The Use of Chest Magnetic Resonance Imaging in Malignant Pleural Mesothelioma Diagnosis
Abstract
:1. Introduction
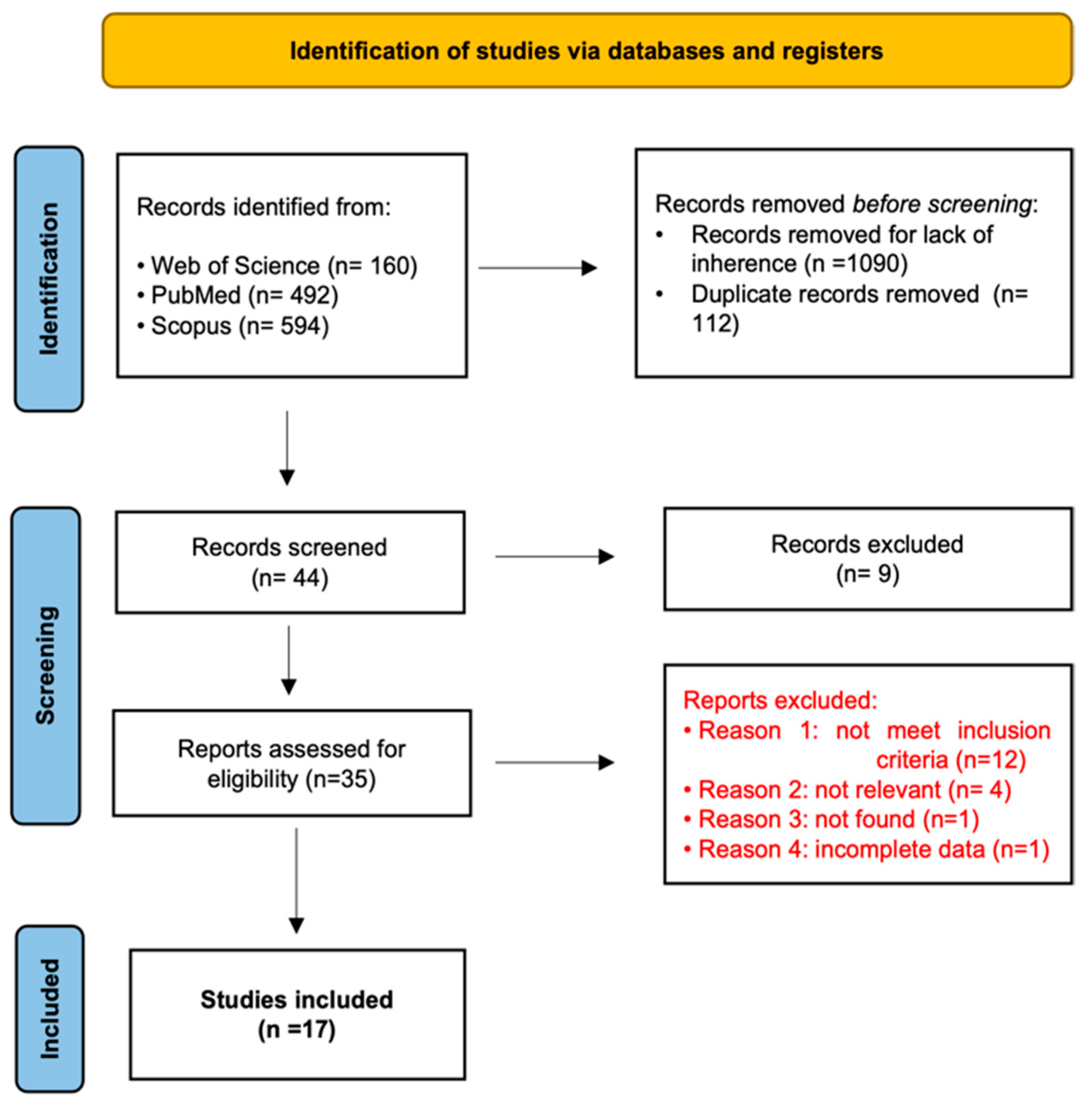
2. Materials and Methods
Search Strategies
3. Results
4. Discussion
4.1. Morphological Evaluation
4.2. Functional Imaging
4.2.1. Dynamic MRI
4.2.2. Contrast Enhancement-MRI
4.2.3. Diffusion-Weighted Imaging and Apparent Diffusion Coefficient
ADC Cut-Off
ADC-ROIs
ADC-Histologic Subtype
DWI-Visual Assessment
5. MRI Protocol: A Proposal
6. Conclusions
Author Contributions
Funding
Informed Consent Statement
Conflicts of Interest
References
- Beasley, M.B.; Galateau-Salle, F.; Dacic, S. Pleural mesothelioma classification update. Virchows Arch. 2021, 478, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Odisio, E.G.; Marom, E.M.; Shroff, G.S.; Wu, C.C.; Benveniste, A.P.A.; Truong, M.T.; Benveniste, M.F. Malignant Pleural Mesothelioma: Diagnosis, Staging, Pitfalls and Follow-up. Semin. Ultrasound CT MRI 2017, 38, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Testa, J.R.; Carbone, M. Mesothelioma Epidemiology, Carcinogenesis, and Pathogenesis. Curr. Treat. Options Oncol. 2008, 9, 147–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinn, K.; Mosleh, B.; Hoda, M.A. Malignant pleural mesothelioma: Recent developments. Curr. Opin. Oncol. 2021, 33, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Bibby, A.C.; Tsim, S.; Kanellakis, N.; Ball, H.; Talbot, D.C.; Blyth, K.; Maskell, N.A.; Psallidas, I. Malignant pleural mesothelioma: An update on investigation, diagnosis and treatment. Eur. Respir. Rev. 2016, 25, 472–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherpereel, A.; Wallyn, F.; Albelda, S.M.; Munck, C. Novel therapies for malignant pleural mesothelioma. Lancet Oncol. 2018, 19, e161–e172. [Google Scholar] [CrossRef]
- Schillebeeckx, E.; van Meerbeeck, J.P.; Lamote, K. Clinical utility of diagnostic biomarkers in malignant pleural mesothelioma: A systematic review and meta-analysis. Eur. Respir. Rev. 2021, 30, 210057. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Swift, A.; Kamil, M.; Matthews, S.; Bull, M.; Fisher, P.; De Fonseka, D.; Saha, S.; Edwards, J.; Johns, C. The role of imaging in malignant pleural mesothelioma: An update after the 2018 BTS guidelines. Clin. Radiol. 2020, 75, 423–432. [Google Scholar] [CrossRef]
- Podobnik, J.; Kocijančič, I.; Kovac, V.; Serša, I. 3T MRI in evaluation of asbestos-related thoracic diseases-preliminary results. Radiol. Oncol. 2010, 44, 92–96. [Google Scholar] [CrossRef]
- Romei, C.; Fanni, S.C.; Volpi, F.; Milazzo, A.; D’Amore, C.A.; Colligiani, L.; Neri, E.; De Liperi, A.; Stella, G.M.; Bortolotto, C. New Updates of the Imaging Role in Diagnosis, Staging, and Response Treatment of Malignant Pleural Mesothelioma. Cancers 2021, 13, 4377. [Google Scholar] [CrossRef]
