The Astrophysical Formation of Asymmetric Molecules and the Emergence of a Chiral Bias †
Abstract
:1. Introduction
1.1. The Asymmetry of Life
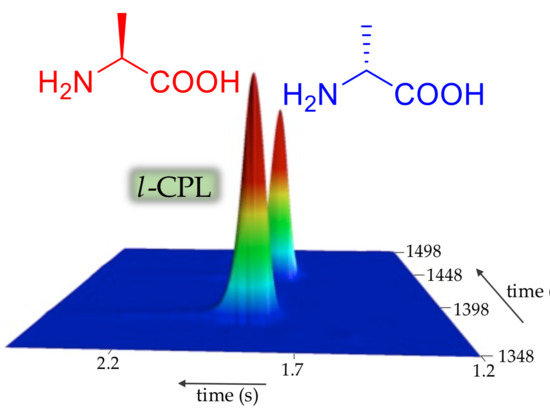
1.2. Circularly Polarized Light (CPL): A Chiral Bias
1.3. The Astrophysical Scenario
2. Amino Acids
2.1. Amino Acids in Meteorites
2.2. Amino Acids in Comets
2.3. Amino Acids in Simulated Interstellar Ices
3. Chiral Sugars
3.1. Chiral Sugars in Meteorites
3.2. Chiral Sugars in Comets
3.3. Chiral Sugars in Simulated Interstellar Ices
4. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Meierhenrich, U. Amino Acids and the Asymmetry of Life; Springer: Berlin/Heidelberg, Germany, 2008; ISBN 978-3-540-76886-9. [Google Scholar]
- Griesbeck, A.G.; Meierhenrich, U.J. Asymmetric photochemistry and photochirogenesis. Angew. Chem. Int. Ed. 2002, 41, 3147–3154. [Google Scholar] [CrossRef]
- Karagunes, G.; Coumoulos, G. A new method of resolving a racemic compound. Nature 1938, 142, 162–163. [Google Scholar] [CrossRef]
- Bonner, W.A.; Kavasmaneck, P.R.; Martin, F.S.; Flores, J.J. Asymmetric adsorption of alanine by quartz. Science 1974, 186, 143–144. [Google Scholar] [CrossRef] [PubMed]
- Soai, K.; Osanai, S.; Kadowaki, K.; Yonekubo, S.; Shibata, T.; Sato, I. d- and l-quartz-promoted highly enantioselective synthesis of a chiral organic compound. J. Am. Chem. Soc. 1999, 121, 11235–11236. [Google Scholar] [CrossRef]
- Yamagata, Y. A hypothesis for the asymmetric appearance of biomolecules on earth. J. Theor. Biol. 1966, 11, 495–498. [Google Scholar] [CrossRef]
- Barron, L.D. True and false chirality and absolute asymmetric synthesis. J. Am. Chem. Soc. 1986, 108, 5539–5542. [Google Scholar] [CrossRef]
- Riehl, J.P.; Richardson, F.S. Circularly polarized luminescence spectroscopy. Chem. Rev. 1986, 86, 1–16. [Google Scholar] [CrossRef]
- Balavoine, G.; Moradpour, A.; Kagan, H.B. Preparation of chiral compounds with high optical purity by irradiation with circularly polarized light, a model reaction for the prebiotic generation of optical activity. J. Am. Chem. Soc. 1974, 96, 5152–5158. [Google Scholar] [CrossRef]
- Snyder, P.A.; Vipond, P.M.; Johnson, W.C., Jr. Circular Dichroism of the Alkyl Amino Acids in the Vacuum Ultraviolet. Biopolymers 1973, 12, 975–992. [Google Scholar] [CrossRef]
- Feeley, T.M.; Hargreaves, M.K.; Marshall, D.L. The circular dichroism of some α-hydroxyaldehydes. Tetrahedron Lett. 1968, 46, 4831–4834. [Google Scholar] [CrossRef]
- Totty, R.N.; Hudec, J.; Hayward, L.D. The circular dichroism of pentoses. Carbohyd. Res. 1972, 23, 152–154. [Google Scholar] [CrossRef]
- Nelson, R.G.; Johnson, W.C., Jr. Optical Properties of Sugars. I. Circular Dichroism of Monomers at Equilibrium. J. Am. Chem. Soc. 1972, 94, 3343–3345. [Google Scholar] [CrossRef]
- Nelson, R.G.; Johnson, W.C., Jr. Optical Properties of Sugars. 3. Circular Dichroism of Aldo- and Ketopyranose Anomers. J. Am. Chem. Soc. 1976, 98, 4290–4295. [Google Scholar] [CrossRef] [PubMed]
- Matsuo, K.; Gekko, K. Vacuum-ultraviolet circular dichroism study of saccharides by synchrotron radiation spectrophotometry. Carbohyd. Res. 2004, 339, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.B.; Chakraborty, T.; Hoffmann, V. Synchrotron Radiation Circular Dichroism Spectroscopy of Ribose and Deoxyribose Sugars, Adenosine, AMP and dAMP Nucleotides. ChemPhysChem 2005, 6, 2619–2624. [Google Scholar] [CrossRef] [PubMed]
- Myrgorodska, I.; Meinert, C.; Hoffmann, S.V.; Jones, N.C.; Nahon, L.; Meierhenrich, U.J. Light on Chirality: Absolute Asymmetric Formation of Chiral Molecules Relevant in Prebiotic Evolution. ChemPlusChem 2017, 82, 74–87. [Google Scholar] [CrossRef]
- Kaneko, F.; Yagi-Watanabe, K.; Tanaka, M.; Nakagawa, K. Natural Circular Dichroism Spectra of Alanine and Valine Films in Vacuum Ultraviolet Region. J. Phys. Soc. Jpn. 2009, 78, 013001. [Google Scholar] [CrossRef]
- Tanaka, M.; Yagi-Watanabe, K.; Kaneko, F.; Nakagawa, K. Chiroptical Study of α-Aliphatic Amino Acid Films in the Vacuum Ultraviolet Region. J. Phys. Chem. A 2010, 114, 11928–11932. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J.; Filippi, J.-J.; Meinert, C.; Bredehöft, J.H.; Takahashi, J.; Nahon, L.; Jones, N.C.; Hoffmann, S.V. Circular Dichroism of Amino Acids in the Vacuum-Ultraviolet Region. Angew. Chem. Int. Ed. 2010, 49, 7799–7802. [Google Scholar] [CrossRef]
