Usefulness of the IgA and IgG Responses to Macrophage Migration Inhibitory Factor for the Diagnosis of Tuberculosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Subjects
2.2. Preparation of MIF
2.3. Enzyme-Linked Immunosorbent Assay (ELISA)
2.4. Data Analysis
2.5. Ethics Statement
3. Results
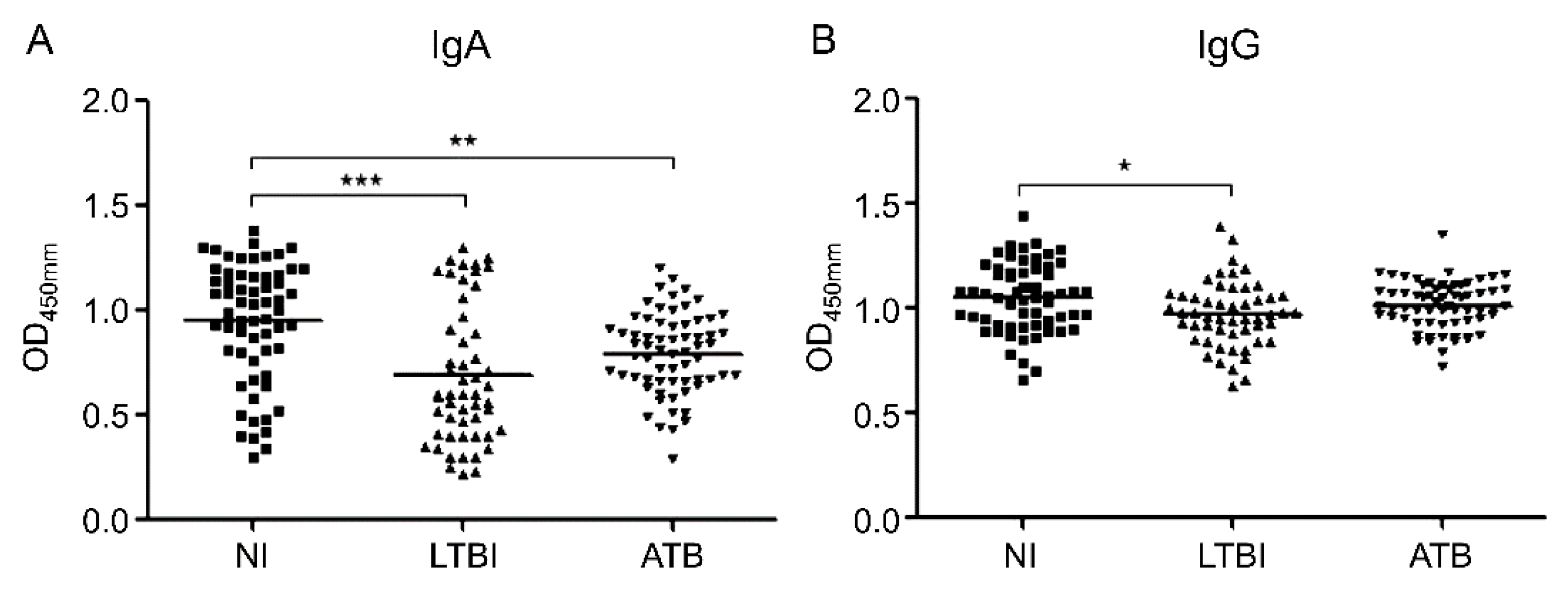
3.1. Serum Levels of IgA and IgG against MIF
3.2. IgA and IgG Serum Levels in Subgroup Analysis
3.3. Correlations Among IgA and IgG Serum Levels, and IFN-γ, Age, BMI, Sex, and Drug Resistance
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Tuberculosis Report 2019. Available online: https://www.who.int/tb/publications/global_report/en/ (accessed on 31 August 2020).
- Pfyffer, G.E.; Wittwer, F. Incubation time of mycobacterial cultures: How long is long enough to issue a final negative report to the clinician? J. Clin. Microbiol. 2012, 50, 4188–4189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canadian Thoracic Society. Canadian Tuberculosis Standards, 7th edition. Available online: https://www.canada.ca/en/public-health/services/infectious-diseases/canadian-tuberculosis-standards-7th-edition.html (accessed on 31 August 2020).
