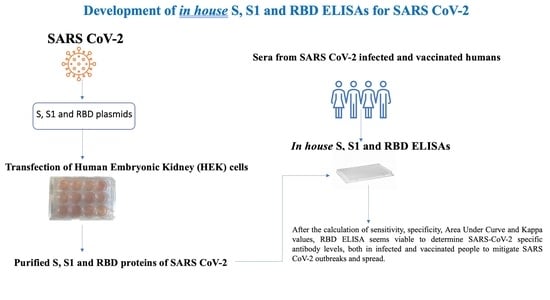
Development of in House ELISAs to Detect Antibodies to SARS-CoV-2 in Infected and Vaccinated Humans by Using Recombinant S, S1 and RBD Proteins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population and Sampling
2.2. Production of Recombinant Spike (S), S1 and Receptor Binding Domain (RBD) Proteins of SARS-CoV-2
2.3. Validation of S, S1 and RBD ELISAs with Human Sera Analyzed by Commercial Test Kits
2.4. Surrogate Virus Neutralization Test (sVNT)
2.5. Calculation of Performance Characteristics
3. Results
3.1. Assessment of S, S1 and RBD-Specific iELISAs Developed in this Study
3.2. Performance Characteristics
3.3. Surrogate Virus Neutralization Test (sVNT)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, F.; Zhao, S.; Yu, B.; Chen, Y.-M.; Wang, W.; Song, Z.-G.; Hu, Y.; Tao, Z.-W.; Tian, J.-H.; Pei, Y.-Y.; et al. A new coronavirus associated with human respiratory disease in China. Nature 2020, 579, 265–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghinai, I.; McPherson, T.D.; Hunter, J.C.; Kirking, H.L.; Christiansen, D.; Joshi, K.; Rubin, R.; Morales-Estrada, S.; Black, S.R.; Pacilli, M.; et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 2020, 395, 1137–1144. [Google Scholar] [CrossRef] [PubMed]
- Tuite, A.R.; Fisman, D.N.; Greer, A.L. Mathematical modelling of COVID-19 transmission and mitigation strategies in the population of Ontario, Canada. Can. Med. Assoc. J. 2020, 192, E497–E505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moazemi-Goudarzi, L.; Ziafatikafi, Z.; Seyedasgari, F.; Najafi, H.; Hashemzadeh, M.; Aghaeean, L.; Ghalyanchilangeroudi, A. Molecular Detection of Middle East Respiratory Syndrome Coronavirus from Dromedary Camels Illegally Transferred to Iran. Acta Veter Eurasia 2022, 48, 117–122. [Google Scholar] [CrossRef]
- Ozer, K.; Yilmaz, A.; Carossino, M.; Ozturk, G.Y.; Bamac, O.E.; Tali, H.E.; Mahzunlar, E.; Cizmecigil, U.Y.; Aydin, O.; Tali, H.B.; et al. Clinical, virological, imaging and pathological findings in a SARS-CoV-2 antibody positive cat. J. Veter Sci. 2022, 23, e52. [Google Scholar] [CrossRef]
- Telenti, A.; Hodcroft, E.B.; Robertson, D.L. The evolution and biology of SARS-CoV-2 variants. Cold Spring Harb. Perspect. Med. 2022, 12, a041390. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, Y.; Guo, Z.; Li, J.; Li, J.; Cheng, H.; Lu, B.; Sun, Q. Transmission and prevention of SARS-CoV-2. Biochem. Soc. Trans. 2020, 48, 2307–2316. [Google Scholar] [CrossRef]
- Chansaenroj, J.; Yorsaeng, R.; Posuwan, N.; Puenpa, J.; Sudhinaraset, N.; Chirathaworn, C.; Poovorawan, Y. Detection of SARS-CoV-2-specific antibodies via rapid diagnostic immunoassays in COVID-19 patients. Virol. J. 2021, 18, 52. [Google Scholar] [CrossRef]
- Pfefferle, S.; Reucher, S.; Nörz, D.; Lütgehetmann, M. Evaluation of a quantitative RT-PCR assay for the detection of the emerging coronavirus SARS-CoV-2 using a high throughput system. Eurosurveillance 2020, 25, 2000152. [Google Scholar] [CrossRef]