- Kuhn, W. The physical significance of optical rotatory power. Trans. Faraday Soc. 1930, 26, 293–308. [Google Scholar] [CrossRef]
- Meinert, C.; Bredehöft, J.H.; Filippi, J.-J.; Baraud, Y.; Nahon, L.; Wien, F.; Jones, N.C.; Hoffmann, S.V.; Meierhenrich, U.J. Anisotropy Spectra of Amino Acids. Angew. Chem. Int. Ed. 2012, 51, 4484–4487. [Google Scholar] [CrossRef] [PubMed]
- Bredehöft, J.H.; Jones, N.C.; Meinert, C.; Evans, A.C.; Hoffmann, S.V.; Meierhenrich, U.J. Understanding Photochirogenesis: Solvent Effects on Circular Dichroism and Anisotropy Spectroscopy. Chirality 2014, 26, 373–378. [Google Scholar] [CrossRef]
- Kuhn, W.; Braun, E. Photochemical preparation of optically active substances. Naturwissenschaften 1929, 17, 227–228. [Google Scholar] [CrossRef]
- Flores, J.J.; Banner, W.A.; Massey, G.A. Asymmetric photolysis of (RS)-leucine with circularly polarized ultraviolet light. J. Am. Chem. Soc. 1977, 99, 3622–3625. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J.; Nahon, L.; Alcaraz, C.; Bredehöft, J.H.; Hoffmann, S.V.; Barbier, B.; Brack, B. Asymmetric Vacuum UV photolysis of the Amino Acid Leucine in the Solid State. Angew. Chem. Int. Ed. 2005, 44, 5630–5634. [Google Scholar] [CrossRef] [PubMed]
- Meierhenrich, U.J.; Filippi, J.-J.; Meinert, C.; Hoffmann, S.V.; Bredehöft, J.H.; Nahon, L. Photolysis of rac-Leucine with Circularly Polarized Synchrotron Radiation. Chem. Biodivers. 2010, 7, 1651–1659. [Google Scholar] [CrossRef]
- Meinert, C.; Hoffmann, S.V.; Cassam-Chenaï, P.; Evans, A.C.; Giri, C.; Nahon, L.; Meierhenrich, U.J. Photonenergy-controlled symmetry breaking with circularly polarized light. Angew. Chem. Int. Ed. 2014, 53, 210–214. [Google Scholar] [CrossRef]
- Meinert, C.; Cassam-Chenaï, P.; Jones, N.C.; Nahon, L.; Hoffmann, S.V.; Meierhenrich, U.J. Anisotropy-guided enantiomeric enhancement in alanine using far-UV circularly polarized light. Orig. Life Evol. Biospheres 2015, 45, 149–161. [Google Scholar] [CrossRef]
- Tia, M.; Cunha de Miranda, B.; Daly, S.; Gaie-Levrel, F.; Garcia, G.A.; Nahon, L.; Powis, I. VUV Photodynamics and Chiral Asymmetry in the Photoionization of Gas Phase Alanine Enantiomers. J. Phys. Chem. A 2014, 118, 2765–2779. [Google Scholar] [CrossRef]
- Rubenstein, E.; Bonner, W.A.; Noyes, H.P.; Brown, G.S. Supernovae and life. Nature 1983, 306, 118. [Google Scholar] [CrossRef]
- Bonner, W.A. The origin and amplification of biomolecular chirality. Orig. Life Evol. Biosph. 1991, 21, 59–111. [Google Scholar] [CrossRef]
- Bailey, J. Astronomical sources of circularly polarized light and the origin of homochirality. Orig. Life Evol. Biosph. 2001, 31, 167–183. [Google Scholar] [CrossRef] [PubMed]
- Bailey, J.; Chrysostomou, A.; Hough, J.H.; Gledhill, T.M.; McCall, A.; Clark, S.; Ménard, F.; Tamura, M. Circular polarization in star-formation regions: Implications for biomolecular homochirality. Science 1998, 281, 672–674. [Google Scholar] [CrossRef]
- Fukue, T.; Tamura, M.; Kandori, R.; Kusakabe, N.; Hough, J.H.; Bailey, J.; Whittet, D.C.B.; Lucas, P.W.; Nakajima, Y.; Hashimoto, J. Extended high circular polarization in the Orion massive star forming region: Implications for the origin of homochirality in the solar system. Orig. Life Evol. Biosph. 2010, 40, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.; Tamura, M.; Lucas, P.W.; Hashimoto, J.; Kusakabe, N.; Kandori, R.; Nakajima, Y.; Nagayama, T.; Nagata, T.; Hough, J.H. Near-infrared circular polarization images of NGC 6334-V. Astrophys. J. Lett. 2013, 765, L6. [Google Scholar] [CrossRef]
- Miller, G.E.; Scalo, J.M. On the birthplaces of stars. Publ. Astron. Soc. Pac. 1978, 90, 506–513. [Google Scholar] [CrossRef]
- Hester, J.J.; Desch, S.J.; Healy, K.R.; Leshin, L.A. The cradle of the solar system. Science 2004, 304, 16–17. [Google Scholar] [CrossRef] [PubMed]
- Botta, O.; Bada, J.L. Extraterrestrial organic compounds in meteorites. Surv. Geophys. 2002, 23, 411–467. [Google Scholar] [CrossRef]
