Acute Kidney Injury Predictors and Outcomes after Cardiac Surgery in Children with Congenital Heart Disease: An Observational Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Data Collection—Definition of Variables
2.3. Intraoperative Procedure
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, A.; Chakraborty, R.; Sharma, K.; Sethi, S.K.; Raina, R. Development of acute kidney injury following pediatric cardiac surgery. Kidney Res. Clin. Pr. 2020, 39, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Gist, K.M.; Kwiatkowski, D.M.; Cooper, D.S. Acute kidney injury in congenital heart disease. Curr. Opin. Cardiol. 2018, 33, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P. Acute kidney injury after pediatric cardiac surgery. Ann. Card. Anaesth. 2016, 19, 306–313. [Google Scholar] [CrossRef]
- Yuan, S.-M. Acute kidney injury after pediatric cardiac surgery. Pediatr. Neonatol. 2019, 60, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sethi, S.K.; Sharma, R.; Gupta, A.; Tibrewal, A.; Akole, R.; Dhir, R.; Soni, K.; Bansal, S.B.; Jha, P.K.; Bhan, A.; et al. Long-Term Renal Outcomes in Children with Acute Kidney Injury Post Cardiac Surgery. Kidney Int. Rep. 2021, 6, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Van den Eynde, J.; Salaets, T.; Louw, J.J.; Herman, J.; Breysem, L.; Vlasselaers, D.; Desmet, L.; Meyns, B.; Budts, W.; Gewillig, M.; et al. Persistent Markers of Kidney Injury in Children Who Developed Acute Kidney Injury After Pediatric Cardiac Surgery: A Prospective Cohort Study. J. Am. Heart Association. 2022, 11, e024266. [Google Scholar] [CrossRef]
- Madsen, N.L.; Goldstein, S.L.; Frøslev, T.; Christiansen, C.F.; Olsen, M. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int. 2017, 92, 751–756. [Google Scholar] [CrossRef] [PubMed]
- Cooper, D.S.; Claes, D.; Goldstein, S.L.; Bennett, M.R.; Ma, Q.; Devarajan, P.; Krawczeski, C.D. Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury (FRAIL-AKI). Clin. J. Am. Soc. Nephrol. 2016, 11, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Schoenmaker, N.J.; Weeda, J.A.; van der Palen, R.L.; Hazekamp, M.G.; Bunker-Wiersma, H.E. Acute kidney injury after the arterial switch operation: Incidence, risk factors, and outcomes. Cardiol. Young 2021, 32, 794–799. [Google Scholar] [CrossRef]
- Jang, W.S.; Kim, W.-H.; Choi, K.; Nam, J.; Jung, J.C.; Kwon, B.S.; Kim, G.B.; Kang, H.G.; Lee, J.R.; Kim, Y.J. Incidence, risk factors and clinical outcomes for acute kidney injury after aortic arch repair in paediatric patients. Eur. J. Cardio. Thoracic Surg. 2014, 45, e208–e214. [Google Scholar] [CrossRef]
- Harmer, M.J.; Southgate, G.; Smith, V.; Bharucha, T.; Viola, N.; Griksaitis, M.J. Acute kidney injury and short-term renal support in the post-operative management of neonates following repair of transposition of the great arteries. Prog. Pediatr. Cardiol. 2019, 52, 26–32. [Google Scholar] [CrossRef]
