COPD Imaging on a 3rd Generation Dual-Source CT: Acquisition of Paired Inspiratory-Expiratory Chest Scans at an Overall Reduced Radiation Risk
Abstract
:1. Introduction
2. Methods
2.1. Subjects, CT Systems, and Imaging Protocols
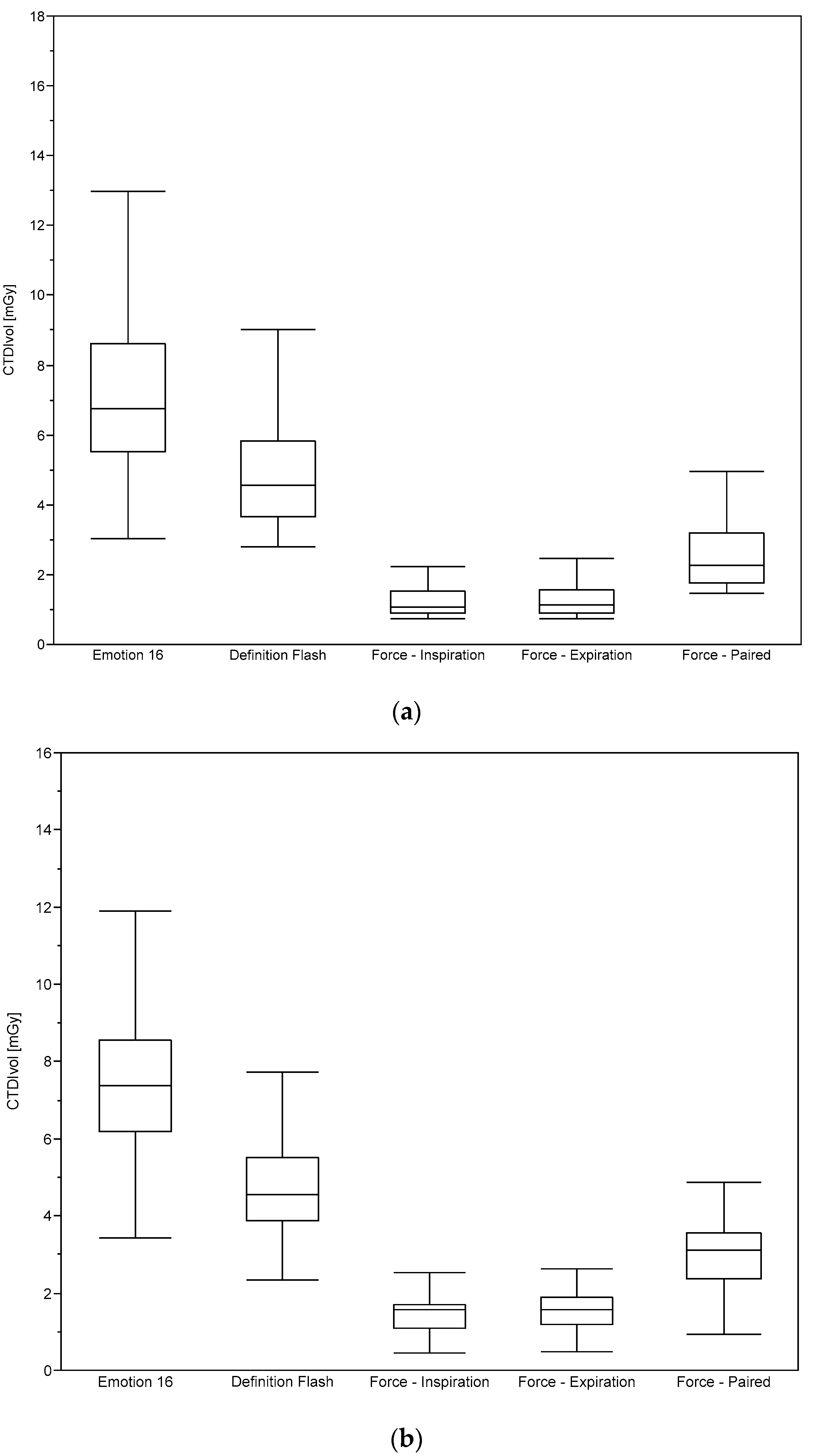
2.2. Dosimetry
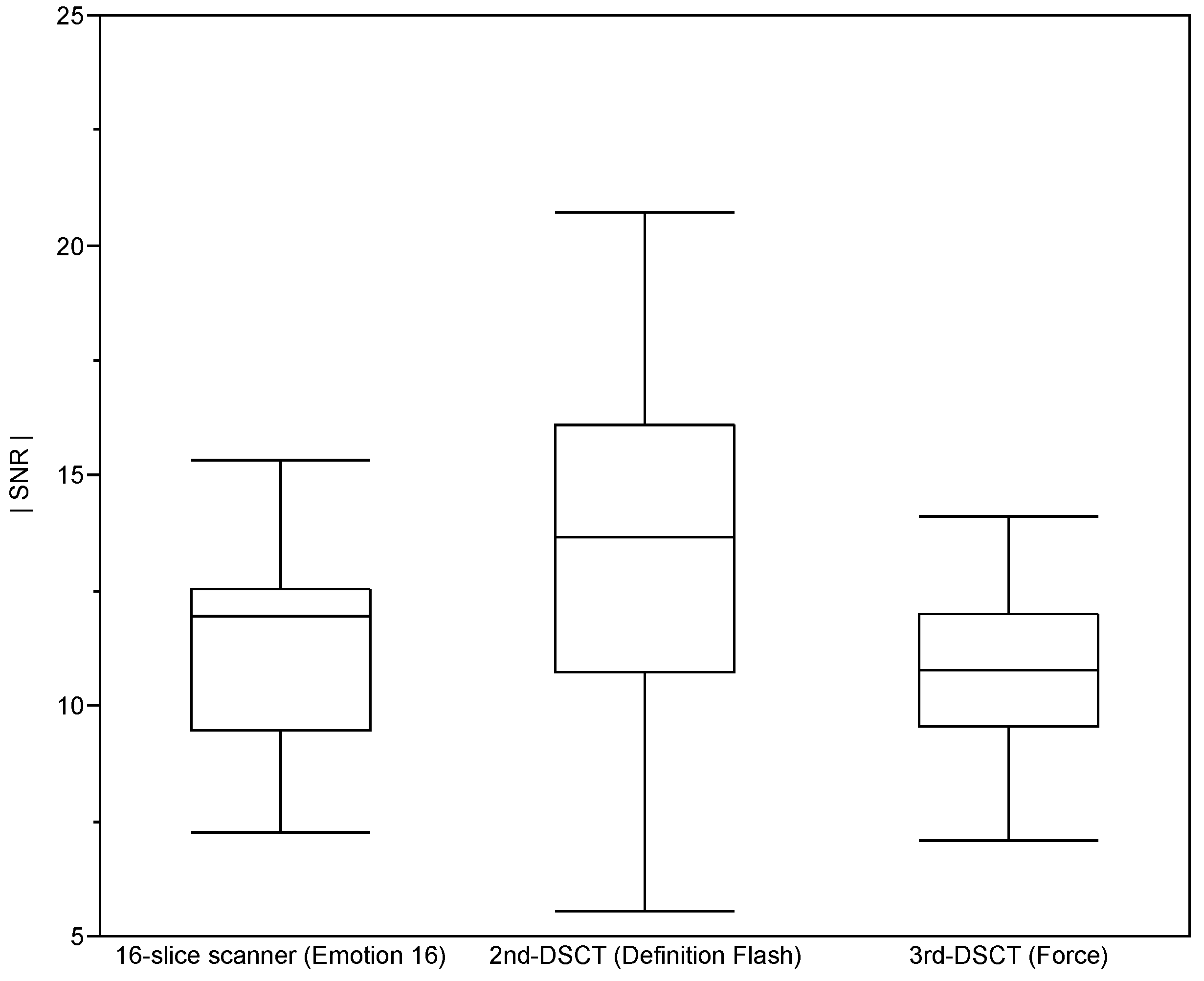
2.3. Assessment of Image Quality
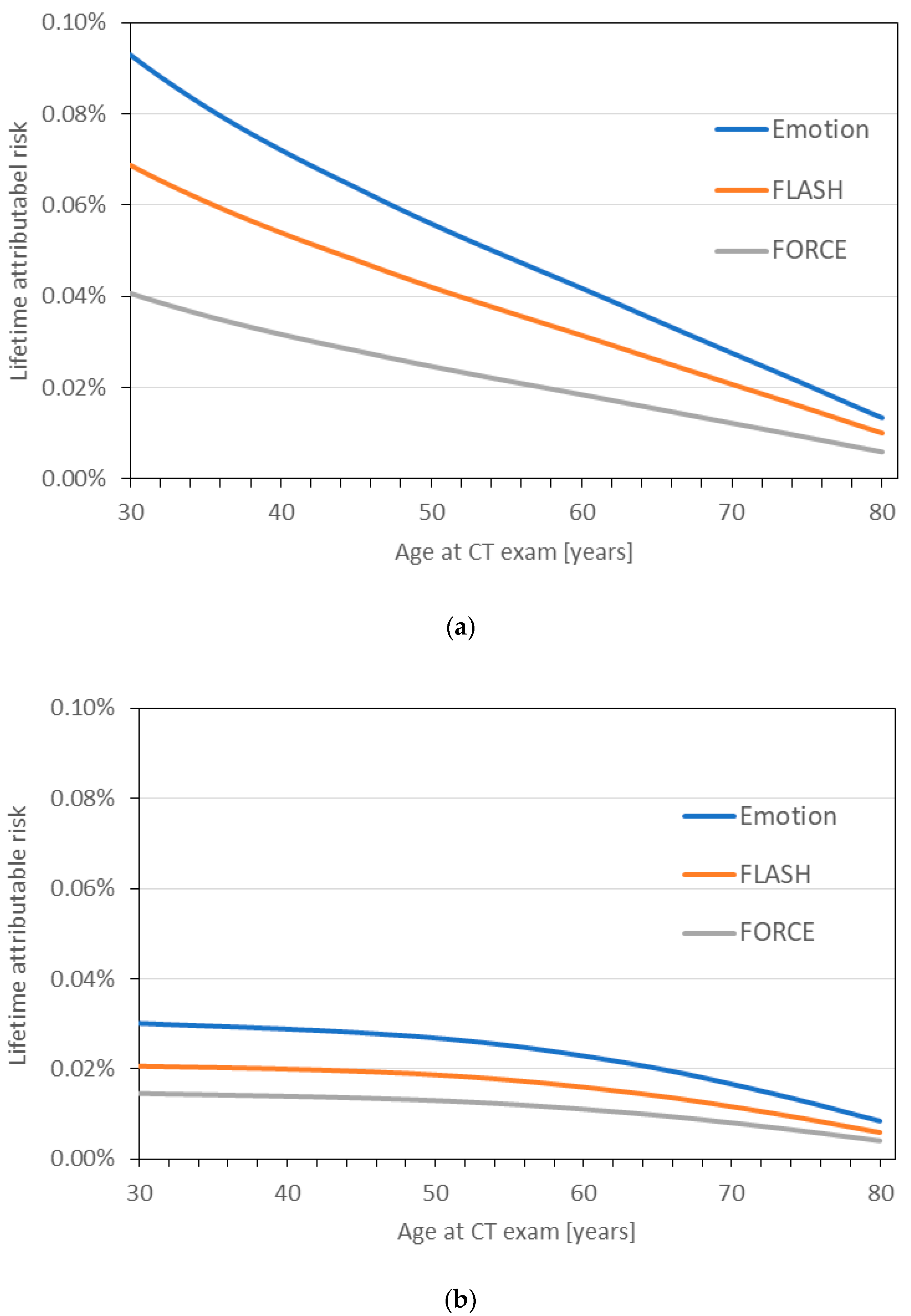
2.4. Lifetime Attributable Cancer Risk
2.5. Data Analysis
3. Results
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Bhutta, Z.A.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar]
- The Top 10 Causes of Death. Available online: http://www.who.int/mediacentre/factsheets/fs310/en/ (accessed on 18 September 2020).