- Krot, A.N.; Keil, K.; Scott, E.R.D.; Goodrich, C.A.; Weisberg, M.K. Classification of meteorites and their genetic relationships. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 1, pp. 1–63. ISBN 9780080959757. [Google Scholar]
- Alexander, C.M.O’.D.; Bowden, R.; Fogel, M.L.; Howard, K.T.; Herd, C.D.K.; Nittler, L.R. The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science 2012, 337, 721–723. [Google Scholar] [CrossRef] [PubMed]
- Berzelius, J.J. Ueber Meteorsteine. Ann. Phys. Chem. 1834, 33, 113–148. [Google Scholar] [CrossRef]
- Wöhler, M.F.; Hörnes, M. Die organische Substanz im Meteorsteine von Kaba. Sitzber. Akad. Wiss. Wien, Math. Naturw. Kl. 1859, 34, 7–8. [Google Scholar] [CrossRef]
- Berthelot, M. Sur la matière charbonneuse des météorites. C. R. Acad. Sci. 1868, 67, 849–851. [Google Scholar]
- Hayes, J.M. Organic constituents of meteorites—A review. Geochim. Cosmochim. 1967, 31, 1395–1440. [Google Scholar] [CrossRef]
- Kvenvolden, K.; Lawless, J.; Pering, K.; Peterson, E.; Flores, J.; Ponnamperuma, C.; Kaplan, I.R.; Moore, C. Evidence for extraterrestrial amino-acids and hydrocarbons in the Murchison meteorite. Nature 1970, 228, 923–926. [Google Scholar] [CrossRef] [PubMed]
- Kvenvolden, K.A.; Lawless, J.G.; Ponnamperuma, C. Nonprotein Amino Acids in the Murchison meteorite. Proc. Natl. Acad. Sci. USA 1971, 68, 486–490. [Google Scholar] [CrossRef] [PubMed]
- Lawless, J.G.; Kvenvolden, K.A.; Peterson, E.; Ponnamperuma, C.; Moore, C. Amino Acids Indigenous to the Murray Meteorite. Science 1971, 173, 626–627. [Google Scholar] [CrossRef]
- Pollock, G.E.; Cheng, C.-N.; Cronin, S.E.; Kvenvolden, K.A. Stereoisomers of isovaline in the Murchison meteorite. Geochim. Cosmochim. Acta. 1975, 39, 1571–1573. [Google Scholar] [CrossRef]
- Cronin, J.R.; Gandy, W.E.; Pizzarello, S. Amino Acids of the Murchison Metorite: I. Six Carbon Acyclic Primary α-Amino Alkanoic Acids. J. Mol. Evol. 1981, 17, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.R.; Pizzarello, S.; Yuen, G.E. Amino Acids of the Murchison Metorite: II. Five carbon acyclic primary β-, γ-, and δ-amino alkanoic acids. Geochim. Cosmochim. Acta. 1985, 49, 2259–2265. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Amino Acids of the Murchison Meteorite: III. Seven carbon acyclic primary α-amino alkanoic acids. Geochim. Cosmochim. Acta. 1986, 50, 2419–2427. [Google Scholar] [CrossRef]
- Meierhenrich, U.J.; Muñoz Caro, G.M.; Bredehöft, J.H.; Jessberger, E.K.; Thiemann, H.-P. Identification of diamino acids in the Murchison meteorite. Proc. Natl. Acad. Sci. USA 2004, 101, 9182–9186. [Google Scholar] [CrossRef] [Green Version]
- Joyce, G.F. The antiquity of RNA-based evolution. Nature 2002, 418, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Naraoka, H. Anew family of extraterrestrial amino acids in the Murchison meteorite. Sci. Rep. 2017, 7, 636. [Google Scholar] [CrossRef]
- Martins, Z.; Alexander, C.M.O’.D.; Orzechowska, G.E.; Fogel, M.L.; Ehrenfreund, P. Indigenous amino acids in primitive CR meteorites. Meteorit. Planet. Sci. 2007, 42, 2125–2136. [Google Scholar] [CrossRef] [Green Version]
- Glavin, D.P.; Callahan, M.P.; Dworkin, J.P.; Elsila, J.E. The effects of parent body processes on amino acids in carbonaceous chondrites. Meteorit. Planet. Sci. 2011, 45, 1948–1972. [Google Scholar] [CrossRef]
- Burton, A.S.; Elsila, J.E.; Callahan, M.P.; Martin, M.G.; Glavin, D.P.; Johnson, N.M.; Dworkin, J.P. A propensity for n-ω-amino acids in thermally altered Antarctic meteorites. Meteorit. Planet. Sci. 2012, 47, 374–386. [Google Scholar] [CrossRef] [Green Version]
- Burton, A.S.; McLain, H.; Glavin, D.P.; Elsila, J.E.; Davidson, J.; Miller, K.E.; Andronikov, A.V.; Lauretta, D.; Dworkin, J.P. Amino acid analyses of R and CK chondrites. Meteorit. Planet. Sci. 2015, 50, 470–482. [Google Scholar] [CrossRef] [Green Version]
- Botta, O.; Martins, Z.; Ehrefreund, P. Amino acids in Antarctic CM1 meteorites and their relationship to other carbonaceous chondrites. Meteorit. Planet. Sci. 2010, 42, 81–92. [Google Scholar] [CrossRef]
