1. Introduction
Age-related macular degeneration (AMD) is the fourth most prevalent ocular disease resulting in vision loss in the macula [
1]. The macula is located in the optical center of the human eye and is an important part of the retina. It is required for reading, driving, watching TV, and performing many other daily activities [
2]. Of all cases of blindness worldwide, 8.7% are caused by AMD and the number of patients with AMD was estimated at around 196 million in 2020, predicted to rise to 288 million by 2040 [
3].
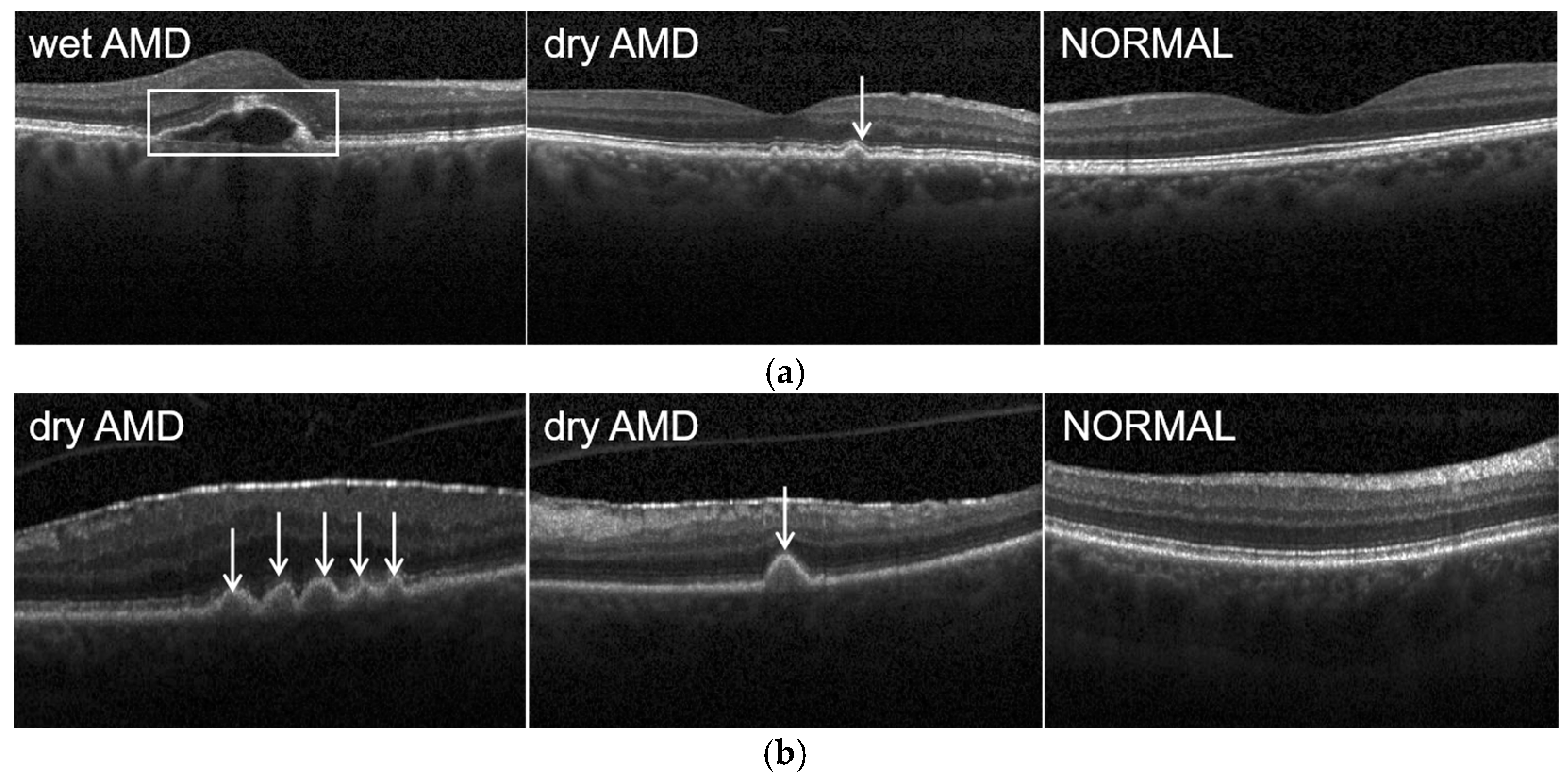
AMD is broadly classified into non-exudative or dry AMD and exudative or wet AMD. The difference between dry and wet AMD is that dry AMD does not have any blood or serum leakage. Around 85% to 90% of AMD cases are dry [
4]. Patients suffering from dry AMD have a significant anomaly known as drusen in the retinal pigment epithelium (RPE) layer. The formation of drusen leads to a thinning and drying out of the macula, which results in the loss of macular function. Although patients with dry AMD may still have a good central vision, they may have significant functional limitations, including limited night vision, vision fluctuations, and reading difficulties due to a limited area of central vision. Moreover, a certain percentage of dry AMD may develop into wet AMD as time goes by [
5]. In wet AMD, patients may see dark spots in their central vision due to blood or fluid leakage under the macula. The main pathogenesis of wet AMD is choroidal neovascularization (CNV), which occurs under the retina and macula. This neovascularization may lead to macular swelling and a reversible loss of vision, or bleeding, which can be highly toxic to the overlying photoreceptors, sometimes even causing irreversible vision loss [
6,
7]. In wet AMD, vision loss may be rapid and progressive. Once CNV has developed in one eye, the other eye is in a high-risk state and requires periodic eye examination [
8]. Therefore, regular screening of the retina is crucial for the diagnosis and treatment of AMD and the prevention of further deterioration.
Ophthalmologists can detect AMD-related lesions through a variety of methods with the continuous development of imaging technology [
9,
10,
11,
12]. Optical coherence tomography (OCT) uses the basic principle of a weak coherence interferometer to carry out high-resolution cross-sectional tomography of the internal microstructure of materials or biological systems [
13]. It has been widely used in many fields and has great scientific research and application potential in biomedical, agricultural and industrial detection [
14,
15,
16,
17,
18]. In recent years, OCT has become a major tool to diagnose AMD and monitor its progress [