- Aydin, S.I.; Seiden, H.S.; Blaufox, A.D.; Parnell, V.A.; Choudhury, T.; Punnoose, A.; Schneider, J. Acute Kidney Injury After Surgery for Congenital Heart Disease. Ann. Thorac. Surg. 2012, 94, 1589–1595. [Google Scholar] [CrossRef]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron Exp. Nephrol. 2012, 120, c179–c184. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-K.; Hur, M.; Kim, E.; Kim, W.H.; Park, J.B.; Kim, Y.; Yang, J.-H.; Jun, T.-G.; Kim, C.S. Risk Factors for Acute Kidney Injury after Congenital Cardiac Surgery in Infants and Children: A Retrospective Observational Study. PLoS ONE 2016, 11, e0166328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jenkins, K.J.; Gauvreau, K.; Newburger, J.W.; Spray, T.L.; Moller, J.H.; Iezzoni, L.I. Consensus-based method for risk adjustment for surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 2002, 123, 110–118. [Google Scholar] [CrossRef] [Green Version]
- Selewski, D.T.; Cornell, T.T.; Heung, M.; Troost, J.P.; Ehrmann, B.J.; Lombel, R.M.; Blatt, N.B.; Luckritz, K.; Hieber, S.; Gajarski, R.; et al. Validation of the KDIGO acute kidney injury criteria in a pediatric critical care population. Intensiv. Care Med. 2014, 40, 1481–1488. [Google Scholar] [CrossRef]
- Micheletti, A. Congenital Heart Disease Classification, Epidemiology, Diagnosis, Treatment, and Outcome. In Congenital Heart Disease: The Nursing Care Handbook; Flocco, S.F., Lillo, A., Dellafiore, F., Goossens, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 1–67. [Google Scholar]
- Pedersen, K.R.; Povlsen, J.V.; Christensen, S.; Pedersen, J.; Hjortholm, K.; Larsen, S.H.; Hjortdal, V.E. Risk factors for acute renal failure requiring dialysis after surgery for congenital heart disease in children. Acta Anaesthesiol. Scand. 2007, 51, 1344–1349. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.L.; Carmona, F.; Thiagarajan, R.R.; Westgate, L.; Ferguson, M.A.; del Nido, P.J.; Rajagopal, S.K. Mild postoperative acute kidney injury and outcomes after surgery for congenital heart disease. J. Thorac. Cardiovasc. Surg. 2013, 146, 146–152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Krawczeski, C.D.; Zappitelli, M.; Devarajan, P.; Thiessen-Philbrook, H.; Coca, S.G.; Kim, R.W.; Parikh, C.R. Incidence, risk factors, and outcomes of acute kidney injury after pediatric cardiac surgery: A prospective multicenter study. Crit. Care Med. 2011, 39, 1493–1499. [Google Scholar] [CrossRef] [Green Version]
- Blinder, J.J.; Goldstein, S.L.; Lee, V.-V.; Baycroft, A.; Fraser, C.D.; Nelson, D.; Jefferies, J.L. Congenital heart surgery in infants: Effects of acute kidney injury on outcomes. J. Thorac. Cardiovasc. Surg. 2012, 143, 368–374. [Google Scholar] [CrossRef]
