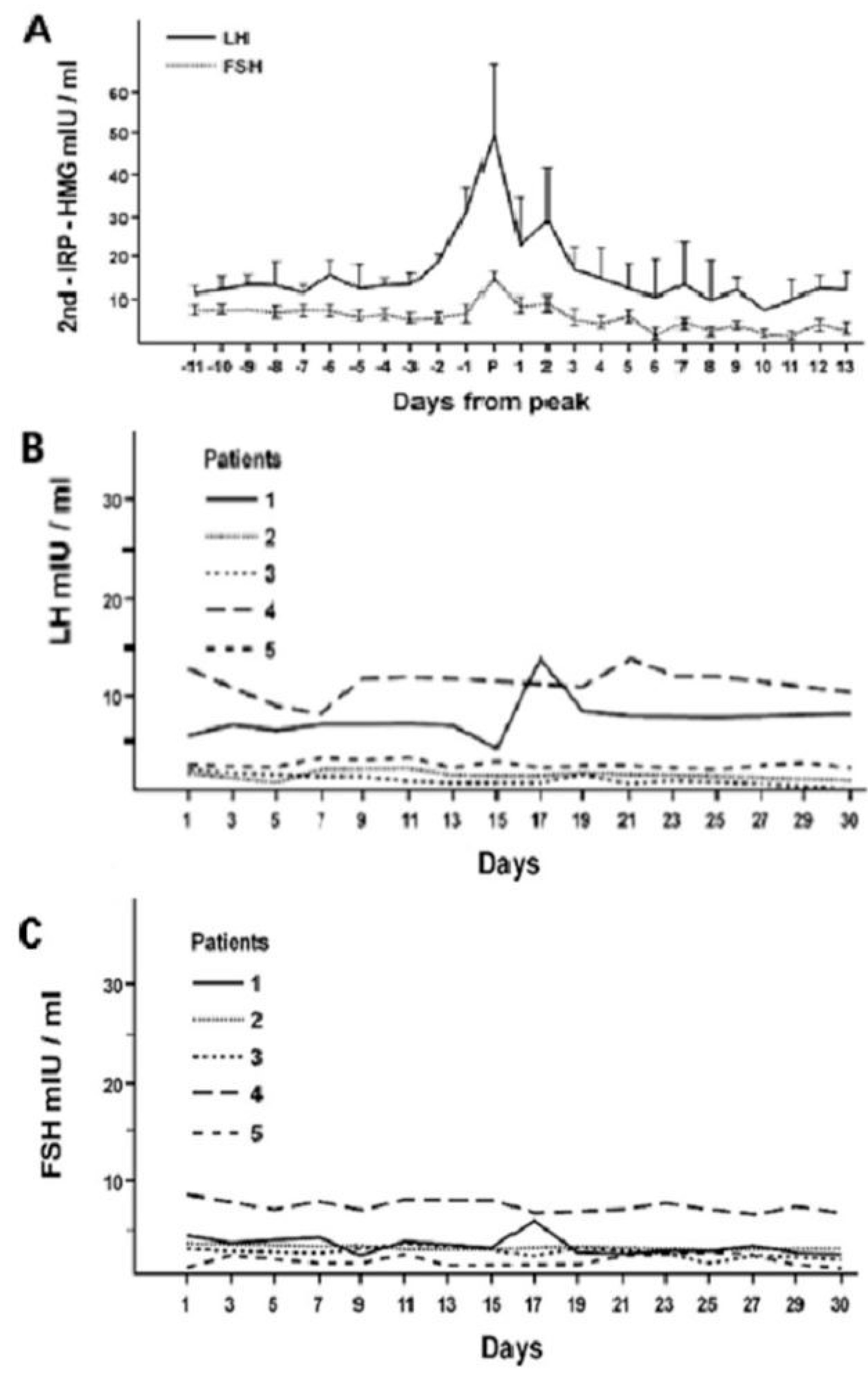
3.5. Hypothalamic–Pituitary–Gonadal Axis
3.6. Insulin, Leptin, and Ghrelin
4. Elementary Principles of Chronotherapy
- Maintain an optimal circadian organization of the individual to be treated;
- Timing the administration of drugs and targeting the biological clock;
- Replacement therapy carried out, if possible, mimicking the circadian rhythm of the variable to be replaced;
- Looking for and use of chronobiotic drugs capable of recovering desynchronized rhythms.