- Deeks, J.J.; Dinnes, J.; Takwoingi, Y.; Davenport, C.; Spijker, R.; Taylor-Phillips, S.; Adriano, A.; Beese, S.; Dretzke, J.; Ferrante di Ruffano, L.; et al. Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst. Rev. 2020, 6, CD013652. [Google Scholar] [PubMed]
- Tang, Y.-W.; Schmitz, J.E.; Persing, D.H.; Stratton, C.W. The Laboratory Diagnosis of COVID-19 Infection: Current Issues and Challenges. J. Clin. Microbiol. 2020, 58, 512–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, E.U.; Bloch, E.M.; Clarke, W.; Hsieh, Y.H.; Boon, D.; Eby, Y.; Fernandez, R.E.; Baker, O.R.; Keruly, M.; Kirby, C.S.; et al. Comparative Performance of Five Commercially Available Serologic Assays to Detect Antibodies to SARS-CoV-2 and Identify Individuals with High Neutralizing Titers. J. Clin. Microbiol. 2021, 59, e02257-20. [Google Scholar] [CrossRef] [PubMed]
- Lukosaityte, D.; Sadeyen, J.-R.; Shrestha, A.; Sealy, J.E.; Bhat, S.; Chang, P.; Digard, P.; Iqbal, M. Engineered Recombinant Single Chain Variable Fragment of Monoclonal Antibody Provides Protection to Chickens Infected with H9N2 Avian Influenza. Vaccines 2020, 8, 118. [Google Scholar] [CrossRef] [Green Version]
- Park, J.G.; Oladunni, F.S.; Rohaim, M.A.; Whittingham-Dowd, J.; Tollitt, J.; Assas, B.M.; Alhazmi, W.; Almilaibary, A.; Iqbal, M.; Chang, P.; et al. Immunogenicity and protective efficacy of an intranasal live-attenuated vaccine against SARS-CoV-2 in preclinical animal models. iScience 2021, 24, 102941. [Google Scholar] [CrossRef]
- Yilmaz, A.; Kayar, A.; Turan, N.; Iskefli, O.; Bayrakal, A.; Roman-Sosa, G.; Or, E.; Tali, H.E.; Kocazeybek, B.; Karaali, R.; et al. Presence of Antibodies to SARS-CoV-2 in Domestic Cats in Istanbul, Turkey, Before and After COVID-19 Pandemic. Front. Vet. Sci. 2021, 12, 707368. [Google Scholar] [CrossRef]
- Yilmaz, H.; Faburay, B.; Turan, N.; Cotton-Caballero, M.; Cetinkaya, B.; Gurel, A.; Yilmaz, A.; Cizmecigil, U.Y.; Aydin, O.; Tarakci, E.A.; et al. Production of Recombinant N Protein of Infectious Bronchitis Virus Using the Baculovirus Expression System and Its Assessment as a Diagnostic Antigen. Appl. Biochem. Biotechnol. 2019, 187, 506–517. [Google Scholar] [CrossRef]
- Kirkwood, B.R.; Sterne, J.A.C. Essential Medical Statistics, 2nd ed.; Blackwell Science: Malden, MA, USA, 2003; ISBN 978–0–86542–871–3. [Google Scholar]
- Karagul, M.S.; Sarac, F.; Hasoksuz, M. Enhanced significance of laboaratory biosafety abd biosecurity during COVID-19 pandemic. Acta Vet. Eurasia 2021, 47, 108–116. [Google Scholar] [CrossRef]
- WHO Director-General’s Opening Remarks at the Media Briefing on COVID-19—11 March 2020. Available online: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 (accessed on 13 March 2020).
- World Health Organization. Tracking SARS-CoV-2 Variants. Available online: https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/ (accessed on 13 August 2021).