- AlAbbas, A.; Campbell, A.; Skippen, P.; Human, D.; Matsell, D.; Mammen, C. Epidemiology of cardiac surgery-associated acute kidney injury in neonates: A retrospective study. Pediatr. Nephrol. 2013, 28, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, S.-J.; Kim, H.J.; Son, J.S.; Lee, R.; Yoon, T.G. Acute Kidney Injury Following Cardiopulmonary Bypass in Children—Risk Factors and Outcomes. Circ. J. 2017, 81, 1522–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selewski, D.T.; Charlton, J.R.; Jetton, J.G.; Guillet, R.; Mhanna, M.J.; Askenazi, D.J.; Kent, A.L. Neonatal Acute Kidney Injury. Pediatrics 2015, 136, e463–e473. [Google Scholar] [CrossRef] [Green Version]
- Jetton, J.; Askenazi, D.J. Update on acute kidney injury in the neonate. Curr. Opin. Pediatr. 2012, 24, 191–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, P.T.; Devarajan, P.; Levey, A.S.; Eckardt, K.U.; Bonventre, J.V.; Lombardi, R.; Herget-Rosenthal, S.; Levin, A. A Framework and Key Research Questions in AKI Diagnosis and Staging in Different Environments. Clin. J. Am. Soc. Nephrol. 2008, 3, 864–868. [Google Scholar] [CrossRef] [Green Version]
- Brezis, M.; Rosen, S. Hypoxia of the Renal Medulla—Its Implications for Disease. N. Engl. J. Med. 1995, 332, 647–655. [Google Scholar] [CrossRef]
- Tóth-Heyn, P.; Drukker, A.; Guignard, J.-P. The stressed neonatal kidney: From pathophysiology to clinical management of neonatal vasomotor nephropathy. Pediatr. Nephrol. 2000, 14, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S.K.; Goyal, D.; Yadav, D.K.; Shukla, U.; Kajala, P.L.; Gupta, V.K.; Grover, V.; Kapoor, P.; Juneja, A. Predictors of acute kidney injury post-cardiopulmonary bypass in children. Clin. Exp. Nephrol. 2011, 15, 529–534. [Google Scholar] [CrossRef]
- Rosner, M.H.; Okusa, M.D. Acute Kidney Injury Associated with Cardiac Surgery. Clin. J. Am. Soc. Nephrol. 2006, 1, 19–32. [Google Scholar] [CrossRef] [Green Version]
- Ricci, Z.; Luciano, R.; Favia, I.; Garisto, C.; Muraca, M.; Morelli, S.; Di Chiara, L.; Cogo, P.; Picardo, S. High-dose fenoldopam reduces postoperative neutrophil gelatinase-associated lipocaline and cystatin C levels in pediatric cardiac surgery. Crit. Care 2011, 15, R160. [Google Scholar] [CrossRef]
- Koyner, J.L.; Murray, P.T. Mechanical Ventilation and Lung–Kidney Interactions. Clin. J. Am. Soc. Nephrol. 2008, 3, 562–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pannu, N.; Mehta, R.L. Effect of mechanical ventilation on the kidney. Best Pr. Res. Clin. Anaesthesiol. 2004, 18, 189–203. [Google Scholar] [CrossRef]
- van den Akker, J.P.C.; Egal, M.; Groeneveld, A.B.J. Invasive mechanical ventilation as a risk factor for acute kidney injury in the critically ill: A systematic review and meta-analysis. Crit. Care 2013, 17, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.; Yao, Y.; Han, L.; Xiao, Y. Renal function and injury in infants and young children with congenital heart disease. Pediatr. Nephrol. 2013, 28, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Wilson, R.; Ross, O.; Griksaitis, M.J. Tetralogy of Fallot. BJA Educ. 2019, 19, 362–369. [Google Scholar] [CrossRef]