- Fernandes, L.; Fernandes, Y.; Mesquita, A.M. Quantitative computed tomography imaging in chronic obstructive pulmonary disease. Lung India 2016, 33, 646–652. [Google Scholar] [CrossRef] [PubMed]
- Nambu, A.; Zach, J.; Schroeder, J.; Jin, G.; Kim, S.S.; Kim, Y.-I.; Schnell, C.; Bowler, R.; Lynch, D.A. Quantitative computed tomography measurements to evaluate airway disease in chronic obstructive pulmonary disease: Relationship to physiological measurements, clinical index and visual assessment of airway disease. Eur. J. Radiol. 2016, 85, 2144–2151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuoka, S.; Kurihara, Y.; Yagihashi, K.; Hoshino, M.; Nakajima, Y. Airway Dimensions at Inspiratory and Expiratory Multisection CT in Chronic Obstructive Pulmonary Disease: Correlation with Airflow Limitation. Radiology 2008, 248, 1042–1049. [Google Scholar] [CrossRef]
- Gawlitza, J.; Trinkmann, F.; Scheffel, H.; Fischer, A.; Nance, J.W.; Henzler, C.; Vogler, N.; Saur, J.; Akin, I.; Borggrefe, M.; et al. Time to Exhale: Additional Value of Expiratory Chest CT in Chronic Obstructive Pulmonary Disease. Can. Respir. J. 2018, 2018, 9. [Google Scholar] [CrossRef] [Green Version]
- Gawlitza, J.; Haubenreisser, H.; Henzler, T.; Akin, I.; Schönberg, S.; Borggrefe, M.; Trinkmann, F. Finding the right spot: Where to measure airway parameters in patients with COPD. Eur. J. Radiol. 2018, 104, 87–93. [Google Scholar] [CrossRef]
- Lynch, D.A.; Austin, J.H.; Hogg, J.C.; Grenier, P.A.; Kauczor, H.U.; Bankier, A.A.; Barr, R.G.; Colby, T.V.; Galvin, J.R.; Gevenois, P.A.; et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society. Radiology 2015, 277, 192–205. [Google Scholar] [CrossRef] [Green Version]
- Kalra, M.K.; Maher, M.M.; Toth, T.L.; Kamath, R.S.; Halpern, E.F.; Saini, S. Comparison of Z-axis automatic tube current modulation technique with fixed tube current CT scanning of abdomen and pelvis. Radiology 2004, 232, 347–353. [Google Scholar]
- Takx, R.A.; Schoepf, U.J.; Moscariello, A.; Das, M.; Rowe, G.; Schoenberg, S.O.; Fink, C.; Henzler, T. Coronary CT angiography: Comparison of a novel iterative reconstruction with filtered back projection for reconstruction of low-dose CT—Initial experience. Eur. J. Radiol. 2013, 82, 275–280. [Google Scholar]
- Haubenreisser, H.; Meyer, M.; Sudarski, S.; Allmendinger, T.; Schoenberg, S.O.; Henzler, T. Unenhanced third-generation dual-source chest CT using a tin filter for spectral shaping at 100kVp. Eur. J. Radiol. 2015, 84, 1608–1613. [Google Scholar] [CrossRef]
