Metabolic Changes in Maternal and Cord Blood in One Case of Pregnancy-Associated Breast Cancer Seen by Fluorescence Lifetime Imaging Microscopy
Abstract
:1. Introduction
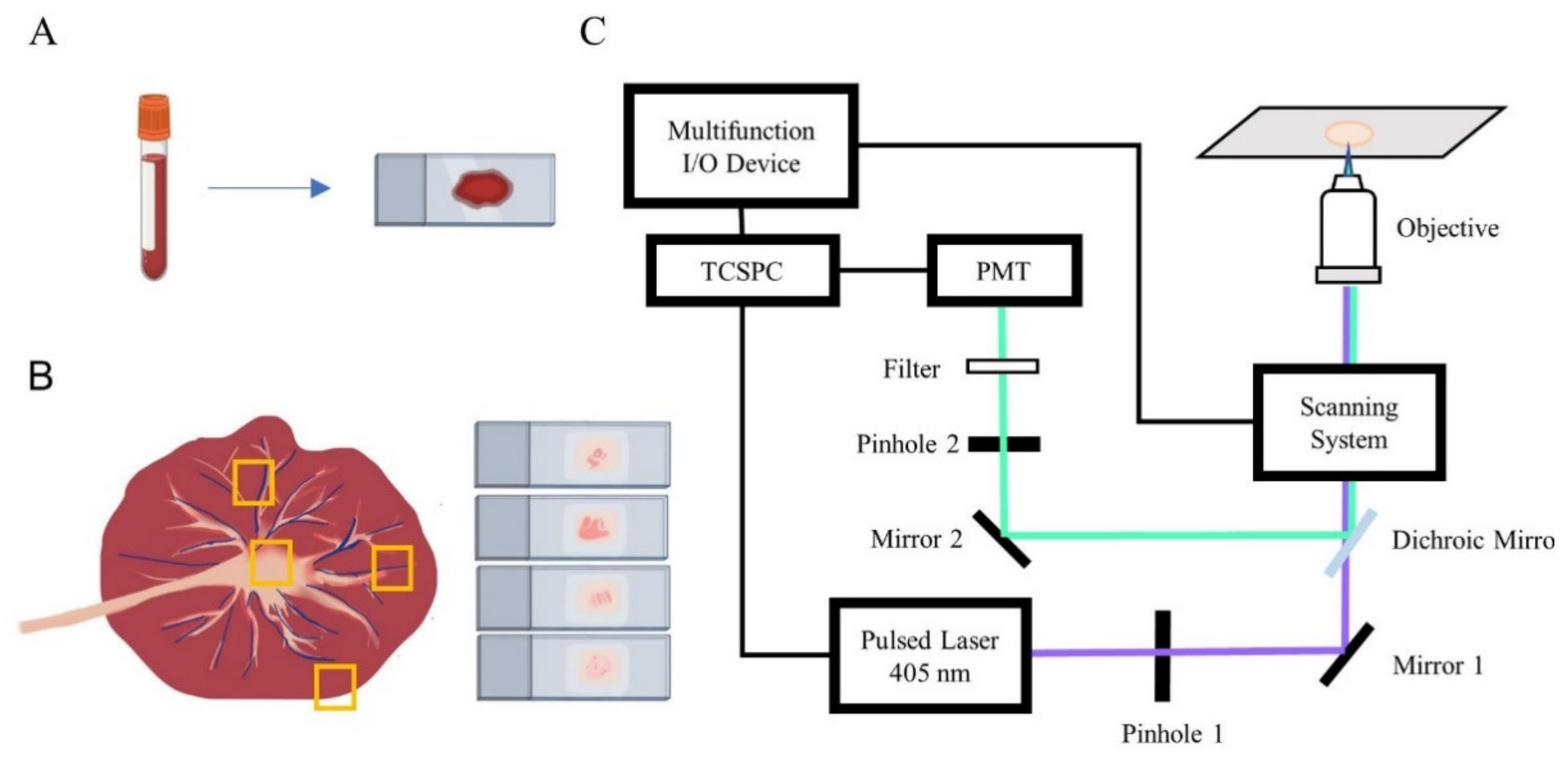
2. Materials and Methods
2.1. Sample Preparation
2.2. FLIM Setup
2.3. Image and Data Processing
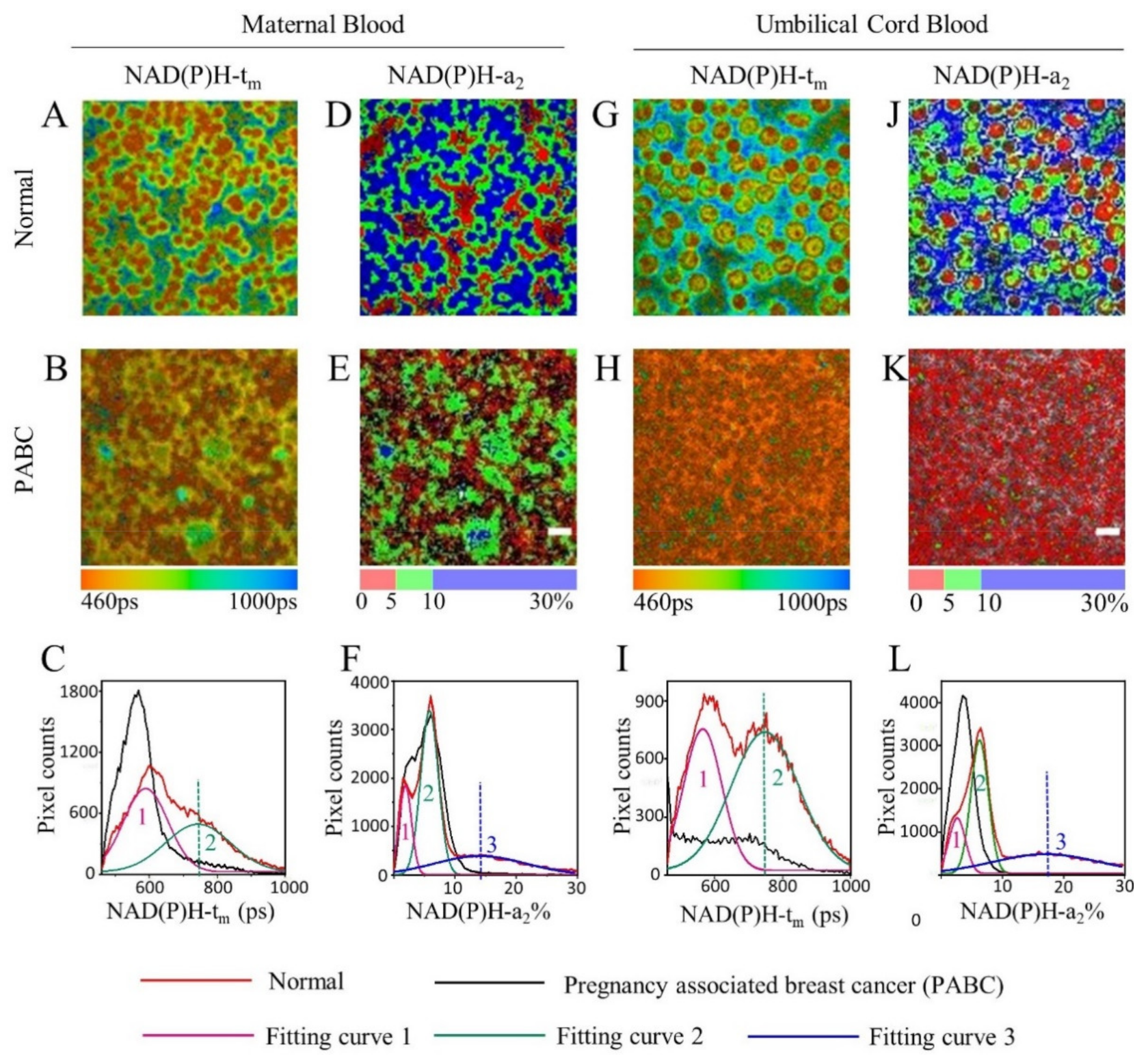
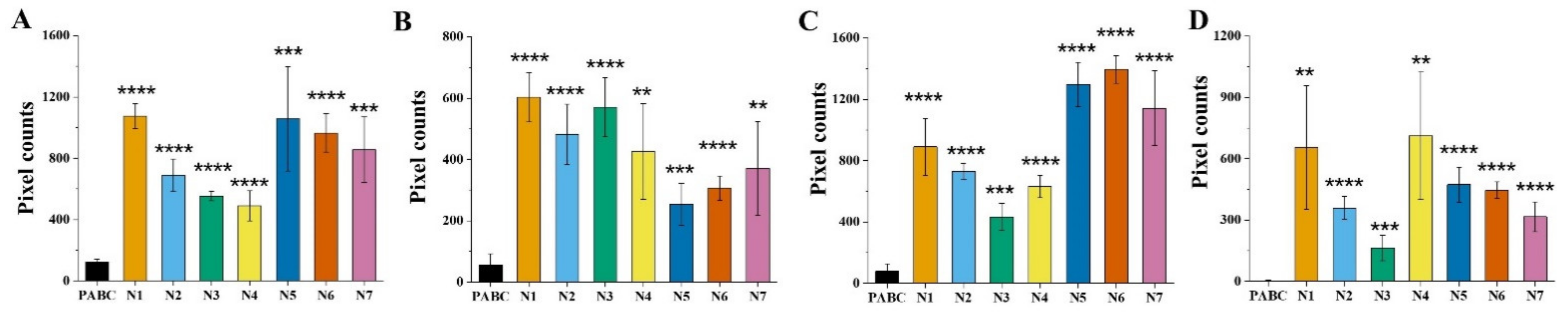
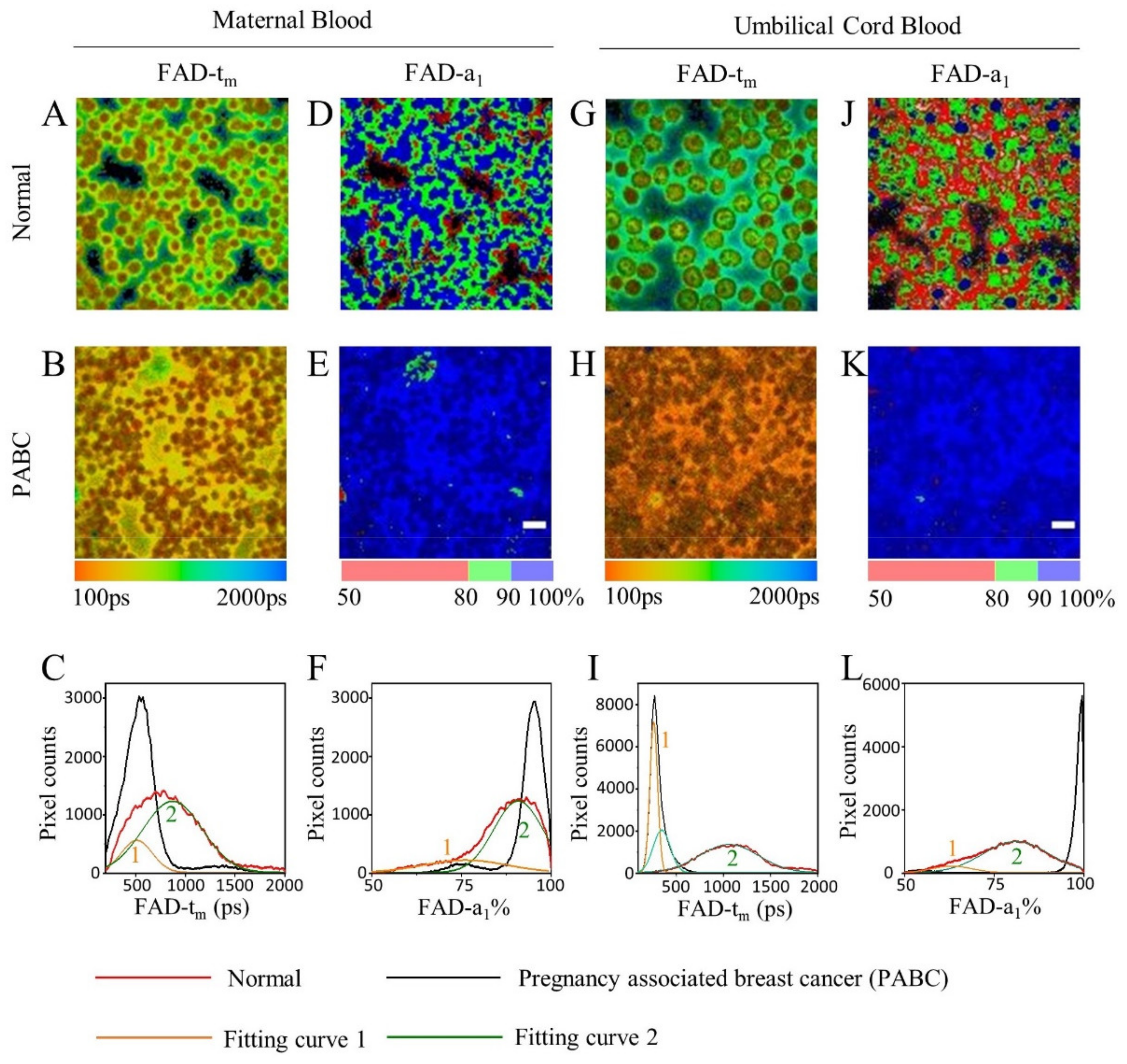
3. Results
3.1. FLIM of NAD(P)H in Blood
3.2. FLIM of FAD in Blood
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA-Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Abenhaim, H.A.; Azoulay, L.; Holcroft, C.A.; Bure, L.A.; Assayag, J.; Benjamin, A. Incidence, risk factors, and obstetrical outcomes of women with breast cancer in pregnancy. Breast J. 2012, 18, 564–568. [Google Scholar] [CrossRef]
- Ahn, B.Y.; Kim, H.H.; Moon, W.K.; Pisano, E.D.; Kim, H.S.; Cha, E.S.; Kim, J.S.; Oh, K.K.; Park, S.H. Pregnancy- and lactation-associated breast cancer: Mammographic and sonographic findings. J. Ultrasound Med. 2003, 22, 491–497. [Google Scholar] [CrossRef]
- Amant, F.; Loibl, S.; Neven, P.; Van Calsteren, K. Breast cancer in pregnancy. Lancet 2012, 379, 570–579. [Google Scholar] [CrossRef]
- Collins, L.C.; Gelber, S.; Marotti, J.D.; White, S.; Ruddy, K.; Brachtel, E.F.; Schapira, L.; Come, S.E.; Borges, V.F.; Schedin, P.; et al. Molecular Phenotype of Breast Cancer According to Time Since Last Pregnancy in a Large Cohort of Young Women. Oncologist 2015, 20, 713–718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsyuba, E.; Romani, M.; Hofer, D.; Auwerx, J. NAD+ homeostasis in health and disease. Nat. Metab. 2020, 2, 9–31. [Google Scholar] [CrossRef] [PubMed]