- Wang, X.; Chen, H.; Liu, D.; Zhang, Q.; Su, L. The correlation between CVP-derived parameters and the prognosis of critically ill patients. J. Crit. Care 2017, 40, 257–264. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Zhou, Y.; Wang, P.; Qi, E.-Y.; Gu, W.-J. Elevated central venous pressure is associated with increased mortality and acute kidney injury in critically ill patients: A meta-analysis. Crit. Care 2020, 24, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Wang, X.; Honore, P.M.; Spapen, H.D.; Liu, D. Renal failure in critically ill patients, beware of applying (central venous) pressure on the kidney. Ann. Intensiv. Care 2018, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Chaturvedi, R.R.; Shore, D.F.; Lincoln, C.; Mumby, S.; Kemp, M.; Brierly, J.; Petros, A.; Gutteridge, J.M.G.; Hooper, J.; Redington, A.N. Acute right ventricular restrictive physiology after repair of tetralogy of Fallot: Association with myocardial injury and oxidative stress. Circulation 1999, 100, 1540–1547. [Google Scholar] [CrossRef] [Green Version]
- Damman, K.; van Deursen, V.M.; Navis, G.; Voors, A.A.; van Veldhuisen, D.J.; Hillege, H.L. Increased central venous pressure is associated with impaired renal function and mortality in a broad spectrum of patients with cardiovascular disease. J. Am. Coll. Cardiol. 2009, 53, 582–588. [Google Scholar] [CrossRef]
- Amoozgar, H.; Basiratnia, M.; Ghasemi, F. Renal Function in Children with Cyanotic Congenital Heart Disease: Pre- and Post-Cardiac Surgery Evaluation. Iran. J. Pediatr. 2014, 24, 81–86. [Google Scholar]
- Mah, K.E.; Hao, S.; Sutherland, S.M.; Kwiatkowski, D.M.; Axelrod, D.M.; Almond, C.S.; Krawczeski, C.D.; Shin, A.Y. Fluid overload independent of acute kidney injury predicts poor outcomes in neonates following congenital heart surgery. Pediatr. Nephrol. 2018, 33, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Lex, D.J.; Tóth, R.; Czobor, N.R.; Alexander, S.I.; Breuer, T.; Sápi, E.; Szatmári, A.; Székely, E.; Gál, J.; Székely, A. Fluid Overload Is Associated with Higher Mortality and Morbidity in Pediatric Patients Undergoing Cardiac Surgery*. Pediatr. Crit. Care Med. 2016, 17, 307–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilder, N.S.; Yu, S.; Donohue, J.E.; Goldberg, C.S.; Blatt, N.B. Fluid Overload Is Associated with Late Poor Outcomes in Neonates Following Cardiac Surgery*. Pediatr. Crit. Care Med. 2016, 17, 420–427. [Google Scholar] [CrossRef] [Green Version]
- Claure-Del Granado, R.; Mehta, R.L. Fluid overload in the ICU: Evaluation and management. BMC Nephrol. 2016, 17, 109. [Google Scholar] [CrossRef] [Green Version]