- Hu, X.; Ding, X.; Wu, R.; Zhang, M. Radiation dose of non-enhanced chest CT can be reduced 40% by using iterative reconstruction in image space. Clin. Radiol. 2011, 66, 1023–1029. [Google Scholar] [PubMed]
- Nagayama, Y.; Oda, S.; Nakaura, T.; Tsuji, A.; Urata, J.; Furusawa, M.; Utsunomiya, D.; Funama, Y.; Kidoh, M.; Yamashita, Y. Radiation Dose Reduction at Pediatric CT: Use of Low Tube Voltage and Iterative Reconstruction—Erratum. RadioGraphics 2019, 39, 912. [Google Scholar] [PubMed] [Green Version]
- Zinsser, D.; Marcus, R.; Othman, A.E.; Bamberg, F.; Nikolaou, K.; Flohr, T.; Notohamiprodjo, M. Dose reduction and dose management in computed tomography–state of the art. RoFo Fortschr. Gebiete Rontgenstrahlen Bildgeb. Verfahr. 2018, 190, 531–541. [Google Scholar]
- Brix, G.; Lechel, U.; Sudarski, S.; Trumm, C.; Henzler, T. Spectral optimization of iodine-enhanced CT: Quantifying the effect of tube voltage on image quality and radiation exposure determined at an anthropomorphic phantom. Phys. Med. 2016, 32, 999–1006. [Google Scholar] [PubMed]
- International Commission on Radiological Protection. The 2007 Recommendations of the International Commission on Radiological Protection. Ann. ICRP 2007, 37, 32–34. [Google Scholar]
- Stamm, G.; Nagel, H.D. CT-expo—A novel program for dose evaluation in CT. RoFo Fortschr. Geb. Rontgenstrahlen Bildgeb. Verfahr. 2002, 174, 1570–1576. [Google Scholar]
- Brix, G.; Lechel, U.; Veit, R.; Truckenbrodt, R.; Stamm, G.; Coppenrath, E.; Griebel, J.; Nagel, H.-D. Assessment of a theoretical formalism for dose estimation in CT: An anthropomorphic phantom study. Eur. Radiol. 2004, 14, 1275–1284. [Google Scholar]
- McCollough, C.; Bakalyar, D.; Bostani, M.; Brady, S.; Boedeker, K.; Boone, J.; Chen-Mayer, H.H.; Christianson, O.; Leng, S.; Li, B.; et al. Use of Water Equivalent Diameter for Calculating Patient Size and Size-Specific Dose Estimates (SSDE) in CT; AAPM: Alexandria, VA, USA, 2014. [Google Scholar]
- Bostani, M.; McMillan, K.; Lu, P.; Kim, H.J.; Cagnon, C.H.; DeMarco, J.J.; McNitt-Gray, M.F. Attenuation-based size metric for estimating organ dose to patients undergoing tube current modulated CT exams. Med Phys. 2015, 42, 958–968. [Google Scholar] [CrossRef]
- National Research Council. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2; National Academies Press: Washington, DC, USA, 2006; Volume 7.24. [Google Scholar]