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Touitou, Y.; Haus, E. (Eds.) Biological rhythms from biblical to modern times. In Biological Rhythms in Clinical and Laboratory Medicine; Springer: Berlin/Heidelberg, Germany, 1992; pp. 1–5. [Google Scholar]
- Allada, R.; Bass, J. Circadian mechanisms in Medicine. N. Engl. J. Med. 2021, 384, 550–561. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Sassone-Corsi, P. The emerging link between cancer, metabolism and circadian rhythms. Nat. Med. 2019, 24, 1795–1803. [Google Scholar] [CrossRef] [PubMed]
- Sancar, A.; Van Gelder, R.N. Clocks, cancer, and chronotherapy. Science 2021, 371, eabb0738. [Google Scholar] [CrossRef]
- Hedlund, L.W.; Franz, J.M.; Kenny, A.D. Biological Rhythms and Endocrine Function; Plenum Press: New York, NY, USA, 1976; pp. 1–194. [Google Scholar]
- Halberg, F.; Katinas, G.S.; Chiba, Y.; Garcia-Sanz, M.; Krovats, T.G.; Kinnel, H.; Montalbetti, N.; Reinberg, A.; Scharf, R.; Simpson, H. Chronobiology glossary of the International Society for the study of biological rhythms. Int. J. Chronobiol. 1973, 1, 31–63. [Google Scholar] [PubMed]
- Kalsbeek, A.; Fliers, E. Circadian and endocrine rhythms. Best. Pract. Res. Clin. Endocrinol. Metab. 2017, 31, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, G.; De Bellis, A.; Maiorino, M.; Paglionico, V.A.; Esposito, K.; Bellastella, A. Endocrine rhythms and sport: It is time to take time into account. J. Endocrinol. Investig. 2019, 42, 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, G.; Maiorino, M.I.; Scappaticcio, L.; De Bellis, A.; Mercadante, S.; Esposito, K.; Bellastella, A. Chronothyroidology: Chronobiological aspects in thyroid function and diseases. Life 2021, 11, 426. [Google Scholar] [CrossRef] [PubMed]
- Talamanca, L.; Gobet, C.; Naef, F. Sex-dimorphic and age-dependent organization of 24-hour gene expression rhythm in humans. Science 2023, 377, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Doi, M.; Hirayama, J.; Sassone-Corsi, P. Circadian regulator CLOCK is a histone acetyltransferase. Cell 2006, 125, 497–508. [Google Scholar] [CrossRef]
- Greco, C.M.; Sassone-Corsi, P. Circadian blueprint of metabolic pathways in the brain. Nat. Rev. Neurosci. 2018, 20, 71–82. [Google Scholar] [CrossRef]
- Ralph, M.R.; Foster, R.G.; Davis, F.C.; Menaker, M. Transplanted suprachiasmatic nucleus determines circadian period. Science 1990, 247, 975–978. [Google Scholar] [CrossRef] [PubMed]
- Hardin, P.E.; Hall, J.C.; Rosbash, M. Feed-back of the Drosophila period gene product on circadian cycling of its messanger RNA levels. Nature 1990, 343, 536–540. [Google Scholar] [CrossRef] [PubMed]
- King, D.P.; Zhao, Y.; Sangoram, A.M.; Wilsbacher, L.D.; Tanaka, M.; Antoch, M.P.; Steeves, T.D.; Vitaterna, M.H.; Kornhauser, J.M.; Lowrey, P.L.; et al. Positional cloning of mouse circadian clock gene. Cell 1997, 89, 641–653. [Google Scholar] [CrossRef]
- Takahashi, J.R.; Hong, H.K.; Ko, C.H.; McDearmon, E.L. The genetics of mammalian circadian order and disorder: Implications for physiology and disease. Nat. Rev. Genet. 2008, 9, 764–775. [Google Scholar] [CrossRef]
- Bur, E.D.; Takahashi, J.S. Molecular components of the mammalian circadian clock. Handb. Exp. Pharmacol. 2013, 217, 3–27. [Google Scholar]
- Wagner, P.M.; Prucca, C.G.; Caputto, B.L.; Guido, M.E. Adjusting the molecular clock: The importance of circadian rhythms in the development of glioblastomas and its intervention as a therapeutic strategy. Int. J. Mol. Sci. 2021, 22, 8289. [Google Scholar] [CrossRef]
- Guido, M.E.; Monjes, N.M.; Wagner, P.M.; Salvador, G.A. Circadian regulation and clock-controlled mechanisms of glycerophospholipid metabolism from neuronal cells and tissues to fibroblast. Mol. Neurobiol. 2022, 59, 326–353. [Google Scholar] [CrossRef]
- Zhang, E.E.; Liu, Y.; Dentin, R.; Pongsawakul, P.Y.; Liu, A.C.; Hirota, T.; Nusinow, D.A.; Sun, X.; Landais, S.; Kodama, Y.; et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat. Med. 2010, 16, 1152–1156. [Google Scholar] [CrossRef]
- Hirota, T.; Wooklee, J.; St John, P.C.; Sawa, M.; Iwaisako, K.; Noguchi, T.; Pongsawakul, P.Y.; Sonntag, T.; Welsh, D.K.; Brenner, D.A.; et al. Identification of small molecule activators of cryptochrome. Science 2012, 337, 1094–1097. [Google Scholar] [CrossRef]
- Shafi, A.A.; McNair, C.M.; McCann, J.J.; Alshalalfa, M.; Shostak, A.; Severson, T.M.; Zhu, Y.; Bergman, A.; Gordon, N.; Mandigo, A.C.; et al. The circadian cryptochrome, CRY1, is a pro-tumorigenic factor that rhythmically modulates DNA repair. Nat Comm. 2021, 12, 401. [Google Scholar] [CrossRef]