19]. It reflects the physical structure of the retina accurately and effectively in a non-contact and non-invasive way [
20,
21]. It can clearly describe the particular pathology relating to AMD, such as drusen, intra-retinal fluid (IRF), sub-retinal fluid (SRF), sub-retinal hyper-reflective material and RPE detachment [
22].
The OCT images to be analyzed increase dramatically with a more widespread screening of the retina. The number of professional ophthalmologists is limited because training new professional doctors is a long process. It is often difficult for patients of AMD to receive timely diagnosis and treatment, especially in places where medical resources are insufficient. Therefore, a computer-aided diagnosis (CAD) system that can automatically detect AMD from a large number of retinal OCT images is urgently needed. In recent years, researchers have launched kinds of studies on AMD detection and classification based on retinal OCT images with the development and improvement of computer technology and image processing algorithms [
23,
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34].
2. Related Work
Layer segmentation is crucial in many automatic analysis algorithms based on retinal OCT images. The position and thickness of each retinal layer are obtained according to the result of the layer segmentation algorithm, then by analyzing the similarities and differences between the layer index of the tested image and the reference image, a variety of issues, including lesion detection and positioning, can be addressed.
Farsiu et al. [
23] introduced a semi-automatic segmentation of RPE, RPE drusen complex (RPEDC) and total retina (TR) boundaries. Then, volumes of TR, RPEDC and abnormal RPEDC of each subject were measured and compared with the normal thickness generated by control subjects to detect AMD. The area under the curve (AUC) of the receiver operating characteristic (ROC) for this classifier was 0.9900.
Naz et al. [
24] proposed an algorithm to detect the AMD-effected OCT scans by calculating the difference between the RPE layer and a second-order polynomial curve. The method was made time efficient by using an intensity-based threshold method for the RPE segmentation. A dataset with 25 AMD and 25 healthy images was used, and the study obtained an accurate detection of AMD with 96.00% accuracy.
Arabi et al. [
25] used the binary threshold method to extract the RPE layer, sampled the extracted layers and counted the number of white pixels in each sample. The mean value of the numbers of pixels was calculated and classified. They tested the approach on 16 images and obtained an accuracy of 75.00%.