- Mohit, E.; Rostami, Z.; Vahidi, H. A comparative review of immunoassays for COVID-19 detection. Expert Rev. Clin. Immunol. 2021, 17, 573–599. [Google Scholar] [CrossRef]
- Guo, L.; Ren, L.; Yang, S.; Xiao, M.; Chang, D.; Yang, F.; Cruz, C.S.D.; Wang, Y.; Wu, C.; Xiao, Y.; et al. Profiling Early Humoral Response to Diagnose Novel Coronavirus Disease (COVID-19). Clin. Infect. Dis. 2020, 71, 778–785. [Google Scholar] [CrossRef]
- Jääskeläinen, A.J.; Kekäläinen, E.; Kallio-Kokko, H.; Mannonen, L.; Kortela, E.; Vapalahti, O.; Kurkela, S.; Lappalainen, M. Evaluation of commercial and automated SARS-CoV-2 IgG and IgA ELISAs using coronavirus disease (COVID-19) patient samples. Eurosurveillance 2020, 25, 2000603. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.-H.; Cai, L.; Cheng, Z.-S.; Cheng, H.; Deng, T.; Fan, Y.-P.; Fang, C.; Huang, D.; Huang, L.-Q.; Huang, Q.; et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Liu, L.; Kou, G.; Zheng, Y.; Ding, Y.; Ni, W.; Wang, Q.; Tan, L.; Wu, W.; Tang, S.; et al. Evaluation of Nucleocapsid and Spike Protein-Based Enzyme-Linked Immunosorbent Assays for Detecting Antibodies against SARS-CoV-2. J. Clin. Microbiol. 2020, 58, e00461-20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; De Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2−Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect. Dis. 2020, 26, 478–1488. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.A.; Nicholson, J.; Gill, D. International solidarity: Medical school collaborations during the COVID-19 pandemic. Clin. Teach. 2020, 17, 547–548. [Google Scholar] [CrossRef]
- Haveri, A.; Smura, T.; Kuivanen, S.; Österlund, P.; Hepojoki, J.; Ikonen, N.; Pitkäpaasi, M.; Blomqvist, S.; Rönkkö, E.; Kantele, A.; et al. Serological and molecular findings during SARS-CoV-2 infection: The first case study in Finland, January to February 2020. Eurosurveillance 2020, 25, 2000266. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Feng, Y.; Mo, X.; Zheng, P.; Wang, Q.; Li, P.; Peng, P.; Liu, X.; Chen, Z.; Huang, H.; et al. Kinetics of SARS-CoV-2 specific IgM and IgG responses in COVID-19 patients. Emerg. Microbes Infect. 2020, 9, 940–948. [Google Scholar] [CrossRef]
- Zhong, L.; Chuan, J.; Gong, B.; Shuai, P.; Zhou, Y.; Zhang, Y.; Jiang, Z.; Zhang, D.; Liu, X.; Ma, S.; et al. Detection of serum IgM and IgG for COVID-19 diagnosis. Sci. China Life Sci. 2020, 63, 777–780. [Google Scholar] [CrossRef] [Green Version]
- Zhang, P.; Gao, Q.; Wang, T.; Ke, Y.; Mo, F.; Jia, R.; Liu, W.; Liu, L.; Zheng, S.; Liu, Y.; et al. Evaluation of recombinant nucleocapsid and spike proteins for serological diagnosis of novel coronavirus disease 2019 (COVID-19). bioRxiv, 2020; submitted. [Google Scholar]
- West, R.; Kobokovich, A.; Connell, N.; Gronvall, G.K. COVID-19 Antibody Tests: A Valuable Public Health Tool with Limited Relevance to Individuals. Trends Microbiol. 2021, 29, 214–223. [Google Scholar] [CrossRef]
- Herroelen, P.H.; Martens, G.A.; De Smet, D.; Swaerts, K.; Decavele, A. Kinetics of the humoral immune response to SARS-CoV-2: Comparative analytical performance of seven commercial serology tests. bioRxiv, 2020; submitted. [Google Scholar]
- Espejo, R. COVID-19. Syst. Res. Behav. Sci. 2020, 38, 184–186. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, Z.; Huang, C.; Sun, J.; Xue, M.; Feng, T.; Pan, W.; Wang, K.; Dai, J. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Membrane (M) and Spike (S) Proteins Antagonize Host Type I Interferon Response. Front. Cell Infect. Microbiol. 2021, 11, 766922. [Google Scholar] [CrossRef] [PubMed]
- Bastos, M.L.; Tavaziva, G.; Abidi, S.K.; Campbell, J.R.; Haraoui, L.-P.; Johnston, J.; Lan, Z.; Law, S.; MacLean, E.; Trajman, A.; et al. Diagnostic accuracy of serological tests for COVID-19: Systematic review and meta-analysis. Mil. Med. Res. 2020, 370, m:2516. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Tuaillon, E.; Bolloré, K.; Pisoni, A.; Debiesse, S.; Renault, C.; Marie, S.; Groc, S.; Niels, C.; Pansu, N.; Dupuy, A.M.; et al. Detection of SARS-CoV-2 antibodies using commercial assays and seroconversion patterns in hospitalized patients. J. Infect. 2020, 81, 39–45. [Google Scholar] [CrossRef]
- Ni, L.; Ye, F.; Cheng, M.L.; Feng, Y.; Deng, Y.Q.; Zhao, H.; Wei, P.; Ge, J.; Gou, M.; Li, X.; et al. Detection of SARS-CoV-2-Specific Humoral and Cellular Immunity in COVID-19 Convalescent Individuals. Immunity 2020, 52, 971–977. [Google Scholar] [CrossRef]
- Spicuzza, L.; Montineri, A.; Manuele, R.; Crimi, C.; Pistorio, M.P.; Campisi, R.; Vancheri, C.; Crimi, N. Reliability and usefulness of a rapid IgM-IgG antibody test for the diagnosis of SARS-CoV-2 infection: A preliminary report. J. Infect. Prev. 2020, 81, 53–54. [Google Scholar] [CrossRef]
- US Food and Drug Administration. Available online: https://www.fda.gov/media/137698/download. (accessed on 27 September 2022).