- Melandez-Fernandez, O.H.; Liu, J.A.; Nelson, R.J. Circadiand rhythms disrupted by light at night and mistimed food intake alter hormonal rhythms and metabolism. Int. J. Mol. Sci. 2023, 24, 3392. [Google Scholar] [CrossRef] [PubMed]
- Challet, E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019, 15, 393–403. [Google Scholar] [CrossRef] [PubMed]
- Parker, D.; Sassin, J.F.; Mace, J.W.; Godin, R.W.; Grossman, L.G. Human growth hormone release during sleep: Electroencephalographic correlation. J. Clin. Endocrinol. Metab. 1969, 29, 871–874. [Google Scholar] [CrossRef] [PubMed]
- Oda, H. Chrononutrition. J. Nutr. Sci. Vitaminol. 2015, 61, S92–S94. [Google Scholar] [CrossRef] [PubMed]
- Mirstberger, R.E. Neurobiology of food anticipatory circadian rhyhms. Physiol. Behav. 2011, 104, 535–545. [Google Scholar] [CrossRef] [PubMed]
- Heden, T.D.; Kanaley, J.A. Syncing exercise with meals and circadian clocks. Exerc. Sport. Sci. Rev. 2019, 47, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Brezinski, A. Melatonin in human. N. Engl. J. Med. 1997, 336, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Roennenberg, T.; Wirz-Justice, A.; Merrow, M. Life between clocks: Daily temporal pattern of human chronotypes. J. Biol. Rhythms 2003, 18, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Muscogiuri, G.; Barrea, L.; Aprano, S.; Framondi, L.; Di Matteo, R.; Laudisio, D.; Colao, A. Chronotype and adherence to the mediterranean diet in obesity: Results from the opera prevention project. Nutrients 2020, 12, 1354. [Google Scholar] [CrossRef]
- Horne, J.A.; Ostberg, O. A self assessment questionnaire to determine morningness/eveningness in human circadian rhythms. Int. J. Chronobiol. 1976, 4, 97–110. [Google Scholar]
- Mazri, F.H.; Manaf, Z.A.; Shahar, S.; Ludin, A.F.M. The association between chronotype and dietary pattern among adults: A scoping review. Int. J. Environ. Res. Public. Health 2020, 17, 68. [Google Scholar] [CrossRef] [PubMed]
- Makarem, N.; Paul, J.; Giardina, E.G.V.; Liao, M.; Aggarwal, B. Evening chronotype is associated with poor cardiovascular health and adverse health behaviors in a diverse population of women. Chronobiol. Int. 2020, 37, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Von Behren, J.; Hurley, S.; Goldberg, D.; Clague DeHart, J.; Wang, S.S.; Reynolds, P. Chronotype and risk of post-menopausal endometrial cancer in the California Teachers Study. Chronobiol. Int. 2021, 38, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Kinouchi, K.; Sassone-Corsi, P. Circadian clocks, epigenetics, and cancer. Current Opin. Oncol. 2015, 27, 50–56. [Google Scholar] [CrossRef]
- Saehong Oh, E.; Petronis, A. Origins of human disease. The chrono-epigenetic perspective. Nat. Rev. Genet. 2021, 22, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Pilorz, V.; Olejniczack, I.; Oster, H. Studying circadian clock entrainment by hormonal signals. Methods Mol. Biol. 2022, 2482, 137–152. [Google Scholar] [CrossRef] [PubMed]
- Melendez-Fernandez, O.H.; Walton, J.C.; Courtney DeVries, A.; Nelson, R.J. Clocks, rhythms, sex, and hearts: How disrupted circadian rhythms, time-of-day, and sex influence cardiovascular health. Biomolecules 2021, 11, 883. [Google Scholar] [CrossRef] [PubMed]
- Bailey, M.; Silver, R. Sex differences in circadian timing systems: Implications for disease. Front. Neuroendocrinol. 2014, 35, 111–139. [Google Scholar] [CrossRef] [PubMed]
- Haus, E. Chronobiology in the endocrine system. Adv. Drug Deliv. Rev. 2007, 59, 985–1014. [Google Scholar] [CrossRef]
- Tonsfeldt, K.J.; Chappel, P.E. Clocks on top: The role of the circadian clock in the hypothalamic and pituitary regulation of endocrine physiology. Mol. Cell Endocrinol. 2012, 349, 3–12. [Google Scholar] [CrossRef]
- Gamble, K.L.; Berry, R.; Frank, S.J.; Young, M.E. Circadian clock control of endocrine factors. Nat. Rev. Endocrinol. 2014, 10, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Lightman, S.; Birnie, M.T.; Conway-Capbell, B.L. Dynamics of ACTH and cortisol secretion and implication for disease. Endocr. Rev. 2021, 41, bnaa002. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, K.; Iwasaki, Y.; Daimom, M. Hypothalamic regulation of corticotrophin-releasing factor under stress and stress resilience. Int. J. Mol. Sci. 2021, 22, 12242. [Google Scholar] [CrossRef]
- Pincus, G. A diurnal rhythm of excretion of urinary ketosteroids in young men. J. Clin. Endocrinol. 1943, 3, 195–198. [Google Scholar] [CrossRef]
- Jones, J.R.; Chaturvedi, S.; Granados-Fuentes, D.; Herzog, E.D. Circadian neurons in the paraventricular nucleus entrain and sustain daily rhythms in glucocorticoids. Nat. Commun. 2021, 12, 5763. [Google Scholar] [CrossRef] [PubMed]