- Skala, M.C.; Riching, K.M.; Gendron-Fitzpatrick, A.; Eickhoff, J.; Eliceiri, K.W.; White, J.G.; Ramanujam, N. In vivo multiphoton microscopy of NADH and FAD redox states, fluorescence lifetimes, and cellular morphology in precancerous epithelia. Proc. Natl. Acad. Sci. USA 2007, 104, 19494–19499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conklin, M.W.; Provenzano, P.P.; Eliceiri, K.W.; Sullivan, R.; Keely, P.J. Fluorescence lifetime imaging of endogenous fluorophores in histopathology sections reveals differences between normal and tumor epithelium in carcinoma in situ of the breast. Cell Biochem. Biophys. 2009, 53, 145–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.; Zhang, Z.; Wang, X.; Xie, Y.; Fei, Y.; Ma, J.; Wang, J.; Chen, L.; Mi, L.; Wang, Y. Detecting benign uterine tumors by autofluorescence lifetime imaging microscopy through adjacent healthy cervical tissues. J. Innov. Opt. Health Sci. 2019, 12, 1940006. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, Y.; Zhang, Z.; Huang, M.; Fei, F.; Ma, J.; Mi, L. Discriminating different grades of cervical intraepithelial neoplasia based on label-free phasor fluorescence lifetime imaging microscopy. Biomed. Opt. Express 2020, 11, 1977–1990. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, F.; Pan, X.; Yao, L.; Wang, X.; Jing, Y.; Ma, J.; Wang, G.; Mi, L. Rapid diagnosis and intraoperative margin assessment of human lung cancer with fluorescence lifetime imaging microscopy. BBA Clin. 2017, 8, 7–13. [Google Scholar] [CrossRef]
- Liu, L.; Yang, Q.; Zhang, M.; Wu, Z.; Xue, P. Fluorescence lifetime imaging microscopy and its applications in skin cancer diagnosis. J. Innov. Opt. Health Sci. 2019, 12, 1930004. [Google Scholar] [CrossRef] [Green Version]
- Sohn, E. Diagnosis: Frontiers in blood testing. Nature 2017, 549, S16–S18. [Google Scholar] [CrossRef] [PubMed]