Thomas et al. [
26] proposed an algorithm based on RPE layer detection and baseline estimation using statistical methods and randomization for the detection of AMD from retinal OCT images. The method was tested on a public dataset including 2130 images and achieved an overall accuracy of 96.66%.
Sharif et al. [
27] presented a method based on feature extraction and the support vector machine (SVM). First, the RPE layer was extracted by utilizing the graph theory dynamic programming technique, then a unique feature set consisting of features extracted from the difference signal of RPE and the inner segment outer segment layer of RPE was obtained. Finally, the SVM classifier was used to detect AMD-affected images from 950 OCT scans, and an accuracy of 95.00% was obtained.
Although the above methods based on layer segmentation obtained promising results, they are not suitable for large-scale AMD detection. The convolutional neural network (CNN), which emerged at the end of the 20th century, has significantly improved the ability to classify images [
28].
Lee et al. [
29] classified 52,690 normal and 48,312 AMD OCT images utilizing a modified version of the VGG-16 CNN model and obtained an overall accuracy of 93.40%. Serener et al. [
30] compared two pre-trained CNN, namely AlexNet and ResNet-18, to automatically classify OCT images for dry and wet AMD diseases, respectively. In both cases, the ResNet-18 model outperformed the AlexNet model, and the AUC of the ResNet model for each AMD stage was 0.9400 and 0.9300, respectively.
Thomas et al. [
31,
32] conducted a number of studies based on AMD detection using OCT images. In [
31], a multiscale and multipath CNN with six convolutional layers was proposed and finally achieved an overall accuracy of 98.79% with the random forest (RF) classifier. Later, in [
32], they introduced another novel multiscale CNN with seven convolutional layers to classify AMD and normal OCT images. The multiscale convolution layer enables a large number of local structures to be generated with various filter sizes. The proposed CNN network finally achieved an accuracy of 99.73% on the UCSD dataset.
Yoo et al. [
33] utilized VGG-19 pre-trained with images from ImageNet as a feature extractor, and a multiclass RF classifier was operated to detect AMD images. The overall accuracy using OCT alone was 82.60% on a small dataset including both OCT and matched fundus images. Kadry et al. [