- Engel, M.H.; Nagy, B. Distribution and enantiomeric composition of amino acids in the Murchison meteorite. Nature 1982, 296, 837–840. [Google Scholar] [CrossRef]
- Bada, J.L.; Cronin, J.R.; Ho, M.-S.; Kvenvolden, K.A.; Lawless, J.G.; Miller, S.L.; Oro, J.; Steinberg, S. On the reported optical activity of amino acids in the Murchison meteorite. Nature 1983, 301, 494–496. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Enantiomeric Excesses in Meteoritic Amino Acids. Science 1997, 275, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Cronin, J.R.; Pizzarello, S. Amino acid enantiomer excesses in meteorites: Origin and significance. Adv. Space Res. 1999, 23, 293–299. [Google Scholar] [CrossRef]
- Pizzarello, S.; Cronin, J.R. Non-racemic amino acids in the Murray and Murchison meteorites. Geochim. Cosmochim. Acta 2000, 64, 329–338. [Google Scholar] [CrossRef]
- Glavin, D.P.; Dworkin, J.P. Enrichment of the amino acid L-isovaline by aqueous alteration on CI and CM meteorite parent bodies. Proc. Natl. Acad. Sci. USA 2009, 106, 5487–5492. [Google Scholar] [CrossRef] [PubMed]
- Meinert, C.; Meierhenrich, U.J. A New Dimension in Separation Science: Comprehensive Two-Dimensional Gas Chromatography. Angew. Chem. Int. Ed. 2012, 51, 10460–10470. [Google Scholar] [CrossRef]
- Ishii, C.; Miyamoto, T.; Ishigo, S.; Miyoshi, Y.; Mita, M.; Homma, H.; Ueda, T.; Hamase, K. Two-Dimensional HPLC-MS/MS Determination of Multiple D-Amino Acid Residues in the Proteins Stored Under Various pH Conditions. Chromatography 2017, 38, 65–72. [Google Scholar] [CrossRef]
- Myrgorodska, I.; Meinert, C.; Martins, Z.; le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Quantitative enantioseparation of amino acids by comprehensive two-dimensional gas chromatography applied to non-terrestrial samples. J. Chromatogr. A 2016, 1433, 131–136. [Google Scholar] [CrossRef]
- Meinert, C.; Meierhenrich, U.J. Derivatization and Multidimensional Gas-Chromatographic Resolution of α-Alkyl and α-Dialkyl Amino Acid Enantiomers. ChemPlusChem 2014, 79, 781–785. [Google Scholar] [CrossRef]
- Pizzarello, S.; Zolenski, M.; Turk, K.A. Nonracemic isovaline in the Murchison meteorite: Chiral distribution and mineral association. Geochim. Cosmochim. Acta 2003, 67, 1589–1595. [Google Scholar] [CrossRef]
- Hamase, K.; Nakauchi, Y.; Miyoshi, Y.; Koga, R.; Kusano, N.; Onigahara, H.; Naraoka, H.; Mita, H.; Kadota, Y.; Nishio, Y.; et al. Enantioselective Determination of Extraterrestrial Amino Acids Using a Two-Dimensional Chiral High-Performance Liquid Chromatographic System. Chromatography 2014, 35, 103–110. [Google Scholar] [CrossRef] [Green Version]
- Imai, K.; Watanabe, Y. Fluorimetric determination of secondary amino acids by 7-fluoro-4-nitrobenzo-2-oxa-1,3-diazole. Anal. Chim. Acta 1981, 130, 377–383. [Google Scholar] [CrossRef]
- Jungclaus, G.A.; Yuen, G.U.; Moore, C.B.; Lawless, J.G. Evidence for the presence of low molecular weight alcohols and carbonyl compounds in the Murchison meteorite. Meteoritics 1976, 11, 231–237. [Google Scholar] [CrossRef]
- Pizzarello, S.; Holmes, W. Nitrogen-containing compounds in two CR2 meteorites: 15N composition, molecular distribution and precursor molecules. Geochim. Cosmochim. Acta 2009, 73, 2150–2162. [Google Scholar] [CrossRef]
- Monroe, A.A.; Pizzarello, S. The soluble organic compounds of the Bells meteorite: Not a unique or unusual composition. Geochim. Cosmochim. Acta 2011, 75, 7585–7595. [Google Scholar] [CrossRef]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simkus, D.N.; Aponte, J.C.; Hilts, R.W.; Elsila, J.E.; Herd, C.D.K. Compound-specific carbon isotope compositions of aldehydes and ketones in the Murchison meteorite. Meteorit. Planet. Sci. 2019, 54, 142–156. [Google Scholar] [CrossRef]
- Aponte, J.C.; Whitaker, D.; Powner, M.W.; Elsila, J.E.; Dworkin, J.P. Analyses of Aliphatic Aldehydes and Ketones in Carbonaceous Chondrites. ACS Earth Space Chem. 2019. [Google Scholar] [CrossRef]
- Wetherill, G.W. Formation of the Earth. Annu. Rev. Earth Planet. Sci. 1990, 18, 205–256. [Google Scholar] [CrossRef]
- Irvine, W.M. Extraterrestrial organic matter: A review. Orig. Life Evol. Biosph. 1998, 28, 365–383. [Google Scholar] [CrossRef]