- Lim, T.K.; Gough, A.; Chin, N.K.; Kumarasinghe, G. Relationship between estimated pretest probability and accuracy of automated Mycobacterium tuberculosis assay in smear-negative pulmonary tuberculosis. Chest 2000, 118, 641–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zyl-Smit, R.N.; Zwerling, A.; Dheda, K.; Pai, M. Within-subject variability of interferon-g assay results for tuberculosis and boosting effect of tuberculin skin testing: A systematic review. PLoS ONE 2009, 4, e8517. [Google Scholar] [CrossRef] [Green Version]
- Pai, M.; Denkinger, C.M.; Kik, S.V.; Rangaka, M.X.; Zwerling, A.; Oxlade, O.; Metcalfe, J.Z.; Cattamanchi, A.; Dowdy, D.W.; Dheda, K.; et al. Gamma interferon release assays for detection of Mycobacterium tuberculosis infection. Clin. Microbiol. Rev. 2014, 27, 3–20. [Google Scholar] [CrossRef] [Green Version]
- Sester, M.; Sotgiu, G.; Lange, C.; Giehl, C.; Girardi, E.; Migliori, G.B.; Bossink, A.; Dheda, K.; Diel, R.; Dominguez, J.; et al. Interferon-γ release assays for the diagnosis of active tuberculosis: A systematic review and meta-analysis. Eur. Respir. J. 2011, 37, 100–111. [Google Scholar] [CrossRef] [Green Version]
- Metcalfe, J.Z.; Everett, C.K.; Steingart, K.R.; Cattamanchi, A.; Huang, L.; Hopewell, P.C.; Pai, M. Interferon-γ release assays for active pulmonary tuberculosis diagnosis in adults in low- and middle-income countries: Systematic review and meta-analysis. J. Infect. Dis. 2011, 204, S1120–S1129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, B.R.; Bennett, B. Mechanism of a reaction in vitro associated with delayed-type hypersensitivity. Science 1966, 153, 80–82. [Google Scholar] [CrossRef] [PubMed]
- David, J.R. Delayed hypersensitivity in vitro: Its mediation by cell-free substances formed by lymphoid cell-antigen interaction. Proc. Natl. Acad. Sci. USA 1966, 56, 72–77. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, T.; Ohkawara, A.; Nishihira, J.; Sakamoto, W. Identification of macrophage migration inhibitory factor (MIF) in human skin and its immunohistochemical localization. FEBS Lett. 1996, 381, 199–202. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, A.; Tagawa, Y.; Matsuda, H.; Nishihira, J. Identification and immunohistochemical localization of macrophage migration inhibitory factor in human cornea. FEBS Lett. 1996, 385, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Bacher, M.; Metz, C.N.; Calandra, T.; Mayer, K.; Chesney, J.; Lohoff, M.; Gemsa, D.; Donnelly, T.; Bucala, R. An essential regulatory role for macrophage migration inhibitory factor in T-cell activation. Proc. Natl. Acad. Sci. USA 1996, 93, 7849–7854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calandra, T.; Bernhagen, J.; Metz, C.N.; Spiegel, L.A.; Bacher, M.; Donnelly, T.; Cerami, A.; Bucala, R. MIF as a glucocorticoid-induced modulator of cytokine production. Nature 1995, 377, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Das, R.; Koo, M.S.; Kim, B.H.; Jacob, S.T.; Subbian, S.; Yao, J.; Leng, L.; Levy, R.; Murchison, C.; Burman, W.J.; et al. Macrophage migration inhibitory factor (MIF) is a critical mediator of the innate immune response to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2013, 110, E2997–E3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, M.; Uchida, K.; Nakagaki, K.; Kanazawa, H.; Trapnell, B.C.; Hoshino, Y.; Kagamu, H.; Yoshizawa, H.; Keicho, N.; Goto, H.; et al. Anti-cytokine autoantibodies are ubiquitous in healthy individuals. FEBS Lett. 2007, 581, 2017–2021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karin, N. Induction of protective therapy for autoimmune diseases by targeted DNA vaccines encoding pro-inflammatory cytokines and chemokines. Curr. Opin. Mol. Ther. 2004, 6, 27–33. [Google Scholar] [PubMed]