- Shirshin, E.; Cherkasova, O.; Tikhonova, T.; Berlovskaya, E.; Priezzhev, A.; Fadeev, V. Native fluorescence spectroscopy of blood plasma of rats with experimental diabetes: Identifying fingerprints of glucose-related metabolic pathways. J. Biomed. Opt. 2015, 20, 051033. [Google Scholar] [CrossRef] [PubMed]
- Kuan, D.-H.; Wu, C.-C.; Su, W.-Y.; Huang, N.-T. A Microfluidic Device for Simultaneous Extraction of Plasma, Red Blood Cells, and On-Chip White Blood Cell Trapping. Sci. Rep. 2018, 8, 15345. [Google Scholar] [CrossRef] [Green Version]
- Madhuri, S.; Vengadesan, N.; Aruna, P.; Koteeswaran, D.; Venkatesan, P.; Ganesan, S. Native fluorescence spectroscopy of blood plasma in the characterization of oral malignancy. Photochem. Photobiol. 2003, 78, 197–204. [Google Scholar] [CrossRef]
- Bhatta, H.; Goldys, M.E. Characterization of yeast strains by fluorescence lifetime imaging microscopy. FEMS Yeast Res. 2008, 8, 81–87. [Google Scholar] [CrossRef] [Green Version]
- Becker, W.; Bergmann, A.; Ibarrola, S.R.; Müller, P.-F.; Braun, L. Metabolic imaging by simultaneous FLIM of NAD(P)H and FAD. In Multiphoton Microscopy in the Biomedical Sciences XIX, Proceedings of the SPIE BiOS SPIE, San Francisco, CA, USA, 2–7 February 2019; SPIE: Bellingham, WA, USA, 2019; p. 108820B. [Google Scholar]
- Guo, K.; Wu, J.; Kong, Y.; Zhou, L.; Li, W.; Fei, Y.; Ma, J.; Mi, L. Label-free and noninvasive method for assessing the metabolic status in type 2 diabetic rats with myocardium diastolic dysfunction. Biomed. Opt. Express 2021, 12, 480–493. [Google Scholar] [CrossRef] [PubMed]
- Tadrous, P.J.; Siegel, J.; French, P.M.W.; Shousha, S.; Lalani, E.-N.; Stamp, G.W.H. Fluorescence lifetime imaging of unstained tissues: Early results in human breast cancer. J. Pathol. 2003, 199, 309–317. [Google Scholar] [CrossRef]
- Kolenc, O.I.; Quinn, K.P. Evaluating Cell Metabolism Through Autofluorescence Imaging of NAD(P)H and FAD. Antioxid. Redox. Signal 2019, 30, 875–889. [Google Scholar] [CrossRef]