34] extracted handcrafted features, such as the local binary pattern (LBP), the pyramid histogram of oriented gradients (PHOG), and the discrete wavelet transform (DWT) from the test images and concatenated them with the deep features of VGG-16. The proposed technique achieved an accuracy of up to 97.00% for OCT images with different binary classifiers.
In this study, we presented a novel method for the detection of AMD based on OCT images and showed it to be more effective than existing methods. The rest of the paper is structured as follows. The proposed methodology is given in
Section 3, then the datasets used for the experiment and the parameters of the model are given in
Section 4. The experimental results and discussion are shown in
Section 5. The conclusion is given in
Section 6.
3. Method
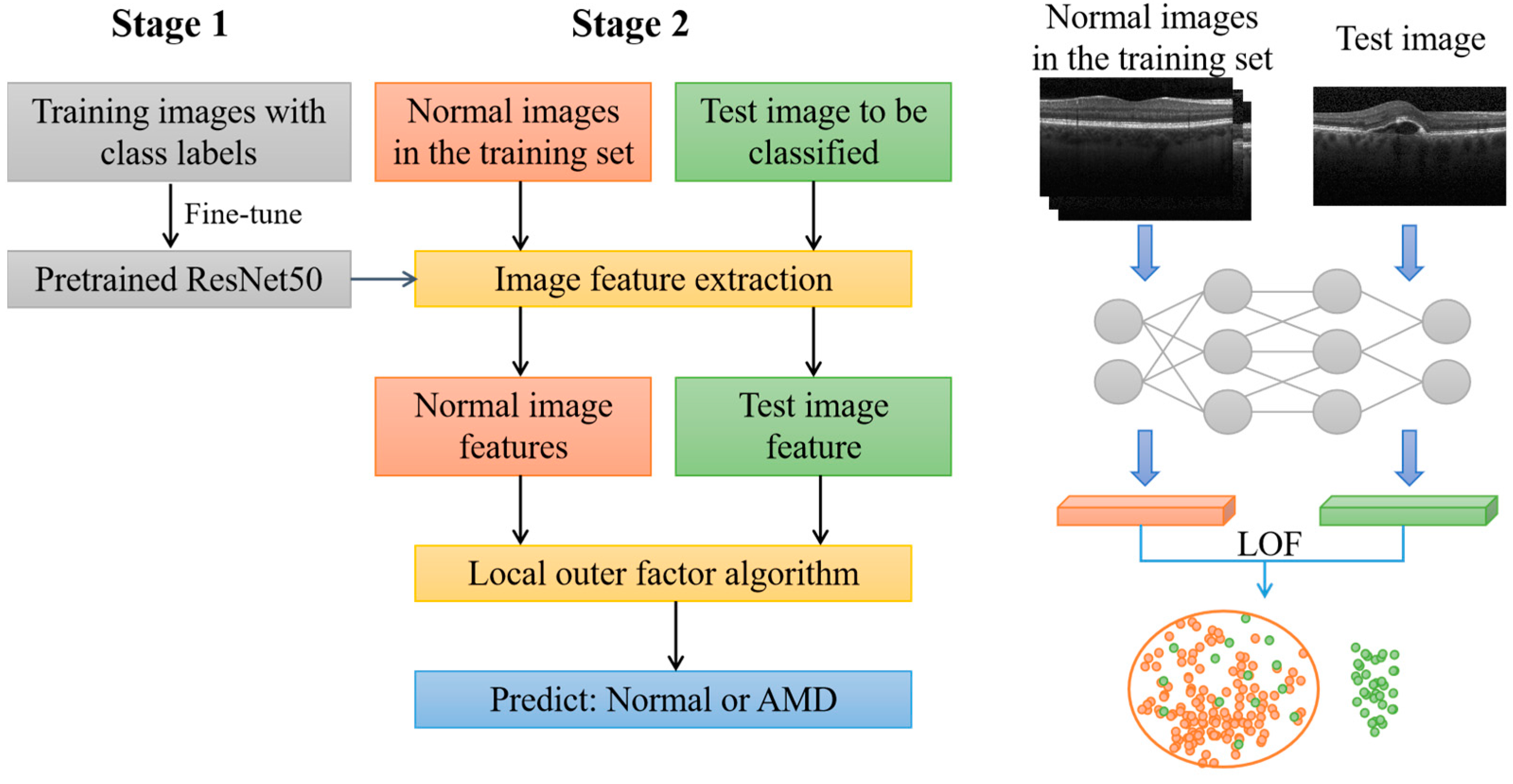
In this study, a two-stage model was proposed for the detection of AMD from OCT images, as shown in
Figure 1. The first stage involved a classification model using a deep CNN, while in the second stage, an outlier detection method was used for detecting AMD.
In the first stage, a deep CNN based on ResNet-50 [
35] was used for classification, and transfer learning was performed using AMD and normal OCT images [
36]. After retraining, the last layer of the network (classification layer) was removed and the model was regarded as an image feature extractor.
In the second stage, the normal images in the training set were imported to the network to obtain a normal image feature vector set. During testing, images in the test set were imported into the network in turn, and each test image could obtain a corresponding feature vector. Both the normal image feature vector set and test image feature vector were used as inputs of the local outlier factor (LOF) algorithm [
37]. Finally, the LOF algorithm classified the test image as normal or abnormal (corresponding to AMD).
3.1. ResNet-50
ResNet was proposed by He et al. [
35]. In the deep structure of CNN, as the layers deepen, gradient disappearance or explosion may occur, resulting in a drop in accuracy. The problem can be solved by the residual network, improving the performance and increasing the depth of the network at the same time. ResNet has been widely used in the field of medical image classification, in applications such as multi-label chest X-ray classification [
38], the diagnosis of COVID-19 [
39] and exudate detection in fundus images [
40].