- Martini, K.; Meier, A.; Opitz, I.; Weder, W.; Veit-Haibach, P.; Stahel, R.A.; Frauenfelder, T. Diagnostic accuracy of sequential co-registered PET+MR in comparison to PET/CT in local thoracic staging of malignant pleural mesothelioma. Lung Cancer 2016, 94, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Armato, S.G.; Labby, Z.E.; Coolen, J.; Klabatsa, A.; Feigen, M.; Persigehl, T.; Gill, R.R. Imaging in pleural mesothelioma: A review of the 11th International Conference of the International Mesothelioma Interest Group. Lung Cancer 2013, 82, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armato, S.G.; Coolen, J.; Nowak, A.K.; Robinson, C.; Gill, R.R.; Straus, C.; Khanwalkar, A. Imaging in pleural mesothelioma: A review of the 12th International Conference of the International Mesothelioma Interest Group. Lung Cancer 2015, 90, 148–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armato, S.G.; Blyth, K.G.; Keating, J.J.; Katz, S.; Tsim, S.; Coolen, J.; Gudmundsson, E.; Opitz, I.; Nowak, A.K. Imaging in pleural mesothelioma: A review of the 13th International Conference of the International Mesothelioma Interest Group. Lung Cancer 2016, 101, 48–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomšič, M.V.; Bisdas, S.; Kovač, V.; Serša, I.; Popovič, K. Dynamic contrast-enhanced MRI of malignant pleural mesothelioma: A comparative study of pharmacokinetic models and correlation with mRECIST criteria. Cancer Imaging 2019, 19, 10. [Google Scholar] [CrossRef] [PubMed]
- Weber, M.A.; Bock, M.; Plathow, C.; Wasser, K.; Fink, C.; Zuna, I.; Schmähl, A.; Berger, I.; Kauczor, H.U.; Schoenberg, S.O. Asbestos-related pleural disease: Value of dedicated magnetic resonance imaging techniques. Investig. Radiol. 2004, 39, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.S.C.; Seethamraju, R.T.; Bueno, R.; Gill, R.R. Clinical Implementation of a Free-Breathing, Motion-Robust Dynamic Contrast-Enhanced MRI Protocol to Evaluate Pleural Tumors. Am. J. Roentgenol. 2020, 215, 94–104. [Google Scholar] [CrossRef] [PubMed]
- Plathow, C.; Klopp, M.; Schoebinger, M.; Thieke, C.; Fink, C.; Puderbach, M.; Ley, S.; Weber, M.A.; Sandner, A.; Claussen, C.D.; et al. Monitoring of lung motion in patients with malignant pleural mesothelioma using two-dimensional and three-dimensional dynamic magnetic resonance imaging: Comparison with spirometry. Investig. Radiol. 2006, 41, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Plathow, C.; Klopp, M.; Thieke, C.; Herth, F.; Thomas, A.; Schmaehl, A.; Zuna, I.; Kauczor, H.-U. Therapy response in malignant pleural mesothelioma-role of MRI using RECIST, modified RECIST and volumetric approaches in comparison with CT. Eur. Radiol. 2008, 18, 1635–1643. [Google Scholar] [CrossRef] [PubMed]