- Bird, D.K.; Yan, L.; Vrotsos, K.M.; Eliceiri, K.W.; Vaughan, E.M.; Keely, P.J.; White, J.G.; Ramanujam, N. Metabolic mapping of MCF10A human breast cells via multiphoton fluorescence lifetime imaging of the coenzyme NADH. Cancer Res. 2005, 65, 8766–8773. [Google Scholar] [CrossRef] [Green Version]
- Alhallak, K.; Rebello, L.G.; Muldoon, T.J.; Quinn, K.P.; Rajaram, N. Optical redox ratio identifies metastatic potential-dependent changes in breast cancer cell metabolism. Biomed. Opt. Express 2016, 7, 4364–4374. [Google Scholar] [CrossRef] [Green Version]
- Kalaivani, R.; Masilamani, V.; AlSalhi, M.S.; Devanesan, S.; Ramamurthy, P.; Palled, S.R.; Ganesh, K.M. Cervical cancer detection by time-resolved spectra of blood components. J. Biomed. Opt. 2014, 19, 057011. [Google Scholar] [CrossRef] [Green Version]
- Froehlich, K.; Stensheim, H.; Markert, U.R.; Turowski, G. Breast carcinoma in pregnancy with spheroid-like placental metastases-a case report. APMIS 2018, 126, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Terakata, M.; Fukuwatari, T.; Sano, M.; Nakao, N.; Sasaki, R.; Fukuoka, S.-I.; Shibata, K. Establishment of true niacin deficiency in quinolinic acid phosphoribosyltransferase knockout mice. J. Nutr. 2012, 142, 2148–2153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becherini, P.; Caffa, I.; Piacente, F.; Damonte, P.; Vellone, V.G.; Passalacqua, M.; Benzi, A.; Bonfiglio, T.; Reverberi, D.; Khalifa, A.; et al. SIRT6 enhances oxidative phosphorylation in breast cancer and promotes mammary tumorigenesis in mice. Cancer Metab. 