- Tang, X.; Wu, C.; Li, X.; Song, Y.; Yao, X.; Wu, X.; Duan, Y.; Zhang, H.; Wang, Y.; Qian, Z.; et al. On the origin and continuing evolution of SARS-CoV-2. Natl. Sci. Rev. 2020, 7, 1012–1023. [Google Scholar] [CrossRef]

| Human Sera | In-House S iELISA | In-House S1 iELISA | In-House RBD iELISA | Commercial ELISA Kit (Abbott) | Surrogate Virus Neutralization Test | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | |
| Infected | 65 | 5 | 65 | 5 | 61 | 9 | 67 | 3 | 63 | 7 |
| Vaccinated | 121 | 101 | 106 | 116 | 116 | 106 | 139 | 83 | 110 | 112 |
| Total | 186 | 106 | 171 | 121 | 177 | 115 | 206 | 86 | 173 | 119 |
| Performance Characteristics | In-House S iELISA | In-House S1 iELISA | In-House RBD iELISA |
|---|---|---|---|
| Sensitivity | 88.44% | 90.17% | 95.38% |
| Specificity | 72.27% | 89.08% | 89.92% |
| Cut-off value | 0.570 | 0.320 | 0.300 |
| AUC | 0.777 | 0.926 | 0.959 |
| Kappa value | 0.62 | 0.79 | 0.86 |
| Test No. | Tests | Number of Positives-(%) |
|---|---|---|
| 1 | S iELISA and sVN | 90 (40.5) |
| 2 | S1 iELISA and sVN | 94 (42.3) |
| 3 | RBD iELISA and sVN | 104 (46.8) |
| 4 | S and S1 iELISA | 91 (40.8) |
| 5 | S and RBD iELISA | 101 (45.4) |
| 6 | S1 and RBD iELISA | 98 (44.1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yilmaz, A.; Turan, N.; Kocazeybek, B.S.; Dinc, H.O.; Tali, H.E.; Aydin, O.; Tali, H.B.; Yilmaz, S.G.; Konukoglu, D.; Borekci, S.; et al. Development of in House ELISAs to Detect Antibodies to SARS-CoV-2 in Infected and Vaccinated Humans by Using Recombinant S, S1 and RBD Proteins. Diagnostics 2022, 12, 3085. https://doi.org/10.3390/diagnostics12123085
Yilmaz A, Turan N, Kocazeybek BS, Dinc HO, Tali HE, Aydin O, Tali HB, Yilmaz SG, Konukoglu D, Borekci S, et al. Development of in House ELISAs to Detect Antibodies to SARS-CoV-2 in Infected and Vaccinated Humans by Using Recombinant S, S1 and RBD Proteins. Diagnostics. 2022; 12(12):3085. https://doi.org/10.3390/diagnostics12123085
Chicago/Turabian StyleYilmaz, Aysun, Nuri Turan, Bekir Sami Kocazeybek, Harika Oyku Dinc, Hasan Emre Tali, Ozge Aydin, Hamid Besim Tali, Semaha Gul Yilmaz, Dildar Konukoglu, Sermin Borekci, and et al. 2022. "Development of in House ELISAs to Detect Antibodies to SARS-CoV-2 in Infected and Vaccinated Humans by Using Recombinant S, S1 and RBD Proteins" Diagnostics 12, no. 12: 3085. https://doi.org/10.3390/diagnostics12123085