- Malek, H.; Ebadzadeh, M.M.; Safabakhsh, R.; Razavi, A. Mathematical analysis of the role of pituitary-adrenal interactions in ultradian rhythms of the HPA axis. Comput. Biol. Med. 2021, 135, 104580. [Google Scholar] [CrossRef]
- Walker, J.J.; Terry, J.R.; Lightman, S.L. Origin of ultradian pulsatility in the hypothalamic–pituitary–adrenal axis. Proc. Biol. Sci. 2010, 277, 1627–1633. [Google Scholar] [CrossRef]
- Flynn-Evans, E.E.; Tabendeh, H.; Skene, D.J.; Lockley, S.W. Circadian rhythm disorders and melatonin production in 127 blind women with and without light perception. J. Biol. Rhythms 2014, 29, 215–224. [Google Scholar] [CrossRef]
- Czeisler, C.A.; Shanahan, T.L.; Klerman, E.B.; Martens, H.; Brotman, D.J.; Ermens, J.S.; Klein, T.; Rizzo, J.F. Suppression of melatonin secretion in some blind patients by exposure to bright light. N. Engl. Med. 1995, 332, 6–11. [Google Scholar] [CrossRef]
- Hull, J.T.; Czeisler, C.A.; Lockley, S.W. Suppression of Melatonin secretion in totally visually blind people by ocular exposure to white light: Clinical characteristics. Ophthalmology 2018, 125, 1160–1171. [Google Scholar] [CrossRef]
- D’Alessandro, B.; Bellastella, A.; Esposito, V.; Colucci, C.F.; Montalbetti, N. Circadian rhythm of cortisol secretion in elderly and blind subjects. Br. Med. J. 1974, 2, 274–276. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, A.; Amato, G.; Bizzarro, A.; Carella, C.; Criscuolo, T.; Iorio, S.; Pisano, G.; Sinisi, A.A.; De Bellis, A. Light, blindness and endocrine secretions. J. Endocrinol. Investig. 1999, 22, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Al-Safi, Z.A.; Polotsky, A.; Chosich, J.; Roth, L.; Allshouse, A.A.; Bradford, A.P.; Santoro, N. Evidence for disruption of normal circadian cortisol rhythm in women with obesity. Gynecol. Endocrinol. 2018, 34, 336–340. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.H.; Joo, Y.; Kim, M.S.; Choe, H.K.; Tomg, Q.; Kwon, Q. Effects of intermittent fasting on the circulating levels and circadian rhythms of hormones. Endocrinol. Metab. 2021, 36, 745–756. [Google Scholar] [CrossRef] [PubMed]
- Boivin, D.B.; Boudreau, P.; Kasmadoupoulos, A. Disturbance of the circadian system in shift work and its health impact. J. Biol. Rhythms 2022, 37, 3–28. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.H., 2nd; Walton, J.C.; Courtney DeVries, A.; Nelson, R.J. Circadian rhythm disruption and mental health. Trend Psychiatry 2020, 10, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Bilgin, A.; Heinonen, K.; Girchenco, P.; Kajantie, E.; Wolke, D.; Raikkonen, K. Early childhood multiple or persistent regulatory problems and diurnal salivary cortisol in young adulthood. Psychoneuroendocrinology 2024, 161, 106940. [Google Scholar] [CrossRef] [PubMed]
- Al-Turk, W.; Al-Dujaili, E.A.S. Effect of age, gender and exercise on salivary deydroepiandrosterone circadian rhythm profile in human volunteers. Steroids 2016, 106, 19–25. [Google Scholar] [CrossRef]
- Quabbe, H.J. Chronobiology of growth hormone secretion. Chronobiologia 1977, 4, 217–246. [Google Scholar]
- Bellastella, A.; Colucci, C.F.; D’Alessadro, B.; Lo Cicero, M. L-Dopa stimulated growth hormone in the blind. J. Clin. Endocrinol. Metab. 1977, 44, 194–195. [Google Scholar] [CrossRef]
- Bellastella, A.; Sinisi, A.A.; Raiola, C.; Perrone, L.; Iorio, S.; Parlato, F.; Mazzuca, A.; Faggiano, M. Blindness influences the growth of institutionalized prepubertal subjects. J. Endocrinol. Investig. 1989, 12, 805–809. [Google Scholar] [CrossRef]
- Amato, G.; Carella, C.; Fazio, S.; La Montagna, G.; Cittadini, A.; Sabatini, D.; Marciano-Mone, C.; Saccà, L.; Bellastella, A. Body composition, bone metabolism, and heart structure and function in growth hormone (GH)-deficient adults before and after GH replacement therapy at low doses. J. Clin. Endocrinol. Metab. 1993, 77, 1671–1676. [Google Scholar] [CrossRef]
- Dos Santos, W.O.; Gusmao, D.O.; Wasinski, F.; List, E.O.; Kopchick, J.J.; Donato, J., Jr. Effects of growth hormone receptor ablation in corticotropin-releasing hormone cells. Int. J. Mol. Sci. 2021, 22, 9908. [Google Scholar] [CrossRef]
- Bellastella, A.; Criscuolo, T.; Mango, A.; Perrone, L.; Sinisi, A.A.; Faggiano, M. Circannual rhythms of plasma kuteinizing hormone, follicle-stimulating hormone, testosterone, prolactin and cortisol in prepuberty. Clin. Endocrinol. 1983, 19, 453–459. [Google Scholar] [CrossRef]
- Donato, J., Jr.; Frazao, R. Interactions between prolactin and kisspeptin to control reproduction. Arch. Endocrinol. Metab. 2016, 60, 5087–5095. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.C. Somatostatin and its receptor family. Front. Neuroendocrinol. 1999, 20, 157–198. [Google Scholar] [CrossRef] [PubMed]