2021, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Culp-Hill, R.; Zheng, C.; Reisz, A.J.; Smith, K.; Rachubinski, A.; Nemkov, T.; Butcher, E.; Granrath, R.; Hansen, C.K.; Espinosa, M.J.; et al. Red blood cell metabolism in Down syndrome: Hints on metabolic derangements in aging. Blood Adv. 2017, 1, 2776–2780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemkov, T.; Reisz, A.J.; Xia, Y.; Zimring, C.J.; D’Alessandro, A. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev. Proteomic 2018, 15, 855–864. [Google Scholar] [CrossRef]
- Micheli, V.; Sestini, S. Determining NAD synthesis in erythrocytes. Method Enzym. 1997, 280, 211–221. [Google Scholar]
- Brunnbauer, P.; Leder, A.; Kamali, C.; Kamali, K.; Keshi, E.; Splith, K.; Wabitsch, S.; Haber, P.; Atanasov, G.; Feldbrügge, L.; et al. The nanomolar sensing of nicotinamide adenine dinucleotide in human plasma using a cycling assay in albumin modified simulated body fluids. Sci. Rep. 2018, 8, 16110. [Google Scholar] [CrossRef]
- O’Reilly, T.; Niven, D.F. Levels of nicotinamide adenine dinucleotide in extracellular body fluids of pigs may be growth–limiting for actinobacillus pleuropneumoniae and haemophilus parasuis. Can. J. Vet. Res. 2003, 67, 229–231. [Google Scholar]
- Phipps, J.E.; Gorpas, D.; Unger, J.; Darrow, M.; Bold, R.J.; Marcu, L. Automated detection of breast cancer in resected specimens with fluorescence lifetime imaging. Phys. Med. Biol. 2017, 63, 015003. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Tan, M.; Cai, Q. The Warburg effect in tumor progression: Mitochondrial oxidative metabolism as an anti-metastasis mechanism. Cancer Lett. 2015, 356, 156–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, K.C.; Hay, N. The pentose phosphate pathway and cancer. Trends Biochem. Sci. 2014, 39, 347–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, L.; Suo, C.; Li, S.; Zhang, H.; Gao, P. Metabolic reprogramming for cancer cells and their microenvironment: Beyond the Warburg Effect. BBA-Rev. Cancer 2018, 1870, 51–66. [Google Scholar] [CrossRef]