- Capini, C.J.; Richardson, M.W.; Hendel, H.; Sverstiuk, A.; Mirchandani, J.; Régulier, E.G.; Khalili, K.; Zagury, J.F.; Rappaport, J. Autoantibodies to TNFα in HIV-1 infection: Prospects for anti-cytokine vaccine therapy. Biomed. Pharmacother. 2001, 55, 23–31. [Google Scholar] [CrossRef]
- Revoltella, R.P.; Robbio, L.L.; Moscato, S.; Vinante, F.; Fasciani, A.; Liberati, A.M.; Reato, G.; Foa, R. Naturally-occurring anti-G-CSF antibodies produced by human cord blood B-cell lines infected with Epstein-Barr virus. Hematol. J. 2001, 2, 161–171. [Google Scholar] [CrossRef]
- Ross, C.; Hansen, M.B.; Schyberg, T.; Berg, K. Autoantibodies to crude human leucocyte interferon (IFN), native human IFN, recombinant human IFN-alpha 2b and human IFN-gamma in healthy blood donors. Clin. Exp. Immunol. 1990, 82, 57–62. [Google Scholar] [CrossRef]
- Watanabe, M.; Uchida, K.; Nakagaki, K.; Trapnell, B.C.; Nakata, K. High avidity cytokine autoantibodies in health and disease: Pathogenesis and mechanisms. Cytokine Growth Factor Rev. 2010, 21, 263–273. [Google Scholar] [CrossRef]
- Lee, J.Y.; Kim, B.J.; Koo, H.K.; Kim, J.; Kim, J.M.; Kook, Y.H.; Kim, B.J. Diagnostic potential of IgG and IgA responses to Mycobacterium tuberculosis antigens for discrimination among active tuberculosis, latent tuberculosis infection, and non-infected individuals. Microorganisms 2020, 8, 979. [Google Scholar] [CrossRef]
- Kim, B.J.; Gong, J.R.; Kim, G.N.; Kim, B.R.; Lee, S.Y.; Kook, Y.H.; Kim, B.J. Recombinant Mycobacterium smegmatis with a pMyong2 vector expressing Human Immunodeficiency Virus Type I Gag can induce enhanced virus-specific immune responses. Sci. Rep. 2017, 7, 44776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghababa, H.; Mobarez, A.M.; Behmanesh, M.; Khoramabadi, N.; Mobarhan, M. Production and purification of mycolyl transferase B of Mycobacterium tuberculosis. Tanaffos 2011, 10, 23–30. [Google Scholar] [PubMed]
- Lawn, S.D.; Edwards, D.J.; Kranzer, K.; Vogt, M.; Bekker, L.G.; Wood, R. Urine lipoarabinomannan assay for tuberculosis screening before antiretroviral therapy diagnostic yield and association with immune reconstitution disease. AIDS 2009, 23, 1875–1880. [Google Scholar] [CrossRef] [PubMed]
- Raja, A.; Devi, K.R.U.; Ramalingam, B.; Brennan, P.J. Improved diagnosis of pulmonary tuberculosis by detection of free and immune complex-bound anti-30 kDa antibodies. Diagn. Microbiol. Infect. Dis. 2004, 50, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Jafari, C.; Olaru, I.D.; Daduna, F.; Ernst, M.; Heyckendorf, J.; Lange, C.; Kalsdorf, B. Rapid diagnosis of pulmonary tuberculosis by combined molecular and immunological methods. Eur. Respir. J. 2018, 51, 1702189. [Google Scholar] [CrossRef] [PubMed]
- Yamada, G.; Shijubo, N.; Takagi-Takahashi, Y.; Nishihira, J.; Mizue, Y.; Kikuchi, K.; Abe, S. Elevated levels of serum macrophage migration inhibitory factor in patients with pulmonary tuberculosis. Clin. Immunol. 2002, 104, 123–127. [Google Scholar] [CrossRef]
- Nattee, P.; Honsawek, S.; Chongsrisawat, V.; Vejchapipat, P.; Thamboonlers, A.; Poovorawan, Y. Elevated serum macrophage migration inhibitory factor levels in post-operative biliary atresia. Asian J. Surg. 2009, 32, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Bernhagen, J.; Calandra, T.; Mitchell, R.A.; Martin, S.B.; Tracey, K.J.; Voelter, W.; Manogue, K.R.; Cerami, A.; Bucala, R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature 1993, 365, 756–759. [Google Scholar] [CrossRef]