- Dean, M. Opioids in renal failure and dialysis patients. J. Pain Symptom Manag. 2004, 28, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.M.; Ritz, R.; Haberthür, C.; Haefeli, W.E.; Scollo-Lavizzari, G.; Ha, H.R.; Hunkeler, W.; Sleight, A.J. Prolonged sedation due to accumulation of conjugated metabolites of midazolam. Lancet 1995, 346, 145–147. [Google Scholar] [CrossRef]


| Characteristic | Total | Acute Kidney Injury | p-Value | |
|---|---|---|---|---|
| No | Yes | |||
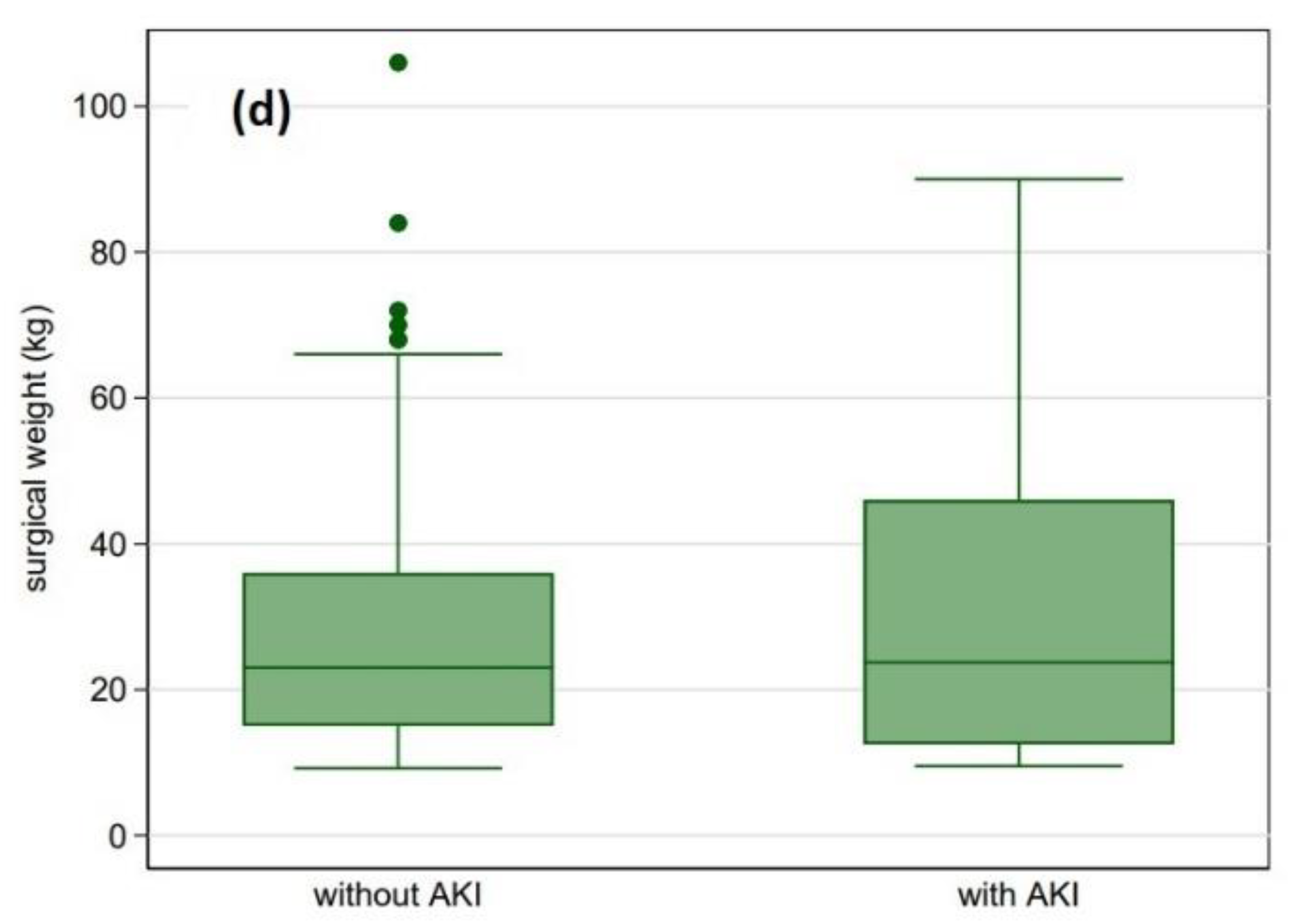
| Surgical weight (kg) | 9 (4.2–23) | 10.6 (6–25) | 3.6 (3–8.9) | <0.001 |
| Surgical age (years) | 1.3 (0.3–6.4) | 2 (0.5–7.3) | 0.099 (0.02–1.41) | <0.001 |
| CPB time (min) (n = 272) | 89 (53–132) | 79 (51–116) | 146 (106–189) | <0.001 |
| ACC time (min) (n = 294) | 46 (23–85) | 44 (23–77) | 72 (32–104) | <0.001 |
| Gender | 0.53 | |||
| Female | 157 (43) | 129 (44) | 28 (40) | |
| Male | 205 (57) | 163 (56) | 42 (60) | |
| Surgical age (≤median) | 179 (49) | 128 (44) | 51 (73) | <0.001 |
| Surgical weight (≤median) | 184 (51) | 130 (45) | 54 (77) | <0.001 |
| Previous cardiac surgery | 54 (15) | 44 (15) | 10 (14) | 0.87 |
| Classification a | <0.001 | |||
| Group 1 | 203 (56) | 189 (65) | 14 (20) | |
| Group 2 | 67 (19) | 45 (15) | 22 (31) | |
| Group 3 | 45 (12) | 34 (12) | 11 (16) | |
| Group 4 | 13 (4) | 5 (2) | 8 (11) | |
| Group 5 | 18 (5) | 9 (3) | 9 (13) | |
| Group 6 | 10 (3) | 4 (1) | 6 (9) | |
| Common Group b (4,5,6) | 41 (11) | 18 (6) | 23 (33) | |
| Preoperative Mechanical Ventilation | 61 (17) | 30 (10) | 31 (44) | <0.001 |
| CT scan ≤ 7 days before surgery | 35 (10) | 18 (6) | 17 (24) | <0.001 |
| Preoperative BAS | 8 (2) | 4 (1) | 4 (6) | 0.048 |
| RACHS-1 | <0.001 | |||
| 1 | 116 (32) | 106 (36) | 10 (14) | |
| 2 | 130 (36) | 110 (38) | 20 (29) | |
| 3 | 89 (25) | 67 (23) | 22 (31) | |
| 4 | 25 (7) | 9 (3) | 16 (23) | |
| 5 | 0 (0) | 0 (0) | 0 (0) | |
| 6 | 2 (0.6) | 0 (0) | 2 (3) | |
| RACHS-1 | <0.001 | |||
| 1–2 | 246 (68) | 216 (74) | 30 (43) | |
| 3–6 | 116 (32) | 76 (26) | 40 (67) | |
| CPB | 272 (75) | 223 (76) | 49 (70) | 0.27 |
| ACC | 294 (81) | 237 (81) | 57 (81) | 0.96 |
| Diagnostic Category | n (%) |
|---|---|
| CHD with shunt between systemic and pulmonary circulation | 203 (57) |
| ASD | 94 (26.4) |
| VSD | 69 (19.4) |
| AVSD | 29 (8.1) |
| PDA | 10 (2.8) |
| APW | 1 (0.3) |
| Left heart CHD | 67 (18.8) |
| Cor triatriatum | 1 (0.3) |
| MV stenosis | 1 (0.3) |
| Subaortic stenosis | 13 (3.7) |
| Aortic stenosis | 3 (0.8) |
| Supravalvar aortic stenosis | 3 (0.8) |
| CoA/Hypoplastic aortic arch | 43 (12.1) |
| IAA | 3 (0.8) |
| Right heart CHD | 45 (12.6) |
| Ebstein’s anomaly | 1 (0.3) |
| PS | 3 (0.8) |
| PA/IVS | 3 (0.8) |
| TOF | 33 (9.3) |
| PA/VSD | 5 (1.4) |
| CHD with anomalous origin of great arteries | 13 (3.7) |
| TGA/IVS | 7 (2) |
| TGA/VSD | 3 (0.8) |
| DORV | 2 (0.6) |
| TA | 1 (0.3) |