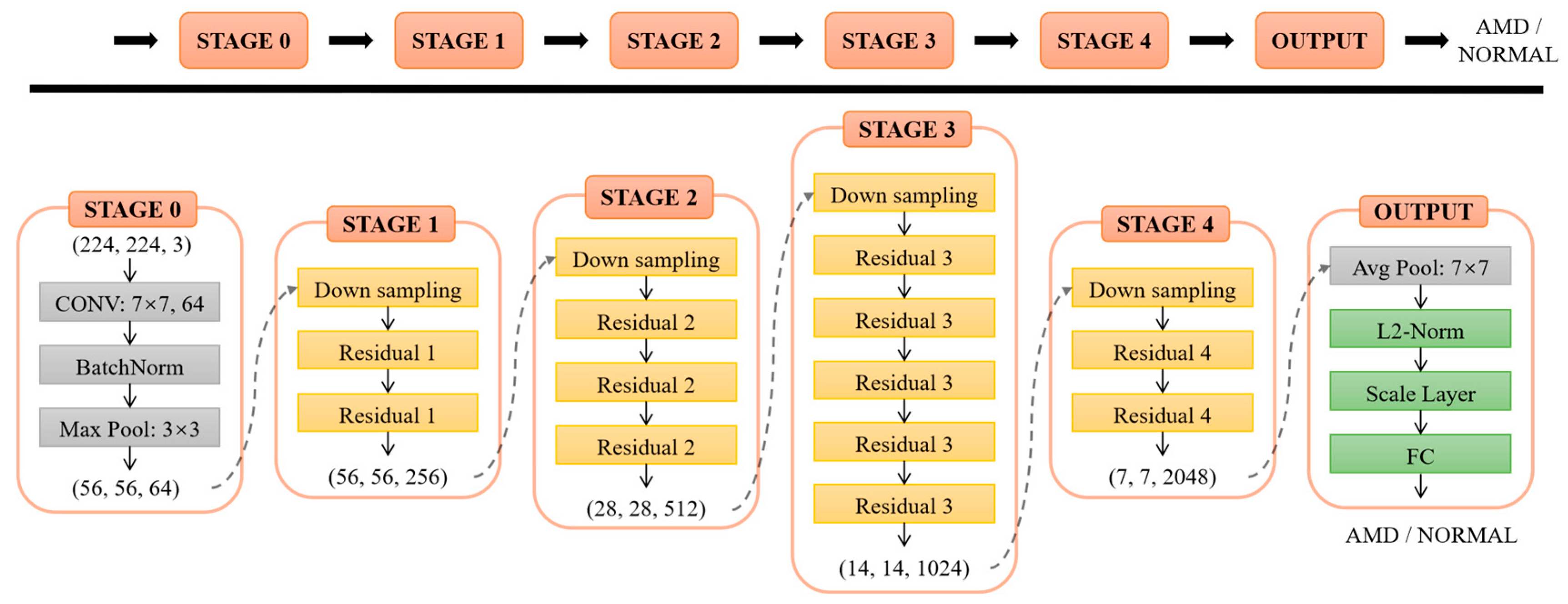
In this work, we utilized a 50-layer structure of ResNet. The residual block structure of ResNet-50 is shown in
Figure 2. The input
x is transferred across layers through a shortcut connection to be added into the output
F(x) after convolution, and then the output
y = F(x) + x is obtained. The residual block can fully train the underlying network, so the accuracy can be significantly improved as the depth increases.
Figure 3 shows the architecture of ResNet-50 used in this study. The last fully-connected layer was adjusted to binary output classes for AMD and normal instead of the 1000 output classes of the ImageNet, and the loss function was L2-constrained softmax loss [
41].
The L2-constrained softmax loss is given by Equation (1) [
41]:
where
xi is the input image in the mini-batch of size
M,
yi is the corresponding class label,
f(xi) is the feature descriptor obtained from the penultimate layer,
C is the number of classes, and
W and
b are the weight and bias of the last layer. An additional L2 constraint is added on the basis of the traditional softmax function, and the constraint is enforced by adding an L2-normalize (L2-Norm) layer, followed by a scale layer, as shown in
Figure 3. This constraint restricts the feature to a hypersphere with a fixed radius through a parameter, which brings the features from the same class closer to each other and separates the features from different classes in the normalized or angular space. In this study,
M = 12,
C = 2, and
α was set to 5.