- Knuuttila, A.; Kivisaari, L.; Kivisaari, A.; Palomäki, M.; Tervahartiala, P.; Mattson, K. Evaluation of pleural disease using MR and CT. With special reference to malignant pleural mesothelioma. Acta Radiol. 2001, 42, 502–507. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Coolen, J.; De Keyzer, F.; Nafteux, P.; De Wever, W.; Dooms, C.; Vansteenkiste, J.; Roebben, I.; Verbeken, E.; De Leyn, P.; Van Raemdonck, D.; et al. Malignant Pleural Disease: Diagnosis by Using Diffusion-weighted and Dynamic Contrast-enhanced MR Imaging—Initial Experience. Radiology 2012, 263, 884–892. [Google Scholar] [CrossRef] [PubMed]
- Tsim, S.; Humphreys, C.A.; Cowell, G.W.; Stobo, D.B.; Noble, C.; Woodward, R.; Kelly, C.A.; Alexander, L.; Foster, J.E.; Dick, C.; et al. Early Contrast Enhancement: A novel magnetic resonance imaging biomarker of pleural malignancy. Lung Cancer 2018, 118, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hierholzer, J.; Luo, L.; Bittner, R.C.; Stroszczynski, C.; Schröder, R.-J.; Schoenfeld, N.; Dorow, P.; Loddenkemper, R.; Grassot, A. MRI and CT in the Differential Diagnosis of Pleural Disease. Chest 2000, 118, 604–609. [Google Scholar] [CrossRef]
- Coolen, J.; De Keyzer, F.; Nafteux, P.; De Wever, W.; Dooms, C.; Vansteenkiste, J.; Derweduwen, A.; Roebben, I.; Verbeken, E.; De Leyn, P.; et al. Malignant Pleural Mesothelioma: Visual Assessment by Using Pleural Pointillism at Diffusion-weighted MR Imaging. Radiology 2015, 274, 576–584. [Google Scholar] [CrossRef] [Green Version]
- Sabri, Y.Y.; Mahmoud, I.H.; El-Gendy, L.T.; El-Mageed, M.R.A.; Tadros, S.F. Added value of diffusion-weighted MRI in assessment of pleural lesions. Egypt. J. Radiol. Nucl. Med. 2021, 52, 184. [Google Scholar] [CrossRef]
- Inan, N.; Sarisoy, H.T.; Çam, I.; Sakçi, Z.; Arslan, A. Diffusion-weighted Magnetic Resonance Imaging in the Differential Diagnosis of Benign and Metastatic Malignant Pleural Thickening. J. Thorac. Imaging 2016, 31, 37–42. [Google Scholar] [CrossRef]
- Gill, R.R.; Umeoka, S.; Mamata, H.; Tilleman, T.R.; Stanwell, P.; Woodhams, R.; Padera, R.F.; Sugarbaker, D.J.; Hatabu, H. Diffusion-Weighted MRI of Malignant Pleural Mesothelioma: Preliminary Assessment of Apparent Diffusion Coefficient in Histologic Subtypes. Am. J. Roentgenol. 2010, 195, W125–W130. [Google Scholar] [CrossRef] [PubMed]
- Priola, A.M.; Priola, S.M.; Gned, D.; Giraudo, M.T.; Brundu, M.; Righi, L.; Veltri, A. Diffusion-weighted quantitative MRI of pleural abnormalities: Intra- and interobserver variability in the apparent diffusion coefficient measurements. J. Magn. Reson. Imaging 2017, 46, 769–782. [Google Scholar] [CrossRef]