- Rice, E.K.; Nikolic-Paterson, D.J.; Hill, P.A.; Metz, C.N.; Bucala, R.; Atkins, R.C.; Tesch, G.H. Interferon-gamma induces macrophage migration inhibitory factor synthesis and secretion by tubular epithelial cells. Nephrology 2003, 8, 156–161. [Google Scholar] [CrossRef]
- Calandra, T.; Bernhagen, J.; Mitchell, R.A.; Bucala, R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J. Exp. Med. 1994, 179, 1895–1902. [Google Scholar] [CrossRef] [Green Version]
- Madariaga, L.; Amurrio, C.; Martín, G.; García-Cebrian, F.; Bicandi, J.; Lardelli, P.; Suarez, M.D.; Cisterna, R. Detection of anti-interferon-gamma autoantibodies in subjects infected by Mycobacterium tuberculosis. Int. J. Tuberc. Lung Dis. 1998, 2, 62–68. [Google Scholar] [PubMed]
- Caruso, A.; Turano, A. Natural antibodies to interferon-gamma. Biotherapy 1997, 10, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.Y.; Ding, L.; Brown, M.R.; Lantz, L.; Gay, T.; Cohen, S.; Martyak, L.A.; Kubak, B.; Holland, S.M. Anti-IFN-gamma autoantibodies in disseminated nontuberculous mycobacterial infections. J. Immunol. 2005, 175, 4769–4776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, K.; Nakata, K.; Trapnell, B.C.; Terakawa, T.; Hamano, E.; Mikami, A.; Matsushita, I.; Seymour, J.F.; Oh-eda, M.; Ishige, I.; et al. High-affinity autoantibodies specifically eliminate granulocyte-macrophage colony-stimulating factor activity in the lungs of patients with idiopathic pulmonary alveolar proteinosis. Blood 2004, 103, 1089–1098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtney, L.P.; Phelps, J.L.; Karavodin, L.M. An anti-IL-2 antibody increases serum half-life and improves anti-tumor efficacy of human recombinant interleukin-2. Immunopharmacology 1994, 28, 223–232. [Google Scholar] [CrossRef]
- Uchida, K.; Nakata, K.; Suzuki, T.; Luisetti, M.; Watanabe, M.; Koch, D.E.; Stevens, C.A.; Beck, D.C.; Denson, L.A.; Carey, C.B.; et al. Granulocyte/macrophage-colony-stimulating factor autoantibodies and myeloid cell immune functions in healthy subjects. Blood 2009, 113, 2547–2556. [Google Scholar] [CrossRef]
- Monti, E.; Pozzi, A.; Tiberio, L.; Morelli, D.; Caruso, A.; Villa, M.L.; Balsari, A. Purification of interleukin-2 antibodies from healthy individuals. Immunol. Lett. 1993, 36, 261–266. [Google Scholar] [CrossRef]
- Galle, P.; Svenson, M.; Bendtzen, K.; Hansen, M.B. High levels of neutralizing IL-6 autoantibodies in 0.1% of apparently healthy blood donors. Eur. J. Immunol. 2004, 34, 3267–3275. [Google Scholar] [CrossRef]



| Groups and Antibody | AUC (p Value) | Cut-Off (OD) | Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|
| IgA response to MIF | ||||
| NI vs. LTBI | 0.7161 (<0.0001) | 0.7825 | 69.8 | 75.8 |
| NI vs. ATB | 0.6919 (<0.001) | 0.8835 | 69.2 | 67.7 |
| LTBI vs. ATB | 0.6398 (<0.01) | 0.7075 | 64.6 | 62.3 |
| IgG response to MIF | ||||
| NI vs. LTBI | 0.6397 (<0.05) | 1.0175 | 64.2 | 59.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.Y.; Kim, B.-J.; Kim, J.-m.; Kim, J.; Joh, J.-S.; Jeong, I.; Kook, Y.-H.; Kim, B.-J. Usefulness of the IgA and IgG Responses to Macrophage Migration Inhibitory Factor for the Diagnosis of Tuberculosis. Diagnostics 2020, 10, 991. https://doi.org/10.3390/diagnostics10110991
Lee JY, Kim B-J, Kim J-m, Kim J, Joh J-S, Jeong I, Kook Y-H, Kim B-J. Usefulness of the IgA and IgG Responses to Macrophage Migration Inhibitory Factor for the Diagnosis of Tuberculosis. Diagnostics. 2020; 10(11):991. https://doi.org/10.3390/diagnostics10110991
Chicago/Turabian StyleLee, Ji Yeon, Byoung-Jun Kim, Jee-min Kim, Junghyun Kim, Joon-Sung Joh, Ina Jeong, Yoon-Hoh Kook, and Bum-Joon Kim. 2020. "Usefulness of the IgA and IgG Responses to Macrophage Migration Inhibitory Factor for the Diagnosis of Tuberculosis" Diagnostics 10, no. 11: 991. https://doi.org/10.3390/diagnostics10110991