- Mumma, M.J.; Charnley, S.B. The Chemical composition of Comets–Emerging Taxonomies and Natal Heritage. Annu. Rev. Astron. Astrophys. 2011, 49, 471–524. [Google Scholar] [CrossRef]
- Oró, J.; Mills, T.; Lazcano, A. Comets and the formation of biochemical compounds on the primitive Earth—A review. Orig. Life Evol. Biosph. 1992, 21, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Schloerb, F.P.; Kinzel, W.M.; Swade, D.A.; Irvine, W.M. HCN production from comet Halley. Astrophys. J. 1986, 310, 55–70. [Google Scholar] [CrossRef]
- Krueger, F.R. Carbonaceous matter in cometary dust and coma. Adv. Space Res. 1995, 15, 407–411. [Google Scholar] [CrossRef]
- Crovisier, J.; Bockelée-Morvan, D.; Colom, P.; Biver, N.; Despois, D.; Lis, D.C.; the Team for target-of-opportunity radio observations of comets. The composition of ices in comet C/1995 O1 (Hale-Bopp) from radio spectroscopy. Astronom. Astrophys. 2004, 418, 1141–1157. [Google Scholar] [CrossRef] [Green Version]
- Goesmann, F.; Rosenbauer, H.; Bredehöft, J.H.; Cabane, M.; Ehrenfreund, P.; Gautier, T.; Giri, C.; Krüger, H.; Le Roy, L.; MacDermott, A.J.; et al. Organic compounds on comet 67P/Churyumov-Gerasimenko revealed by COSAC mass spectrometry. Science 2015, 349, aab0689. [Google Scholar] [CrossRef] [PubMed]
- Ulamec, S.; Fantinati, C.; Maibaum, M.; Geurts, K.; Biele, J.; Jansen, S.; Küchemann, O.; Cozzonin, B.; Finke, F.; Lommatsch, V.; et al. Rosetta Lander—Landing and operations on comet 67P/Churyumov-Gerasimenko. Acta Astronaut. 2016, 125, 80–91. [Google Scholar] [CrossRef]
- Pascal, R.; Boiteau, L.; Commeyras, A. From the Prebiotic Synthesis of α-Amino Acids Towards a Primitive Translation Apparatus for the Synthesis of Peptides. In Prebiotic Chemistry, 2nd ed.; Walde, P., Ed.; Springer: Berlin, Germany, 2005; Volume 259, pp. 69–122. ISBN 978-3-540-27759-0. [Google Scholar]
- Altwegg, K.; Balsiger, H.; Bar-Nun, A.; Berthelier, J.-J.; Bieler, A.; Bochsler, P.; Briois, C.; Calmonte, U.; Combi, M.R.; Cottin, H.; et al. Prebiotic chemicals—amino acid and phosphorus—in the coma of comet 67P/Churyumov-Gerasimenko. Sci. Adv. 2016, 2, e1600285. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, J.M. Chirality in Interstellar Dust and in Comets: Life from Dead Stars. AIP Conf. Proc. 1996, 379, 185–210. [Google Scholar] [CrossRef]
- D’Hendecourt, L.; Modica, P.; Meinert, C.; Nahon, L.; Meierhenrich, U. Interstellar ices: A possible scenario for symmetry breaking of extraterrestrial chiral organic molecules of prebiotic interest. arXiv, 2019; arXiv:1902.04575. [Google Scholar]
- Greenberg, J.M. Cosmic dust and our origins. Surf. Sci. 2002, 500, 793–822. [Google Scholar] [CrossRef]
- Gibb, E.L.; Whittet, D.C.B.; Boogert, A.C.A.; Tielens, A.G.G.M. Interstellar ice: The infrared space observatory legacy. Astrophys. J. Suppl. Ser. 2004, 151, 35–73. [Google Scholar] [CrossRef]
- Ehrenfreund, P.; Kerkhof, O.; Schutte, W.A.; Boogert, A.C.A.; Gerakines, P.A.; Dartois, E.; d’Hendecourt, L.; Tielens, A.G.G.M.; van Dishoeck, E.F.; Whittet, D.C.B. Laboratory studies of thermally processed H2O-CH3OH-CO2 ice mixtures and their astrophysical implications. Astron. Astrophys. 1999, 350, 240–253. [Google Scholar]
- Bernstein, M.P.; Dworkin, J.P.; Sandford, S.A.; Cooper, G.W.; Allamandola, L.J. Racemic amino acids from the ultraviolet photolysis of interstellar ice analogues. Nature 2002, 416, 401–403. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Caro, G.M.; Meierhenrich, U.J.; Schutte, W.A.; Barbler, B.; Segovia, A.A.; Rosenbauer, H.; Thiemann, W.H.P.; Brack, A.; Greenberg, J.M. Amino acids from ultraviolet irradiation of interstellar ice analogues. Nature 2002, 416, 403–406. [Google Scholar] [CrossRef]
- Cronin, J.R.; Pizzarello, S. Amino acids in meteorites. Adv. Space Res. 1983, 3, 5–18. [Google Scholar] [CrossRef]
- Nuevo, M.; Auger, G.; Blanot, D.; d’Hendecourt, L. A Detailed Study of the Amino Acids Produced from the Vacuum UV Irradiation of Interstellar Ice Analogs. Orig. Life Evol. Biosph. 2008, 38, 37–56. [Google Scholar] [CrossRef] [PubMed]