- Allison, K.; Coomber, B.; Bridle, B. Metabolic reprogramming in the tumour microenvironment: A hallmark shared by cancer cells and T lymphocytes. Immunology 2017, 152, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Mehla, K.; Singh, P. Metabolic Regulation of Macrophage Polarization in Cancer. Trends Cancer 2019, 5(12), 822–834. [Google Scholar] [CrossRef]
- Kim, J.; Bae, J. Metabolic regulation of macrophages in tumor microenvironment. Curr. Opin. Hematol. 2018, 1, 52–59. [Google Scholar] [CrossRef]
- Giancaspero, T.A.; Busco, G.; Panebianco, C.; Carmone, C.; Miccolis, A.; Liuzzi, G.M.; Colella, M.; Barile, M. FAD synthesis and degradation in the nucleus create a local flavin cofactor pool. J. Biol. Chem. 2013, 288, 29069–29080. [Google Scholar] [CrossRef] [Green Version]
- Shaw, N.-S. Riboflavin status in late pregnancy, postpartum and cord blood. Nutr. Res. 1993, 13, 147–155. [Google Scholar] [CrossRef]
- Trindade, E.P.C.; Barreiros, C.R.; Kurokawa, C.; Bossolan, G. Fructose in fetal cord blood and its relationship with maternal and 48-hour-newborn blood concentrations. Early Hum. Dev. 2011, 87, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Elshibly, E.M.; Atabani, G.S.; Osman, N.M.; Hassan, D.A.; Saeed, B.O.; Omer, M.I. Cord and maternal glycosylated haemoglobin levels: A study in Sudanese pregnant diabetic women. Ann. Trop. Paediatr. 1990, 10, 373–376. [Google Scholar] [CrossRef]
- Malik, B.H.; Lee, J.; Cheng, S.; Cuenca, R.; Jabbour, J.M.; Cheng, Y.-S.L.; Wright, J.M.; Ahmed, B.; Maitland, K.C.; Jo, J.A. Objective Detection of Oral Carcinoma with Multispectral Fluorescence Lifetime Imaging In Vivo. Photochem. Photobiol. 2016, 92, 694–701. [Google Scholar] [CrossRef] [Green Version]