- Jiang, W.; Han, Z.; Tang, X.; Yin, H.; Zhang, J. Diffusion-weighted imaging diagnostic algorithm in patients with suspected pleural malignancy. Eur. Radiol. 2021, 31, 9038–9047. [Google Scholar] [CrossRef] [PubMed]
- Usuda, K.; Iwai, S.; Funasaki, A.; Sekimura, A.; Motono, N.; Matoba, M.; Doai, M.; Yamada, S.; Ueda, Y.; Uramoto, H. Diffusion-Weighted Imaging Can Differentiate between Malignant and Benign Pleural Diseases. Cancers 2019, 11, 811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malayeri, A.A.; El Khouli, R.H.; Zaheer, A.; Jacobs, M.A.; Corona-Villalobos, C.P.; Kamel, I.R.; Macura, K.J. Principles and Applications of Diffusion-weighted Imaging in Cancer Detection, Staging, and Treatment Follow-Up. RadioGraphics 2011, 31, 1773–1791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Messina, C.; Bignone, R.; Bruno, A.; Bruno, A.; Bruno, F.; Calandri, M.; Caruso, D.; Coppolino, P.; De Robertis, R.; Gentili, F.; et al. Diffusion-Weighted Imaging in Oncology: An Update. Cancers 2020, 12, 1493. [Google Scholar] [CrossRef] [PubMed]



| Publication | Study Design | Study Population (Period, Location) | N Eligible Patients | N Included Patients | N MPD Patients | N MPM Patients | Imaging Technique | MRI Sequences |
|---|---|---|---|---|---|---|---|---|
| Podobnik J. et al., 2010 [9] | Prospective study | 15 | 15 | 10 | 10 | 3T MR and CT | Coronal, axial and sagittal T2w TSE with SPIR; axial T1w TSE black blood | |
| Tomšič M.V. et al., 2019 [15] | Prospective study | October 2013–July 2015 | 29 | 19 | 19 | 19 | 3T MR | Axial T2w TSE FS; VIBE; DCE turbo-FLASH |
| Weber M.A. et al., 2004 [16] | Prospective study | 21 | 21 | 4 | 1.5T MR and CT | T2w TSE; T1w TSE before contrast and T1w TSE FS TSE after contrast; radial before and after contrast | ||
| Ng S. C. et al., 2020 [17] | Prospective study | May 2008–May 2017 | 23 | 23 | 3T and 1.5T MR | 3T: axial and coronal HASTE; coronal VIBE; axial DWI; radial DCE; FLASH DCE; TWIST DCE 1.5T: radial DCE | ||
| Plathow C. et al., 2006 [18] | Prospective study | 22 | 22 | 22 | 22 | 1.5T MR | trueFISP; FLASH 3D | |
| Plathow C. et al., 2008 [19] | Prospective study | 50 | 50 | 50 | 50 | 1.5T MR and CT | Coronal and axial HASTE; coronal and axial pre and postcontrast VIBE; coronal T2-TSE | |
| Knuuttila A. et al., 2001 [20] | Prospective study | January 1997–December 1998 | 34 | 34 | 27 | 18 | 1.5T MR and CT | Precontrast: Axial T1w FLASH; axial T2w true FISP; axial T2w FS HASTE Postcontrast: axial, coronal and sagittal T1w FS FLASH |