| Univentricular lesions | 18 (5.1) |
| HLHS | 6 (1.7) |
| DORV/PS | 5 (1.4) |
| Tricuspid Atresia | 4 (1.1) |
| TGA/VSD/PS | 1 (0.3) |
| DORV/PA | 1 (0.3) |
| PA/IVS | 1 (0.3) |
| Miscellaneous CHD | 10 (2.8) |
| Scimitar syndrome | 1 (0.3) |
| TAPVD | 5 (1.4) |
| ALCAPA | 4 (1.1) |
| Characteristic | Acute Kidney Injury | p-Value | ||
|---|---|---|---|---|
| Odds Ratio (95% CI) | ||||
| Surgical weight (kg) | 0.96 (0.94–0.98) | 0.002 | ||
| Surgical age (years) | 0.87 (0.8–0.94) | 0.001 | ||
| CPB time (minutes) | 1.01 (1.005–1.012) | <0.001 | ||
| ACC time (minutes) | 1.01 (1.003–1.015) | 0.002 | ||
| n (%) with AKI | p | p | ||
| Gender | 0.53 | |||
| Female | 28 (18) | 1 | ||
| Male | 42 (21) | 1.19 (0.7–2.02) | ||
| Previous cardiac surgery | 10 (19) | 0.94 (0.45–1.97) | 0.87 | |
| Classification a | <0.001 | |||
| Group 1 | 14 (7) | 1 | ||
| Group 2 | 22 (33) | 6.6 (3.13–13.9) | <0.001 | |
| Group 3 | 11 (24) | 4.37 (1.83–10.43) | 0.001 | |
| Group 4 | 8 (62) | 21.6 (6.24–74.8) | <0.001 | |
| Group 5 | 9 (50) | 13.5 (4.62–39.42) | <0.001 | |
| Group 6 | 6 (60) | 20.25 (5.11–80.23) | <0.001 | |
| Common Group b (4,5,6) | 23 (56) | 17.25 (7.58–39.23) | <0.001 | |
| Preoperative Mechanical Ventilation | 31 (51) | 6.94 (3.79–12.7) | <0.001 | |
| CT scan ≤ 7 days before surgery | 17 (49) | 4.88 (2.36–10.08) | <0.001 | |
| Preoperative BAS | 4 (50) | 4.36 (1.06–17.9) | 0.041 | |
| RACHS-1 | <0.001 | |||
| 1 | 10 (9) | 1 | ||
| 2 | 20 (15) | 1.93 (0.86–4.31) | 0.11 | |
| 3 | 22 (25) | 3.48 (1.55–7.81) | 0.002 | |
| 4 | 16 (64) | 18.84 (6.64–53.46) | <0.001 | |
| 5 | 0 (0) | – | – | |
| 6 | 2 (100) | – | – | |
| RACHS-1 | <0.001 | |||
| 1–2 | 30 (12) | 1 | ||
| 3–6 | 40 (35) | 3.79 (2.21–6.51) | ||
| CPB | 49 (18) | 0.72 (0.4–1.29) | 0.27 | |
| ACC | 57 (19) | 1.02 (0.52–1.99) | 0.96 | |
| Characteristic | Pseudo R2 | Acute Kidney Injury | p-Value | |
|---|---|---|---|---|
| Odds Ratio (95% CI) | ||||
| MODEL A a (n = 356) | 0.24 | |||
| Surgical age (years) | 0.91 (0.84–0.99) | 0.044 | ||
| CPB time (minutes) | 1.01 (1.004–1.01) | <0.001 | ||
| Classification b | 0.003 | |||
| Group 1 | 1 | |||
| Group 2 | 3.84 (1.66–8.87) | 0.002 | ||
| Group 3 | 3.05 (1.24–7.48) | 0.015 | ||
| Common Group c (4,5,6) | 5.15 (1.91–13.85) | 0.001 | ||
| Preoperative Mechanical Ventilation | 3.38 (1.48–7.7) | 0.004 | ||
| MODEL B a (n = 356) | 0.25 | |||
| Surgical Weight (kg) | 0.004 | |||
| >median | 1 | |||
| ≤median | 2.97 (1.41–6.27) | |||
| CPB time (minutes) | 1.01 (1.004–1.01) | <0.001 | ||
| Classification b | 0.003 | |||
| Group 1 | 1 | |||
| Group 2 | 3.91 (1.68–9.1) | 0.002 | ||
| Group 3 | 2.85 (1.16–7.03) | 0.023 | ||
| Common Group c (4,5,6) | 5.1 (1.88–13.83) | 0.001 | ||
| Preoperative Mechanical Ventilation | 2.8 (1.21–6.5) | 0.017 | ||
| Characteristic | Total | Acute Kidney Injury | p-Value | |
|---|---|---|---|---|
| No | Yes | |||