- Shen, B.; Yan, J.; Wang, S.; Zhou, F.; Zhao, Y.; Hu, R.; Qu, J.; Liu, L. Label-free whole-colony imaging and metabolic analysis of metastatic pancreatic cancer by an autoregulating flexible optical system. Theranostics 2020, 10, 1849–1860. [Google Scholar] [CrossRef]
- Cantor, J.R.; Sabatini, D.M. Cancer cell metabolism: One hallmark, many faces. Cancer Discov. 2012, 2, 881–898. [Google Scholar] [CrossRef] [Green Version]
- Zu, X.L.; Guppy, M. Cancer metabolism: Facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004, 313, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Zawislak, A.; Gannon, C.; Millar, D.; Loughrey, M.B. Maternal Gastric Adenocarcinoma with Placental Metastases: What Is the Fetal Risk? Pediatr. Dev. Pathol. 2012, 15, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Al-Adnani, M.; Kiho, L.; Scheimberg, I. Maternal pancreatic carcinoma metastatic to the placenta: A case report and literature review. Pediatr. Dev. Pathol. 2007, 10, 61–65. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Kong, Y.; Wu, J.; Li, X.; Fei, Y.; Ma, J.; Wang, Y.; Mi, L. Metabolic Changes in Maternal and Cord Blood in One Case of Pregnancy-Associated Breast Cancer Seen by Fluorescence Lifetime Imaging Microscopy. Diagnostics 2021, 11, 1494. https://doi.org/10.3390/diagnostics11081494
Zhou L, Kong Y, Wu J, Li X, Fei Y, Ma J, Wang Y, Mi L. Metabolic Changes in Maternal and Cord Blood in One Case of Pregnancy-Associated Breast Cancer Seen by Fluorescence Lifetime Imaging Microscopy. Diagnostics. 2021; 11(8):1494. https://doi.org/10.3390/diagnostics11081494
Chicago/Turabian StyleZhou, Li, Yawei Kong, Junxin Wu, Xingzhi Li, Yiyan Fei, Jiong Ma, Yulan Wang, and Lan Mi. 2021. "Metabolic Changes in Maternal and Cord Blood in One Case of Pregnancy-Associated Breast Cancer Seen by Fluorescence Lifetime Imaging Microscopy" Diagnostics 11, no. 8: 1494. https://doi.org/10.3390/diagnostics11081494