- Strahlenschutzkommission (SSK). Dose and Dose-Rate Effectiveness Factor (DDREF). Adopted at the 268th Meeting of the SSK on 13/14 February 2014. Article 2014.02.13. Available online: https://www.ssk.de/SharedDocs/Beratungsergebnisse_PDF/2014/DDREF_e.pdf?__blob=publicationFile (accessed on 10 November 2020).
- Brix, G.; Berton, M.; Nekolla, E.; Lechel, U.; Schegerer, A.; Süselbeck, T.; Fink, C. Cumulative radiation exposure and cancer risk of patients with ischemic heart diseases from diagnostic and therapeutic imaging procedures. Eur. J. Radiol. 2013, 82, 1926–1932. [Google Scholar]
- Tukey, J.W. Exploratory Data Analysis; Addisson-Wesley Publishing: Reading, MA, USA, 1977; Volume 2. [Google Scholar]
- Martin, C.J.; Harrison, J.D.; Rehani, M.M. Effective dose from radiation exposure in medicine: Past, present, and future. Phys. Med. 2020, 79, 87–92. [Google Scholar]
- Robert-Koch-Institut, Gesellschaft der Epidemiologischen Krebsregister e.V. (GEKID) und Zentrum für Krebsregisterdaten (ZfKD). Krebs in Deutschland 2015/2016; Robert Koch-Institut: Berlin, Germany, 2019. [Google Scholar] [CrossRef]
- Weis, M.; Henzler, T.; Nance, J.W., Jr.; Haubenreisser, H.; Meyer, M.; Sudarski, S.; Schoenberg, S.O.; Neff, K.W.; Hagelstein, C. Radiation Dose Comparison Between 70 kVp and 100 kVp With Spectral Beam Shaping for Non-Contrast-Enhanced Pediatric Chest Computed Tomography: A Prospective Randomized Controlled Study. Investig. Radiol. 2017, 52, 155–162. [Google Scholar] [CrossRef]
- Gordic, S.; Morsbach, F.; Schmidt, B.; Allmendinger, T.; Flohr, T.; Husarik, D.; Baumueller, S.; Raupach, R.; Stolzmann, P.; Leschka, S.; et al. Ultralow-dose chest computed tomography for pulmonary nodule detection: First performance evaluation of single energy scanning with spectral shaping. Investig. Radiol. 2014, 49, 465–473. [Google Scholar] [CrossRef] [Green Version]
- Meyer, M.; Haubenreisser, H.; Schoepf, U.J.; Vliegenthart, R.; Leidecker, C.; Allmendinger, T.; Lehmann, R.; Sudarski, S.; Borggrefe, M.; Schoenberg, S.O. Closing in on the K Edge: Coronary CT angiography at 100, 80, and 70 kv—initial comparison of a second-versus a third-generation dual-source CT system. Radiology 2014, 273, 373–382. [Google Scholar] [PubMed] [Green Version]
- Braun, F.M.; Johnson, T.R.; Sommer, W.H.; Thierfelder, K.M.; Meinel, F.G. Chest CT using spectral filtration: Radiation dose, image quality, and spectrum of clinical utility. Eur. Radiol. 2015, 25, 1598–1606. [Google Scholar] [PubMed]
- Omar, R.; Hashim, S.; Ghoshal, S.; Shariff, N. Dose assessment of 4-and 16-slice multi-detector computed tomography (MDCT) scanners. Radiat. Phys. Chem. 2020, 168, 108445. [Google Scholar]