- Thoss, V.S.; Perez, J.; Probst, A.; Hoyer, D. Expression of five somatostatin receptor mRNAs in the human brain and pituitary. Naunyn Schmiedebergs Arch. Pharmacol. 1996, 354, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Kalsbeek, A.; Fliers, E.; Franke, A.N.; Wortel, J.; Buijs, R.M. Functional connections between the suprachiasmatic nucleus and the thyroid gland as revealed by lesioning and viral tracing techniques in the rat. Endocrinology 2000, 141, 3832–3841. [Google Scholar] [CrossRef] [PubMed]
- Romijn, J.A.; Wiersinga, W.M. Decreased nocturnal surge of thyrotropin in nonthyroidal illness. J. Clin. Endocrinol. Metab. 1990, 70, 35–42. [Google Scholar] [CrossRef]
- Roelfsema, F.; Boelen, A.; Kalsbeek, A.; Fliers, E. Regulatory aspects of the human hypothalamus-pituitary-thyroid axis. Best. Pract. Res. Clin. Endocrinol. 2017, 31, 487–503. [Google Scholar] [CrossRef]
- Bellastella, G.; Maiorino, M.I.; Scappaticcio, L.; Casciano, O.; Petrizzo, M.; Caputo, M.; Paglionico, V.A.; Giugliano, D.; Esposito, K. TSH oscillations in young patients with type 1 diabetes may be due to glycemic variability. J. Endocrinol. Investig. 2017, 41, 389–393. [Google Scholar] [CrossRef] [PubMed]
- Ono, H.; Hoshimo, Y.; Watanabe, M.; Nakane, Y.; Murai, A.; Ebihara, S.; Korf, H.W.; Yoshimura, T. Involvement of thyrotropin in photoperiodic signal transduction. Proc. Natl. Acad. Sci. USA 2008, 105, 18238. [Google Scholar] [CrossRef] [PubMed]
- Quignon, C.; Beymer, M.; Gauthier, K.; Gauer, F.; Simonneaux, V. Thyroid hormone receptors are required for the melatonin- dependent control of Rfrp gene expression in mice. FASEB J. 2020, 34, 12072–12082. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.V.; Turner, C.W. Effect of light and darkness upon thyroid secretion rate and on the endocrine glands of female rats. Proc. Soc. Exp. Biol. Med. 1969, 131, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Seo, H.; Lernmark, A.; Refetoff, S. Ontogenetic pattern of thyrotropin-releasing hormone-like material in rat hypothalamus, pancreas and retina. Proc. Nat. Acad. Sci. USA 1980, 77, 4345–4350. [Google Scholar] [CrossRef] [PubMed]
- Petterborg, L.J.; Vaughan, M.K.; Johnson, L.Y.; Champney, T.H.; Reiter, R.J. Modification of testicular and thyroid function by chronic exposure to short photoperiod: A comparison in four rodent species. Comp. Biochem. Physiol. 1984, 78, 31–37. [Google Scholar] [CrossRef]
- Sharp, P.J.; Klandorf, H.; Lea, R.W. Influence of lighting cycles on daily rhythms in concentrations of plasma triiodotyronine and thyroxine in intact and pinealectomized immature broiler hens (Gallus domesticus). J. Endocrinol. 1984, 103, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.V.; Narang, G.D.; Turner, C.W. Effect of melatonin and its withdrawal on thyroid hormone secretion rate of female rats. J. Endocrinol. 1969, 43, 489–490. [Google Scholar] [CrossRef]
- Bellastella, A.; Criscuolo, T.; Sinisi, A.A.; Iorio, S.; Mazzuca, A.; Parlato, F.; Perrone, L.; Faggiano, M. Plasma thyrotropin, thyroxine, triiodothyronine, free thyroxine, free triiodothyronine and cortisol levels in in blind prepubertal boys. J. Endocrinol. Investig. 1988, 11, 171–174. [Google Scholar] [CrossRef]
- Ikegami, K.; Refetoff, S.; Van Cauter, E.; Yoshimura, T. Interconnection between circadian clocks and thyroid function. Nat. Rev. Endocrinol. 2019, 15, 590–600. [Google Scholar] [CrossRef]
- Kuzmenko, N.V.; Tsyrlin, V.A.; Pliss, M.G.; Galagudza, M.M. Seasonal variations in levels of human thyroid-stimulating hormone and thyroid hormones: A meta-analysis. Chronobiol. Int. 2021, 38, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, A.; Criscuolo, T.; Mango, A.; Perrone, L.; Sinisi, A.A.; Faggiano, M. Circannual rhythms of plasma growth hormone, thyrotropin and thyroid hormones in prepuberty. Clin. Endocrinol. 1984, 20, 531–535. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, A.; Criscuolo, T.; Sinisi, A.A.; Rinaldi, A.; Faggiano, M. Circannual variations of plasma thyrotropin in Klinefelter’ syndrome. Neuroendocrinology 1986, 43, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Yoshihara, A.I.; Noh Jaeduk, Y.; Watanabe, N.; Iwaki, K.; Kuni, Y.; Ohye, H.; Suzuki, M.; Matsumoto, M.; Suzuki, N.; Sugino, K. Seasonal changes in serum thyrotropin concentrations observed from big data obtained during six years from 2010 to 2015 at a single hospital in Japan. Thyroid 2018, 28, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Smals, A.G.H.; Ross, H.A.; Kloppenborg, P.W.C. Seasonal variation in serum T3 and T4 levels in man. J. Clin. Endocrinol. Metab. 1977, 44, 998–1001. [Google Scholar] [CrossRef] [PubMed]