| Median (IQR) | ||||
| DMV, post operative(days) | 2 (1–4) | 2 (1–3) | 6 (2–12) | <0.001 |
| DMV, total (days) | 2 (1–5) | 2 (1–3) | 8 (3–18) | <0.001 |
| ICU length of stay, postoperative (days) | 4 (2–8) | 3 (2–7) | 9.5 (5–20) | <0.001 |
| ICU length of stay, total (days) | 4 (2–11) | 3 (2–8) | 13.5 (6–26) | <0.001 |
| Hospital length of stay (days) | 12 (9–22) | 10 (8–19) | 23.5 (14–44) | <0.001 |
| Univariate Cox Regression (n = 362) | Multivariate Cox Regression (n = 356) | |||
|---|---|---|---|---|
| Characteristic | Hazard Ratio (95% CI) | p-Value | Hazard Ratio (95% CI) | p-Value |
| Acute Kidney Injury | 13.77 (3–63.27) | 0.001 | 11.08 (2.45–50.01) | 0.002 |
| RACHS-1 | 0.019 | |||
| 1–2 | 1 | 1 | ||
| 3–6 | 6.33 (1.36–29.42) | 4.64 (1.04–20.9) | 0.046 | |
| Classification a | 0.019 | |||
| Group 1 | 1 | |||
| Group 2 | 5.8 (0.6–56.55) | |||
| Group 3 | 11.85 (1.3–107.66) | |||
| Common Group b (4,5,6) | 12.3 (1.43–105.46) | |||
| Preoperative Mechanical Ventilation | 0.053 | |||
| No | 1 | |||
| Yes | 2.95 (0.99–8.82) | |||
| CPB time (min) | 1.01 (1.003–1.02) | 0.002 | ||
| Weight (kg) | 0.91 (0.79–1.04) | 0.16 |
| Patient No | SW | SA | Diagnosis | CPB | ACC | Classification | RACHS-1 | Preop IPPV | Cardiac CT Scan | DMV Postop | DMV Total | ICU Postop | ICU Total | Hospital LOS |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 a | 2.5 | 0.08 | CoA | N/A | 24 | 2 | 2 | No | No | 4 | 4 | 7 | 7 | 28 |
| 2 b | 4.2 | 0.18 | CoA | N/A | 16 | 2 | 2 | No | No | 3 | 3 | 10 | 10 | 41 |
| 3 | 2.8 | 0.01 | CoA | N/A | 16 | 2 | 2 | Yes | Yes | 3 | 4 | 5 | 9 | 12 |
| 4 c | 1.3 | 0.01 | CoA | N/A | 20 | 2 | 2 | Yes | No | 5 | 6 | 6 | 8 | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kourelis, G.; Kanakis, M.; Samanidis, G.; Tzannis, K.; Bobos, D.; Kousi, T.; Apostolopoulou, S.; Kakava, F.; Kyriakoulis, K.; Bounta, S.; et al. Acute Kidney Injury Predictors and Outcomes after Cardiac Surgery in Children with Congenital Heart Disease: An Observational Cohort Study. Diagnostics 2022, 12, 2397. https://doi.org/10.3390/diagnostics12102397
Kourelis G, Kanakis M, Samanidis G, Tzannis K, Bobos D, Kousi T, Apostolopoulou S, Kakava F, Kyriakoulis K, Bounta S, et al. Acute Kidney Injury Predictors and Outcomes after Cardiac Surgery in Children with Congenital Heart Disease: An Observational Cohort Study. Diagnostics. 2022; 12(10):2397. https://doi.org/10.3390/diagnostics12102397
Chicago/Turabian StyleKourelis, Georgios, Meletios Kanakis, George Samanidis, Kimon Tzannis, Dimitrios Bobos, Theofili Kousi, Sotiria Apostolopoulou, Felicia Kakava, Konstantinos Kyriakoulis, Stavroula Bounta, and et al. 2022. "Acute Kidney Injury Predictors and Outcomes after Cardiac Surgery in Children with Congenital Heart Disease: An Observational Cohort Study" Diagnostics 12, no. 10: 2397. https://doi.org/10.3390/diagnostics12102397