- Tahiri, Z.; Jroundi, L.; Laamarni, F.Z.; Mkimel, M. Radiation Dose and Lifetime Risk of Cancer Incidence and Mortality in Patients Undergoing 16 Slice CT Emergency Examinations. Int. J. Recent Technol. Eng. 2019, 8, 2277–3878. [Google Scholar]
- Schegerer, A.A.; Nagel, H.-D.; Stamm, G.; Adam, G.; Brix, G. Current CT practice in Germany: Results and implications of a nationwide survey. Eur. J. Radiol. 2017, 90, 114–128. [Google Scholar]
- Shore, R.E. Radiation impacts on human health: Certain, fuzzy, and unknown. Health Phys. 2014, 106, 196–205. [Google Scholar] [CrossRef]
- Shah, D.J.; Sachs, R.K.; Wilson, D.J. Radiation-induced cancer: A modern view. Br. J. Radiol. 2012, 85, e1166–e1173. [Google Scholar] [CrossRef] [Green Version]
- United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR). Biological mechanisms of radiation actions at low doses: A white paper to guide the Scientific Committee’s future programme of work. United Nations Publ. 2012, 12, 57831. [Google Scholar]
- Oh, A.S.; Strand, M.; Pratte, K.; Regan, E.A.; Humphries, S.; Crapo, J.D.; Lynch, D.A.; For the Genetic Epidemiology of COPDGene Investigators. Visual emphysema at chest CT in GOLD stage 0 cigarette smokers predicts disease progression: Results from the COPDGene study. Radiology 2020, 296, 641–649. [Google Scholar] [PubMed]

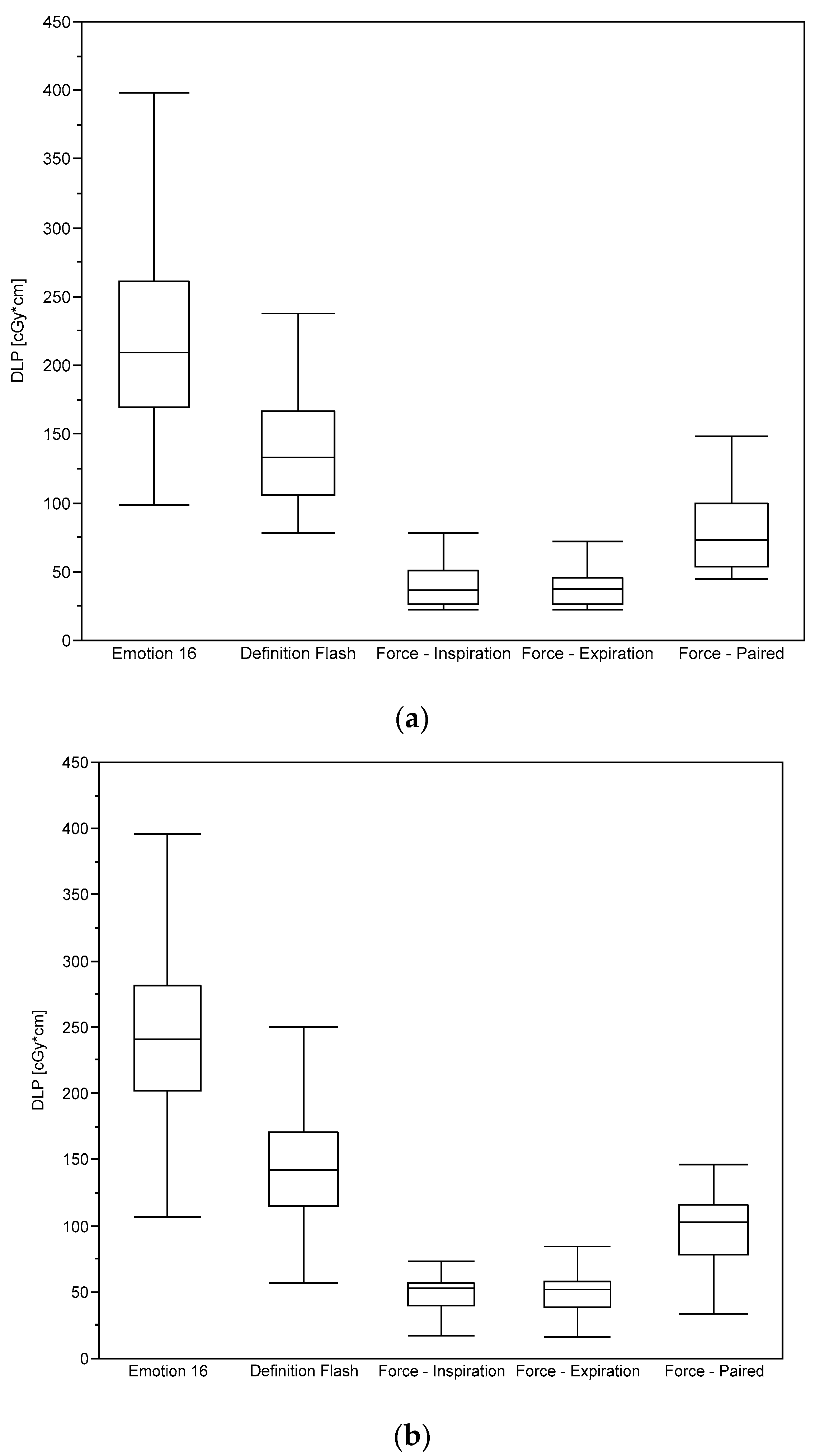

| Emotion 16 | Definition FLASH | FORCE | |
|---|---|---|---|
| Tin filter | No | No | Yes |
| Automated tube voltage modulation | Yes | Yes | Yes |
| Tube voltage [kVp] | 130 | 120 | 120 |
| Reference current [mAs] | 50 | 50 | 96 |
| Pitch | 0.8 | 0.6 | 1.2 |
| Rotation time [s] | 0.6 | 0.3 | 0.25 |
| Detector collimation [mm] | 16 × 1.2 | 128 × 0.6 | 192 × 0.6 |
| Iterative reconstruction | No | Yes | Yes |
| Slice thickness [mm] | 1.5 | 1.5 | 1.5 |
| Size of reconstructed images | 512 × 512 | 512 × 512 | 512 × 512 |
| Emotion 16 | Definition FLASH | FORCE | ||||
|---|---|---|---|---|---|---|
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | |
| No. of patients | 272 | 282 | 64 | 156 | 21 | 29 |
| Age [years] | 64 ± 17 | 64 ± 15 | 58 ± 14 | 59 ± 19 | 62 ± 14 | 60 ± 12 |
| WED [mm] | 285 ± 32 | 297 ± 28 | 281 ± 32 | 292 ± 30 | 309 ± 36 | 331 ± 27 |
| Scan length [mm] | 309 ± 21 | 327 ± 23 | 291 ± 32 | 303 ± 57 | 302 ± 32 | 321 ± 19 |
| Effective doses [mSv] | 4.3 ± 1.5 | 3.0 ± 1.2 | 1.0 ± 0.4 | |||