- Palinkas, L.A.; Reed, H.L.; Reedy, K.R.; Do, N.V.; Case, H.S.; Finney, N.S. Circannual pattern of hypothalamic-pituitary-thyroid (HPT) function and mood during extended Antarctic residence. Psychoneuroendocrinology 2001, 268, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Bollinger, T.; Bollinger, A.; Oster, H.; Solbach, W. Sleep, immunity, and circadian clock: A mechanistic model. Gerontology 2010, 56, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Herghenhan, S.; Holtkamp, S.; Schelermann, C. Molecular interactions between components of the circadian clock and the immune system. J. Mol. Biol. 2020, 432, 3700–3713. [Google Scholar] [CrossRef] [PubMed]
- Bargi-Souza, P.; Peliciari-Garcia, R.A.; Nunes, M.T. Disruption of the pituitary circadian clock induced by hypothyroidism and hyperthyroidism: Consequences on daily Pituitary hormone expression profile. Thyroid. 2019, 29, 502–512. [Google Scholar] [CrossRef]
- Philippe, J.; Dibner, C. Thyroid circadian timing: Roles in physiology and thyroid malignancies. J. Biol. Rhythms 2015, 10, 76–83. [Google Scholar] [CrossRef]
- Angelousi, A.; Kassi, E.; Ansari-Nasiri, N.; Randeva, H.; Kaltsas, G. Clock genes and cancer development in particular in endocrine tissues. Endocr. Relat. Cancer 2019, 26, R305–R317. [Google Scholar] [CrossRef] [PubMed]
- Malaguarnera, R.; Ledda, C.; Filippello, A.; Frasca, F.; Francavilla, V.C.; Ramaci, T.; Parisi, M.C.; Rapisarda, V.; Piro, S. Thyroid cancer and circadian clock disruption. Cancer 2020, 12, 3109. [Google Scholar] [CrossRef] [PubMed]
- Mannic, T.; Meyer, P.; Triponez, F.; Pusztaszeni, M.; Le Martelot, G.; Mariani, O.; Schmitter, D.; Sage, D.; Philippe, J.; Dibner, C. Circadian clock characteristics are altered in human thyroid malignant nodules. J. Clin. Endocrinol. Metab. 2013, 98, 4446–4456. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Yin, Y.; Zhang, W. Ghrelin restores the disruption of the circadian clock in steatotic liver. Int. J. Mol. Sci. 2018, 19, 3134. [Google Scholar] [CrossRef]
- Tendler, A.; Bar, A.; Mendelsohn-Cohen, N.; Karin, O.; Kohanim, Y.K.; Maimon, L.; Milo, T.; Mayo, A.; Tanay, A.; Alon, U. Hormone seasonality In medical records suggests circannual endocrine circuits. Proc. Natl. Acad. Sci. USA 2021, 118, e2003926118. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, A.; Criscuolo, T.; Sinisi, A.A.; Iorio, S.; Sinisi, A.M.; Rinaldi, A.; Faggiano, M. Circannual variations of plasma testosterone, luteinizing hormone, follicle-stimulating hormone and prolactin in Klinefelter’s syndrome. Neuroendocrinology 1986, 42, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Bellastella, G.; Pane, E.; Iorio, S.; De Bellis, A.; Sinisi, A.A. Seasonal variations of plasma gonadotropins, prolactin, and testosterone levels in primary and secondary hypogonadism: Evidence for an independent testicular role. J. Endocrinol. Investig. 2013, 36, 339–342. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Granata, A.R.M.; Setti, M.; Tagliavini, S.; Trenti, T.; Simoni, M. Seasonal changes of serum gonadotropins and testosterone in men revealed by a large data set of real-world observations over nine years. Front. Endocrinol. 2020, 10, 914. [Google Scholar] [CrossRef]
- Bellastella, A.; De Bellis, A.; Bellastella, G.; Esposito, K. Opposite influence of light and blindness on pituitary-gonadal function. Front. Endocrinol. 2014, 4, 205. [Google Scholar] [CrossRef]
- Chen, J.; Okimura, K.; Yoshimura, T. Light and hormones in seasonal regulation of reproduction and mood. Endocrinology 2020, 161, bqaa130. [Google Scholar] [CrossRef]
- Sciarra, F.; Franceschini, E.; Campolo, F.; Gianfrilli, D.; Pallotti, F.; Paoli, D.; Isidori, A.M.; Venneri, M.A. Disruption of circadian rhythms: A crucial factor in the etiology of infertility. Int. J. Mol. Sci. 2020, 21, 3943. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Hou, G.; Wang, X.; Chen, H.; Shi, F.; Liu, C.; Zhang, X.; Han, F.; Yang, H.; Zhou, N.; et al. Adverse effects of circadian desynchrony on the male reproductive system: An epidemiological and experimental study. Hum. Reprod. 2020, 35, 1515–1528. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Bai, Y.; Jiang, Y.; Jiang, K.; Tian, Y.; Gu, J.; Sun, F. The potential impacts of circadian rhythm disturbances on male fertility. Front. Endocrinol. 2022, 13, 1001316. [Google Scholar] [CrossRef] [PubMed]
- Panda, S. Circadian physiology of metabolism. Science 2016, 354, 1008–1115. [Google Scholar] [CrossRef] [PubMed]