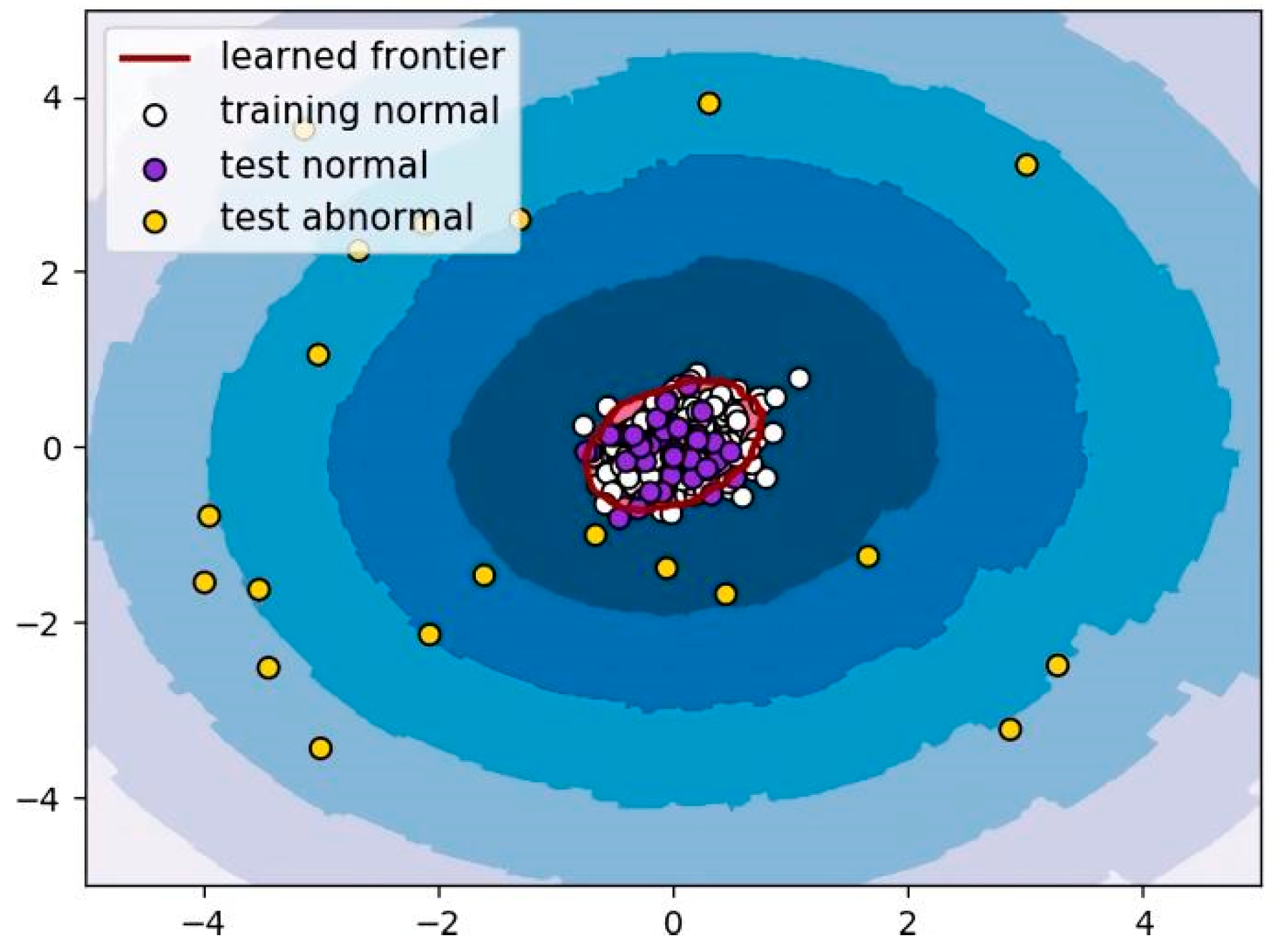
3.2. LOF Algorithm
The LOF algorithm was used to divide OCT images into two groups: normal and AMD. The LOF is an outlier detection method that computes the local density deviation of a given data point with respect to its neighbors [
37]. The local density is given by Equation (2):
where
lrdk(p) is the local density of object
p,
Nk(p) is the k-distance neighborhood of
p, and
reach-distk(p,o) is the reachability distance of object
p with respect to object
o, which is given by Equation (3):
Using
lrdk(p), the LOF of
p is defined by Equation (4):
where
k was set to 20 in this study. The output
LOF value was used to determine whether
p was normal or abnormal (corresponding to AMD), as shown in
Figure 4.