| Coolen J. et al., 2012 [22] | Prospective study | November 2009–May 2010 | 31 | 31 | 14 | 12 | 3T MR and PET/CT | Axial T2w SS-TSE; DWI; DCE T1w 3D FFE |
| Tsim S. et al., 2018 [23] | Prospective study | Not reported | 66 | 58 | 36 | 31 | 3T MR and CT | Pre- and postcontrast coronal T1w FS 3D SPGR |
| Hierholzer J. et al., 2000 [24] | Retrospective study | January 1992–June 1998 | 88 | 42 | 27 | 9 | 1.5T MR and CT | T1w pre- and postcontrast; T2w TSE |
| Coolen J. et al., 2015 [25] | Prospective study | November 2009–December 2012 | 109 | 100 | 67 | 57 | 3T MR, CT and PET/CT | T2w SS-TSE FS; DWI |
| Sabri Y. et al., 2021 [26] | Prospective study | March 2019–November 2020 | 57 | 57 | 28 | 7 | 1.5T MR | Axial and coronal T1w TSE; axial, coronal and sagittal T2w TSE; axial STIR; axial DWI |
| Inan N. et al., 2016 [27] | Prospective study | November 2013–September 2014 | 42 | 34 | 19 | 0 | 3T MR | Axial T1w SPGR-FFE with and without FS; coronal and axial T2w SS-TSE; axial T2w SS-TSE with FS; DWI |
| Gill R. R. et al., 2010 [28] | Prospective study | June 2008–January 2009 | 62 | 62 | 59 | 57 | 3T MR | Coronal and axial HASTE; 3D T1w GE; DWI |
| Priola A.M. et al., 2017 [29] | Retrospective study | January 2014–July 2016 | 37 | 34 | 34 | 18 | 1.5T MR | Axial, coronal and sagittal DWI; T2w SS-TSE; T1w fast field echo |
| Jiang W. et al., 2021 [30] | Retrospective study | March 2014–August 2018 | 730 | 70 | 52 | 1 | 1.5T MR and CT | Axial T1w; axial T2w; DWI |
| Usuda K. et al., 2019 [31] | Prospective study | March 2015–February 2019 | 43 | 43 | 21 | 11 | 1.5T MR, CT, PET/CT | Coronal T1w SE; coronal and axial T2w FSE; DWI |
| Publication | Lesion | Mean ADC Value | Notes |
|---|---|---|---|
| Coolen J. et al., 2012 [25] | Malignant Pleural Disease Benign Alterations | 1.40 ± 0.33 × 10−3 mm2/s 2.49 ± 0.81 × 10−3 mm2/s | ADC MPD vs. benign alterations (p < 0.001) |
| Sabri Y. et al., 2021 [26] | Malignant Pleural Lesions Benign Pleural Lesions MPM Pleural Metastases | 1.10 ± 0.53 × 10−3 mm2/s 2.19 ± 0.42 × 10−3 mm2/s 0.84 ± 0.22 × 10−3 mm2/s 1.19 ± 0.58 × 10−3 mm2/s | ADC malignant vs. benign pleural lesions (p < 0.001) ADC MPM vs. Pleural Metastases (p = 0.090) |
| İnan N. et al., 2016 [27] | Metastatic Malignant Pleural Thickening Benign Pleural Thickening | 1.37 ± 0.65 × 10−3 mm2/s (ADC1) 1.06 ± 0.56 × 10−3 mm2/s (ADC2) 2.11 ± 0.69 × 10−3 mm2/s (ADC1) 1.56 ± 0.71 × 10−3 mm2/s (ADC2) | ADC1 and ADC2 of Metastatic Malignant Pleural Disease vs. Benign Disease (p < 0.05) |
| Gill R. R. et al., 2010 [28] | Epithelioid MPM Biphasic MPM Sarcomatoid MPM | 1.31 ± 0.15 × 10−3 mm2/s 1.01 ± 0.11 × 10−3 mm2/s 0.99 ± 0.07 × 10−3 mm2/s | ADC Epithelioid vs. Sarcomatoid (p < 0.05) ADC Epithelioid vs. Biphasic (p < 0.05) |