- Briggs, R.; Ertem, G.; Ferris, J.P.; Greenberg, J.M.; McCain, P.J.; Mendoza-Gomez, C.X.; Schutte, W. Comet Halley as an aggregate of interstellar dust and further evidence for the photochemical formation of organics in the interstellar medium. Orig. Life Evol. Biosph. 1992, 22, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Takano, Y.; Kaneko, T.; Kobayashi, K.; Hiroishi, D.; Ikeda, H.; Marumo, K. Experimental verification of photostability for free- and bound-amino acids exposed to gamma-rays and UV irradiation. Earth Planets Space 2004, 56, 669–674. [Google Scholar] [CrossRef]
- Takano, Y.; Masuda, H.; Kaneko, T.; Kobayashi, K. Formation of amino acids from possible interstellar media by gamma-rays and UV irradiation. Chem. Lett. 2002, 31, 986–987. [Google Scholar] [CrossRef]
- Takahashi, J.-I.; Masuda, H.; Kaneko, T.; Kobayashi, K.; Saito, T.; Hosokawa, T. Photochemical abiotic synthesis of amino-acid precursors from simulated planetary atmospheres by vacuum ultraviolet light. J. Appl. Phys. 2005, 98, 024907-1–024907-6. [Google Scholar] [CrossRef]
- Takahashi, J.-I.; Hosokawa, T.; Masuda, H.; Kaneko, T.; Kobayashi, K.; Saito, T.; Utsumi, Y. Abiotic synthesis of amino acids by x-ray irradiation of simple inorganic gases. Appl. Phys. Lett. 1999, 74, 877–879. [Google Scholar] [CrossRef]
- Kobayashi, K.; Kaneko, T.; Saito, T. Characterization of complex organic compounds formed in simulated planetary atmospheres by the action of high energy particles. Adv. Space Res. 1999, 24, 461–464. [Google Scholar] [CrossRef]
- Cottin, H.; Szopa, C.; Moore, M.H. Production of hexamethylenetetramine in photolyzed and irradiated interstellar cometary ice analogs. Astrophys. J. 2001, 561, L139–L142. [Google Scholar] [CrossRef]
- Abplanalp, M.J.; Gozem, S.; Krylov, A.I.; Shingledecker, C.N.; Herbst, E.; Kaiser, R.I. A study of interstellar aldehydes and enols as tracers of a cosmic ray-driven nonequilibrium synthesis of complex organic molecules. Proc. Natl. Acad. Sci. USA 2016, 113, 7727–7732. [Google Scholar] [CrossRef] [Green Version]
- Takano, Y.; Marumo, K.; Yabashi, S.; Kaneko, T.; Kobayashi, K. Pyrolysis of complex organics following high energy proton irradiation of a simple inorganic gas mixture. Appl. Phys. Lett. 2004, 85, 1633–1635. [Google Scholar] [CrossRef]
- Lerner, N.R. Influence of Allende minerals on deuterium retention of products of the Strecker synthesis. Geochim. Cosmochim. Acta 1997, 61, 4885–4893. [Google Scholar] [CrossRef]
- Woon, D.E. Pathways to glycine and other amino acids in ultraviolet-irradiated astrophysical ices determined via quantum chemical modeling. Astrophys. J. 2002, 571, L177–L180. [Google Scholar] [CrossRef]
- Elsila, E.J.; Dworkin, J.P.; Bernstein, M.P.; Martin, M.P.; Sandford, S.A. Mechanisms of amino acid formation in interstellarice analogs. Astrophys. J. 2007, 660, 911–918. [Google Scholar] [CrossRef]
- Oba, Y.; Takano, Y.; Watanabe, N.; Kouchi, A. Deuterium fractionation during amino acid formation by photolysis of interstellar ice analogs containin deuterated methanol. Astrophys. J. Lett. 2016, 827, L18. [Google Scholar] [CrossRef]
- Nuevo, M.; Meierhenrich, U.J.; Muñoz Caro, G.M.; Dartois, E.; d’Hendecourt, L.; Deboffle, D.; Auger, G.; Blanot, D.; Bredehöft, J.-H.; Nahon, L. The effects of circularly polarized light on amino acid enantiomers produced by the UV irradiations of interstellar ice analogs. Astronom. Astrophys. 2006, 457, 741–751. [Google Scholar] [CrossRef]
- Nuevo, M.; Meierhenrich, U.J.; d’Hendecourt, L.; Muñoz Caro, G.M.; Dartois, E.; Deboffle, D.; Thiemann, W.H.-P.; Bredehöft, J.-H.; Nahon, L. Enantiomeric separation of complex organic molecules produced from irradiation of interstellar/circumstellar ice analogs. Adv. Space Res. 2007, 39, 400–404. [Google Scholar] [CrossRef]
- Takano, Y.; Takahashi, J.-I.; Kaneko, T.; Marumo, K.; Kobayashi, K. Asymmetric synthesis of amino acid precursors in interstellar complex organics by circularly polarized light. Earth Planet. Sci. Lett. 2007, 254, 106–114. [Google Scholar] [CrossRef] [Green Version]