6. Conclusions
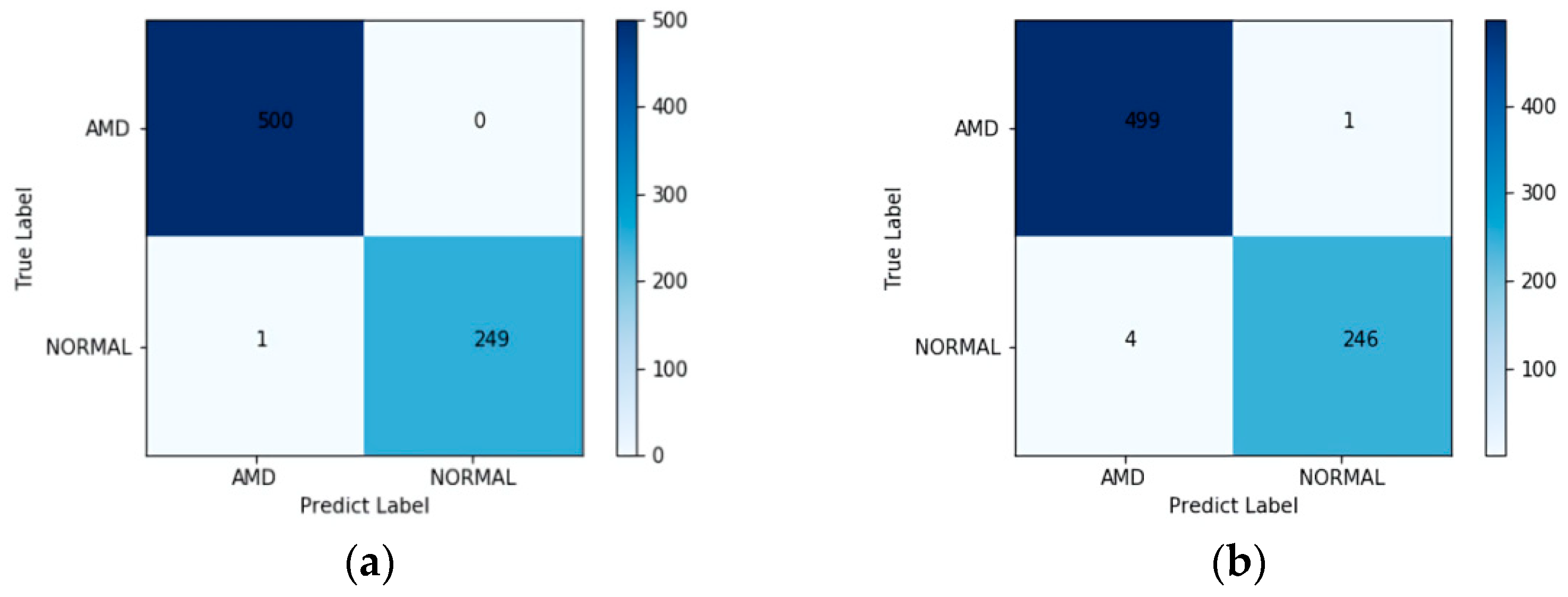
A novel automatic detection method was presented for the detection of AMD from OCT images based on deep learning and an outlier detection method. The ResNet-50 model with L2-constrained softmax loss was retrained to extract features from OCT images, and the LOF algorithm was used as the classifier. The proposed method was trained on the UCSD dataset and tested on both the UCSD dataset and the Duke dataset, with an accuracy of 99.87% and 97.56%, respectively. Even though the model was only trained on the UCSD dataset, it also performed well when tested on the Duke dataset.
Table 4 and
Table 6 show the comparison of the proposed method with existing works, which also indicates the efficiency of the proposed method in detecting AMD.
The advantage of the proposed method is its excellent ability to classify AMD and normal OCT images without preprocessing and with high accuracy, which will help doctors in large-scale OCT image screening.
Our proposed method achieves a good overall performance in the detection of AMD, enabling the proposed method to be used for the early detection of AMD. In future work, we hope to use more datasets to validate the proposed method, and based on this study, we will further subdivide the AMD into dry AMD and wet AMD.