| Emotion 16 | Definition FLASH | FORCE | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Breathing Position | Inspiration | Inspiration | Inspiration | Expiration | Paired | |||||
| ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | ♀ | ♂ | |
| Liver | 5.5 ± 2.0 | 5.6 ± 1.7 | 3.8 ± 1.3 | 3.7 ± 1.6 | 1.3 ± 0.6 | 1.4 ± 0.5 | 1.3 ± 0.6 | 1.4 ± 0.4 | 2.6 ± 1.2 | 2.8 ± 1.0 |
| Adrenals | 7.2 ± 2.6 | 7.4 ± 2.3 | 5.2 ± 1.7 | 5.1 ± 2.2 | 1.6 ± 0.8 | 1.8 ± 0.7 | 1.6 ± 0.8 | 1.8 ± 0.6 | 3.2 ± 1.5 | 3.6 ± 1.2 |
| Skeleton | 7.3 ± 2.6 | 7.6 ± 2.4 | 5.4 ± 1.8 | 5.3 ± 2.3 | 1.6 ± 0.8 | 1.9 ± 0.7 | 1.6 ± 0.8 | 1.8 ± 0.6 | 3.2 ± 1.5 | 3.7 ± 1.3 |
| Heart | 7.9 ± 2.8 | 8.5 ± 2.7 | 6.1 ± 2.0 | 6.1 ± 2.6 | 1.7 ± 0.9 | 2.0 ± 0.8 | 1.7 ± 0.9 | 2.0 ± 0.6 | 3.5 ± 1.6 | 4.0 ± 1.4 |
| Breast | 9.3 ± 3.3 | - | 7.2 ± 2.4 | - | 2.0 ± 1.0 | - | 2.0 ± 1.0 | - | 4.0 ± 1.9 | - |
| Thyroid | 9.6 ± 3.4 | 9.0 ± 2.8 | 4.7 ± 1.6 | 4.0 ± 1.7 | 2.0 ± 1.0 | 2.2 ± 0.8 | 2.0 ± 1.0 | 2.1 ± 0.7 | 4.0 ± 1.9 | 4.3 ± 1.5 |
| Upper Airways | 9.6 ± 3.4 | 9.0 ± 2.8 | 4.7 ± 1.6 | 4.0 ± 1.7 | 2.0 ± 1.0 | 2.0 ± 0.8 | 2.0 ± 1.0 | 2.1 ± 0.7 | 4.0 ± 1.9 | 4.3 ± 1.5 |
| Esophagus | 9.7 ± 3.5 | 9.7 ± 3.0 | 7.4 ± 2.5 | 7.0 ± 3.0 | 2.1 ± 1.0 | 2.3 ± 0.9 | 2.1 ± 1.0 | 2.3 ± 0.7 | 4.2 ± 2.0 | 4.6 ± 1.6 |
| Lungs | 9.7 ± 3.5 | 10.1 ± 3.2 | 7.4 ± 2.5 | 7.2 ± 3.0 | 2.2 ± 1.1 | 2.4 ± 0.9 | 2.2 ± 1.1 | 2.4 ± 0.8 | 4.3 ± 2.0 | 4.8 ± 1.7 |
| Thymus | 9.7 ± 3.5 | 9.8 ± 3.0 | 7.4 ± 2.5 | 7.0 ± 3.0 | 2.1 ± 1.0 | 2.3 ± 0.9 | 2.1 ± 1.0 | 2.3 ± 0.7 | 4.2 ± 2.0 | 4.6 ± 1.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gawlitza, J.; Henzler, T.; Trinkmann, F.; Nekolla, E.; Haubenreisser, H.; Brix, G. COPD Imaging on a 3rd Generation Dual-Source CT: Acquisition of Paired Inspiratory-Expiratory Chest Scans at an Overall Reduced Radiation Risk. Diagnostics 2020, 10, 1106. https://doi.org/10.3390/diagnostics10121106
Gawlitza J, Henzler T, Trinkmann F, Nekolla E, Haubenreisser H, Brix G. COPD Imaging on a 3rd Generation Dual-Source CT: Acquisition of Paired Inspiratory-Expiratory Chest Scans at an Overall Reduced Radiation Risk. Diagnostics. 2020; 10(12):1106. https://doi.org/10.3390/diagnostics10121106
Chicago/Turabian StyleGawlitza, Joshua, Thomas Henzler, Frederik Trinkmann, Elke Nekolla, Holger Haubenreisser, and Gunnar Brix. 2020. "COPD Imaging on a 3rd Generation Dual-Source CT: Acquisition of Paired Inspiratory-Expiratory Chest Scans at an Overall Reduced Radiation Risk" Diagnostics 10, no. 12: 1106. https://doi.org/10.3390/diagnostics10121106