- De Marcellus, P.; Meinert, C.; Nuevo, M.; Filippi, J.-J.; Danger, G.; Deboffle, D.; Nahon, L.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Non-racemic amino acid production by ultraviolet irradiation of achiral interstellar ice analogs with circularly polarized light. Astrophys. J. Lett. 2011, 727, L27. [Google Scholar] [CrossRef]
- Modica, P.; Meinert, C.; De Marcellus, P.; Nahon, L.; Meierhenrich, U.J.; Le Sergeant d’Hendecourt, L. Enantiomeric excesses induced in amino acids by ultraviolet circularly polarized light irradiation of extraterrestrial ice analogs: A possible source of asymmetry for prebiotic chemistry. Astrophys. J. Lett. 2014, 788, 79. [Google Scholar] [CrossRef]
- Tanaka, M.; Yagi-Watanabe, K.; Kaneko, F.; Nakagawa, K. First observation of natural circular dichroism spectra in the extreme ultraviolet region using a polarizing undulator-based optical system and its polarization characteristics. J. Synchrotron Radiat. 2009, 16, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Degens, E.T.; Bajor, M. Amino acid and sugars in the Bruderheim and Murray meteorite. Naturwissenschaften 1962, 49, 605–606. [Google Scholar] [CrossRef]
- Kaplan, I.R.; Degens, E.T.; Reuter, J.H. Organic compounds in stony meteorites. Geochim. Cosmochim. Acta 1963, 27, 805–834. [Google Scholar] [CrossRef]
- Cooper, G.; Kimmich, N.; Belisle, W.; Sarinana, J.; Brabham, K.; Garrel, L. Carbonaceous meteorites as a source of sugar-related organic compounds for the early Earth. Nature 2001, 414, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Cooper, G.; Rios, A.C. Enantiomer excesses of rare and common sugar derivatives in carbonaceous meteorites. Proc. Natl. Acad. Sci. USA 2016, 113, E3322–E3331. [Google Scholar] [CrossRef] [Green Version]
- Butlerow, A.M. Formation synthétique d’une substance sucrée. C. R. Acad. Sci. 1861, 53, 145–147. [Google Scholar]
- Socha, R.F.; Weiss, A.H.; Sakharov, M.M. Autocatalysis in the formose reaction. React. Kinet. Catal. Lett. 1980, 14, 119–128. [Google Scholar] [CrossRef]
- Delidovich, I.V.; Simonov, A.N.; Taran, O.P.; Parmon, V.N. Catalytic formation of monosaccharides: From the formose reaction towards selective synthesis. ChemSusChem 2014, 7, 1833–1846. [Google Scholar] [CrossRef]
- Hollis, J.M.; Lovas, F.J.; Jewell, P.R. Interstellar glycolaldehyde: The first sugar. Astrophys. J. 2000, 540, L107–L110. [Google Scholar] [CrossRef]
- Milam, S.N.; Remijan, A.J.; Womack, M.; Abrell, L.; Ziurys, L.M.; Wyckoff, S.; Apponi, A.J.; Friedel, D.N.; Snyder, L.E.; Veal, J.M.; et al. Formaldehyde in comets C/1995 O1 (Hale-Bopp), C/2002 T7 (LINEAR), and C/2001 Q4 (NEAT): Investigating the cometary origin of H2CO. Astrophys. J. 2006, 649, 1169–1177. [Google Scholar] [CrossRef]
- Lerner, N.R.; Cooper, G.W. Iminodicarboxylic acids in the murchison meteorite: Evidence of Strecker reactions. Geochim. Cosmochim. Acta 2005, 69, 2901–2906. [Google Scholar] [CrossRef]
- De Leuw, S.; Rubin, A.E.; Wasson, J.T. Carbonates in CM chondrites: Complex formational histories and comparison to carbonates in CI chondrites. Meteorit. Planet. Sci. 2010, 45, 513–530. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.R.; Lindgren, P.; Sofe, M.R. Aragonite, breunnerite, calcite and dolomite in the CM carbonaceous chondrites: High fidelity recorders of progressive parent body aqueous alteration. Geochim. Cosmochim. Acta 2014, 144, 126–156. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Eiler, J.M. Temperatures of aqueous alteration and evidence for methane generation on the parent bodies of the CM chondrites. Geochim. Cosmochim. Acta 2007, 71, 5565–5575. [Google Scholar] [CrossRef] [Green Version]
- Biver, N.; Bockelée-Morvan, D.; Moreno, R.; Crovisier, J.; Colom, P.; Lis, D.C.; Sandqvist, A.; Boissier, J.; Despois, D.; Milam, S.N. Ethyl alcohol and sugar in comet C/2014 Q2 (Lovejoy). Sci. Adv. 2015, 1, e1500863. [Google Scholar] [CrossRef] [Green Version]
- Feldman, P.D.; Brune, W.H. Carbon production in comet West 1975n. Astrophys. J. 1976, 209, L45–L48. [Google Scholar] [CrossRef]
- Combes, M.; Moroz, V.I.; Crovisier, J.; Encrenaz, T.; Bibring, J.-P.; Grigoriev, A.V.; Sanko, N.F.; Coron, N.; Crifo, J.F.; Gispert, R.; et al. The 2.5–12 μm Spectrum of Comet Halley fron the IKS–VEGA Experiment. Icarus 1988, 76, 404–436. [Google Scholar] [CrossRef]