- Green, C.B.; Takahashi, J.; Bass, J. The meter of metabolism. Cell 2008, 134, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Sahar, S.; Sassone-Corsi, P. Regulation of metabolism: The circadian clock dictates the time. Trends Endocrinol. Metab. 2012, 23, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Balsalobre, A.; Brown, S.A.; Marcacci, L.; Tronche, F.; Reichardt, H.M.; Schibler, U. Resetting of circadian time in peripheral tissues by glucocorticoid signaling. Science 2000, 289, 2344–2347. [Google Scholar] [CrossRef] [PubMed]
- Hirota, T.; Okano, T.; Kokame, K.; Shirotami-Ikejiema, H.; Miyata, T.; Fukada, Y. Glucose down-regulates Per1 and Per2 mRNA levels and induces circadian gene expression in cultured Rat-1 fibroblasts. J. Biol. Chem. 2002, 277, 44244–44251. [Google Scholar] [CrossRef] [PubMed]
- Homma, K.; Hikosaka, M.; Mochizuki, K.; Goda, T. Loss of circadian rhythm of circulating insulin concentration induced by high-fat diet intake is associated with disrupted clock genes in the liver. Metabolism 2016, 5, 482–492. [Google Scholar] [CrossRef]
- Petrenko, V.; Gandasi, N.R.; Sage, D.; Tengholm, A.; Barg, S.; Dibner, C. In pancreatic islets from type 2 diabetes patients, the dampened circadian oscillators lead to reduced insulin and glucagon exocytosis. Proc. Natl. Acad. Sci. USA 2020, 117, 2484–2495. [Google Scholar] [CrossRef]
- Stenvers, D.J.; Scheer, F.A.J.L.; Schrawen, P.; E la Fleur, S.; Karlsbek, A. Circadian clock and insulin resistance. Nat. Rev. Endocrinol. 2019, 15, 75–89. [Google Scholar] [CrossRef] [PubMed]
- Jouffe, C.; Weger, B.D.; Martin, E.; Atger, F.; Weger, M.; Gobet, C.; Ramnath, D.; Charpagne, A.; Morin-Rivron, D.; Powell, E.E.; et al. Disruption of the circadian clock component BMAL1 elicits an endocrine adaption impacting on insulin sensitivity and liver disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2200083119. [Google Scholar] [CrossRef] [PubMed]
- Marcheva, B.; Moynihan Ramsy, K.; Buhr, E.D.; Kobayashi, Y.; Su, H.; Ko, C.H.; Ivanova, G.; Omura, C.; Mo, S.; Vitaterna, M.H.; et al. Disruption of the clock components CLOCK and BMAL1 leads to hypoinsulinemia and diabetes. Nature 2010, 466, 627–631. [Google Scholar] [CrossRef] [PubMed]
- Grosbellet, E.; Dumont, S.; Schuster-Klein, C.; Guardiola-Lemaitre, B.; Pevet, P.; Criscuolo, F.; Challet, E. Leptin modulates the daily rhythmicity of glucose. Chronobiol. Int. 2011, 32, 637–649. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Patel, M.S.; Bradley, A.; Wagner, E.F.; Karsenty, G. The molecular clock mediates leptin-regulated bone formation. Cell 2005, 122, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Langendonk, J.G.; Pijl, H.; Toorvlit, A.C.; Burggraaf, J.; Frolich, M.; Schoemaker, R.C.; Doornbos, J.; Cohen, A.F.; Meinders, A.E. Circadian rhythm of plasma leptin levels in upper and lower body obese women: Influence of body fat distribution and weight loss. J. Clin. Endocrinol. Metab. 1998, 83, 1706–1712. [Google Scholar] [CrossRef] [PubMed]
- Randeva, H.S.; Karteris, E.; Lewandowski, K.C.; Sailesh, S.; O’Hare, P.; Hilhouse, E.W. Circadian rhythmicity of salivary leptin in healthy subjects. Mol. Genet. Metab. 2003, 78, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Fu, Y.; Li, X.; Li, Y.; Bogdan, A.; Touitou, Y. Age-related modifications of circadian rhythm of serum leptin in healthy men. Gerontology 2002, 48, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Malik, I.A.; Durairajanayagam, D.; Singh, H.J. Leptin and its action on reproduction in males. Asian J. Androl. 2018, 21, 296–299. [Google Scholar] [CrossRef]
- Cundrle, I., Jr.; Suk, P.; Sramek, V.; Lacinova, Z.; Haluzik, M. Circadian leptin concentration changes in critically ill heart failure patients. Physiol. Res. 2018, 67, 505–508. [Google Scholar] [CrossRef]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed]
- Poher, A.L.; Tschop, M.H.; Miller, T.D. Ghrelin regulation of glucose metabolism. Peptides 2018, 100, 236–242. [Google Scholar] [CrossRef] [PubMed]
- Templeman, I.; Smith, H.A.; Walhin, J.; Middleton, B.; Gonzales, J.T.; Karagounis, L.G.; Johnston, J.D.; Betts, J.A. Unacylated ghrelin, leptin, and appetite display diurnal rhythmicity in lean adults. J. Appl. Physiol. 2021, 130, 1534–1543. [Google Scholar] [CrossRef]
- Tajiri, Y. Ghrelin and exercise: A possible virtuous circle. Diabetol. Int. 2017, 8, 347–349. [Google Scholar] [CrossRef] [PubMed]
- Halberg, F.; Haus, E.; Cardoso, S.S.; Scheving, L.E.; Khul, J.F.W.; Shiotsuka, R.; Rosene, G.; Pauly, J.E.; Runge, W.; Spalding, J.F.; et al. Toward a chronotherapy of neoplasia: Tolerance of treatment depends upon host rhythms. Experientia 1973, 29, 909–934. [Google Scholar] [CrossRef] [PubMed]