- Snyder, L.E.; Palmer, P.; De Pater, I. Radio detection of formaldehyde emission from comet Halley. Astron. J. 1989, 97, 246–253. [Google Scholar] [CrossRef]
- Geiss, J.; Altwegg, K.; Anders, E.; Balsiger, H.; Ip, W.-H.; Meier, A.; Neugebauer, M.; Rosenbauer, H.; Shelley, E.G. Interpretation of the ion mass spectra in the mass per charge range 25–35 amu/e obtained in the inner coma of Halley’s comet by the HIS-sensor of the Giotto IMS experiment. Astron. Astrophys. 1991, 247, 226–234. [Google Scholar]
- De Marcellus, P.; Meinert, C.; Myrgorodska, I.; Nahon, L.; Buhse, T.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Aldehydes and sugars from evolved precometary ice analogs: Importance of ices in astrochemical and prebiotic evolution. Proc. Natl. Acad. Sci. USA 2015, 112, 965–970. [Google Scholar] [CrossRef] [Green Version]
- Meinert, C.; Myrgorodska, I.; de Marcellus, P.; Buhse, T.; Nahon, L.; Hoffmann, S.V.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Ribose and related sugars from ultraviolet irradiation of interstellar ice analogs. Science 2016, 352, 208–212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nuevo, M.; Cooper, G.; Sandford, S.A. Deoxyribose and deoxysugar derivatives from photoprocessed astrophysical ice analogues and comparison to meteorites. Nat. Commun. 2018, 9, 5276. [Google Scholar] [CrossRef]
- Myrgorodska, I.; Meinert, C.; Martins, Z.; Le Sergeant d’Hendecourt, L.; Meierhenrich, U.J. Molecular Chirality in Meteorites and Interstellar Ices, and the Chirality Experiment on Board the ESA Cometary Rosetta Mission. Angew. Chem. Int. Ed. 2015, 54, 1402–1412. [Google Scholar] [CrossRef]
- Tachibana, S.; Abe, M.; Arakawa, M.; Fujimoto, M.; Iijima, Y.; Ishiguro, M.; Kitazato, K.; Kobayashi, N.; Namiki, N.; Okada, T.; et al. Hayabusa2: Scientific importance of samples returned from C-type near-Earth asteroid (162173) 1999 JU3. Geochem. J. 2014, 48, 571–587. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, S.; Tsuda, Y.; Yoshikawa, M.; Tanaka, S.; Saiki, T.; Nakazawa, S. Hayabusa2 mission overview. Space Sci. Rev. 2017, 208, 3–16. [Google Scholar] [CrossRef]
- Lauretta, D.S.; Balram-Knutson, S.S.; Beshore, E.; Boynton, W.V.; Drouet d’Aubigny, C.; DellaGiustina, D.N.; Enos, H.L.; Golish, D.R.; Hergenrother, C.W.; Howell, E.S.; et al. OSIRIS-REx: Sample return from asteroid (101955) Bennu. Space Sci. Rev. 2017, 212, 925–984. [Google Scholar] [CrossRef]
- Goesmann, F.; Brinckerhoff, W.B.; Raulin, F.; Goetz, W.; Danell, R.M.; Getty, S.A.; Siljeström, S.; Mißbach, H.; Steininger, H.; Arevalo, R.D., Jr.; et al. The Mars Organic Molecule Analyzer (MOMA) instrument: Characterization of organic material in Martian sediments. Astrobiology 2017, 17, 655–685. [Google Scholar] [CrossRef]
- Goetz, W.; Brinckerhoff, W.B.; Arevalo, R., Jr.; Freissinet, C.; Getty, S.; Glavin, D.P.; Siljeström, S.; Buch, A.; Stalport, F.; Grubisic, A.; et al. MOMA: The challenge to search for organics and biosignatures on Mars. Int. J. Astrobiol. 2016, 15, 239–250. [Google Scholar] [CrossRef]
- Vago, J.L.; Westall, F.; Pasteur Instrument Teams, Landing Site Selection Working Group, Other Contributors. Habitability on early Mars and the search for biosignatures with the ExoMars rover. Astrobiology 2017, 17, 471–510. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia, A.D.; Meinert, C.; Sugahara, H.; Jones, N.C.; Hoffmann, S.V.; Meierhenrich, U.J. The Astrophysical Formation of Asymmetric Molecules and the Emergence of a Chiral Bias. Life 2019, 9, 29. https://doi.org/10.3390/life9010029
Garcia AD, Meinert C, Sugahara H, Jones NC, Hoffmann SV, Meierhenrich UJ. The Astrophysical Formation of Asymmetric Molecules and the Emergence of a Chiral Bias. Life. 2019; 9(1):29. https://doi.org/10.3390/life9010029
Chicago/Turabian StyleGarcia, Adrien D., Cornelia Meinert, Haruna Sugahara, Nykola C. Jones, Søren V. Hoffmann, and Uwe J. Meierhenrich. 2019. "The Astrophysical Formation of Asymmetric Molecules and the Emergence of a Chiral Bias" Life 9, no. 1: 29. https://doi.org/10.3390/life9010029