- Chipchura, D.A.; Freyberg, Z.; Edwards, C.; Leckband, S.G.; McCarty, M.J. Does the time of drug administration alter the metabolic risk of aripiprazole? Front. Psychiatry 2018, 9, 494–498. [Google Scholar] [CrossRef]
- Vera, L.M.; Bello, C.; Paredes, J.F.; Carmona-Antonanzas, G.; Sanchez-Vazquez, F.J. Ethanol toxicity differs depending on the time of day. PLoS ONE 2018, 13, e0190406. [Google Scholar] [CrossRef] [PubMed]
- Cortes-Rios, J.; Rodriguez-Fernandez, M. Understanding the dosing-time-dependent antihypertensive effect of valsartan and aspirin through mathematical modeling. Front. Endocrinol. 2023, 14, 1110459. [Google Scholar] [CrossRef]
- Isidori, A.M.; Venneri, M.A.; Graziadio, C.; Simeoli, C.; Fiore, D.; Hasenmajer, V.; Sbardella, E.; Gianfrilli, D.; Pozza, C.; Pasqualetti, P.; et al. Effect of once-daily, modified-release hydrocortisone versus standard glucocorticoid therapy on metabolism and innate immunity in patients with adrenal insufficiency (DREAM): A single-blind, randomised controlled trial. Lancet Diabetes Endocrinol. 2018, 6, 173–185. [Google Scholar] [CrossRef]
- Venneri, M.A.; Hasenmajer, V.; Fore, D.; Sbardella, E.; Pofi, R.; Graziadio, C.; Gianfrilli, D.; Pivonello, C.; Negri, M.; Naro, F.; et al. Circadian rhythm of glucocorticoid administration entrains clock genes in immune cells: A DREAM trial ancillary study. J. Clin. Endocrinol. Metab. 2018, 103, 2998–3009. [Google Scholar] [CrossRef]
- Forbes-Robertson, S.; Dudley, E.; Vadgama, P.; Cook, C.; Drawer, S.; Kiduf, L. Circadian disruption and remedial interventions: Effects and interventions for jet lag for athletic peak performance. Sports Med. 2012, 42, 185–208. [Google Scholar] [CrossRef]
- Arendt, J. Approaches to the pharmacological management of jet lag. Drugs 2018, 78, 1419–1431. [Google Scholar] [CrossRef]
- Szewczyk-Golec, K.; Wozniak, A.; Reiter, R.J. Inter-relationships of the chronobiotic, melatonin, with leptin and adiponectin: Implication for obesity. J. Pineal Res. 2015, 59, 277–291. [Google Scholar] [CrossRef]
- Aranda, M.L.; Narvaez, O.; Altschuler, F.; Calanni, J.S.; Gonzalez Fleitas, M.F.; Sande, P.H.; Dorfman, D.; Concha, L.; Rosenstein, R.E. Chronobiotic effect of melatonin in experimental optic neuritis. Neuropharmacology 2021, 182, 108401. [Google Scholar] [CrossRef]
- He, B.; Nohara, K.; Park, N.; Park, Y.; Guillory, B.; Zhao, Z.; Garcia, J.M.; Koike, N.; Lee, C.C.; Takahashi, J.S.; et al. The small molecule nobiletin targets the molecular oscillator to enhance circadian rhythms and protect against metabolic syndrome. Cell Metab. 2016, 23, 610–621. [Google Scholar] [CrossRef]
- Ambe, G.N.N.N.; Breda, C.; Bhramba, A.S.; Arroo, R.R.J. Effect of the citrus flavone nobiletin on circadian rhythms and metabolic syndrome. Molecules 2022, 27, 7727. [Google Scholar] [CrossRef]
- Mileykovskaya, E.; Yoo, S.H.; Dowhan, W.; Chen, Z. Nobiletin: Targeting the circadian network to promote bioenergetics and healthy aging. Biochemistry 2020, 85, 1554–1559. [Google Scholar] [CrossRef]
- Wirianto, M.; Wang, C.; Kim, E.; Koike, N.; Gomez-Guiterrez, R.; Nohara, K.; Escobedo, G., Jr.; Choi, J.M.; Han, C.; Yagita, K.; et al. The clock modulator nobiletin mitigates astrogliosis- associated neuroinflammation and disease hallmarks in an Alzheimer’s disease model. FASEB J. 2022, 36, e22186. [Google Scholar] [CrossRef]
- Malik, D.; Attar, R.; Ozbey, U.; Romero, M.A.; Yulaevna, I.M.; Purenovic, J. Multifunctional role of nobiletin in cancer chemoprevention. Cell Mol. Biol. 2022, 68, 200–207. [Google Scholar] [CrossRef]
- Mawatari, K.; Koike, N.; Nohara, K.; Wirianto, M.; Uebanso, T.; Shimohata, T.; Shikishima, Y.; Miura, H.; Nu, Y.; Burish, M.J.; et al. The polymethoxyflavone sudachitin modulates the circadian clock and improves liver physiology. Mol. Nutr. Food Res. 2023, 67, e2200270. [Google Scholar] [CrossRef]

| Light/darkness cycle |
| Sleep/wake alternations |
| Periodic food intake |
| Social environment (physical and mental work, travel) |
| Family and individual chronotype |
| Epigenome |
| Hormone variations |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mercadante, S.; Bellastella, A. Chrono-Endocrinology in Clinical Practice: A Journey from Pathophysiological to Therapeutic Aspects. Life 2024, 14, 546. https://doi.org/10.3390/life14050546
Mercadante S, Bellastella A. Chrono-Endocrinology in Clinical Practice: A Journey from Pathophysiological to Therapeutic Aspects. Life. 2024; 14(5):546. https://doi.org/10.3390/life14050546
Chicago/Turabian StyleMercadante, Silvia, and Antonio Bellastella. 2024. "Chrono-Endocrinology in Clinical Practice: A Journey from Pathophysiological to Therapeutic Aspects" Life 14, no. 5: 546. https://doi.org/10.3390/life14050546