| Jiang W. et al., 2021 [30] | Malignant Group Benign Group | 1.15 ± 0.32 × 10−3 mm2/s 1.46 ± 0.68 × 10−3 mm2/s | ADC Malignant vs. Benign Group (p = 0.161) |
| Usuda K. et al., 2019 [31] | Pleural dissemination MPM Empyema Pleural Effusion | 1.31 ± 0.49 × 10−3 mm2/s 1.22 ± 0.25 × 10−3 mm2/s 2.01 ± 0.45 × 10−3 mm2/s 3.76 ± 0.62 × 10−3 mm2/s | ADC MPM vs. Empyema (p = 0.0007) ADC MPM vs. Pleural Effusion (p < 0.0001) ADC of MPM vs. Pleural Dissemination: not significantly different |
| Sequence | Manufacturer Acronyms | Typical Contrast | Average Acquisition Time | Spatial Resolution Scan Plane | Scan Parameters | Field Strength B0 |
|---|---|---|---|---|---|---|
| Morphology | ||||||
| 2D Echo-planar Fast Spin Echo Sequence | HASTE (SIEMENS) | T2-weighted | Expiration Breath-Hold ≈ 40 s (in 2 different breath-hold) | FOV = 440 mm Thickness = 5 mm Plane = Coronal | TR = 2860 ms TE = 93 ms FA = 160 deg Matrix = (182 × 256) | 1.5T |
| 2D Echo-planar Fast Spin Echo Sequence | HASTE (SIEMENS) | T2-weighted | Expiration Breath-Hold ≈ 40 s (in 2 different breath-hold) | FOV = 420 mm Thickness = 5 mm Plane = Sagittal | TR = 2860 ms TE = 93 ms FA = 160 deg Matrix = (182 × 256) | 1.5T |
| 2D Echo-planar Fast Spin Echo Sequence | HASTE (SIEMENS) | T2-weighted | Expiration Breath-Hold ≈ 40 s (in 2 different breath-hold) | FOV = 360 mm Thickness = 5 mm Plane = Axial | TR = 2860 ms TE = 93 ms FA = 160 deg Matrix = (170 × 256) | 1.5T |
| 2D Echo-planar Fast Spin Echo Sequence (Fat Saturated) | HASTE (SIEMENS) | T2-weighted (Fat saturated) | Expiration Breath-Hold ≈ 40 s (in 2 different breath-hold) | FOV = 440 mm Thickness = 5 mm Plane = Coronal | TR = 2860 ms TE = 93 ms FA = 160 deg Matrix = (182 × 256) | 1.5T |
| 2D Echo-planar Fast Spin Echo Sequence (Fat Saturated) | HASTE (SIEMENS) | T2-weighted (Fat saturated) | Expiration Breath-Hold ≈ 40 s (in 2 different breath-hold) | FOV = 440 mm Thickness = 5 mm Plane = Axial | TR = 2860 ms TE = 93 ms FA = 160 deg Matrix= (170 × 256) | 1.5T |
| 2D Turbo Spin Echo (TSE) | T1-weighted | Free breathing (Navigator) ≈ 2:30 min | FOV = 440 mm Thickness = 5 mm Plane = Coronal | TE =491 ms TE = 19 ms FA = 135 deg Matrix = (179 × 256) | 1.5T | |
| 3D Rapid Acquisition Spoiled Gradient Echo | VIBE (SIEMENS) | T1-weighted (Fat saturated) | Expiration Breath-Hold ≈ 26 s | FOV = 390 mm Thickness = 2.5 mm Plane = Axial | TR = 4.55 ms TE = 2 ms FA = 10 deg Matrix = (153 × 224) | 1.5T |
| DWI | ||||||
| 2D Single-Shot Echo Planar Imaging (EPI) | DWI | T2-weighted diffusion-weighted (SPAIR fat saturation) | Free breathing (Navigator) 7–10 min | FOV = 360 mm Thickness = 5 mm Plane = Axial | TR = 11,500 TE = 90 BW = 1371 B = 0, 50, 400, 800 s/mm2 Matrix = (148 × 192) | 1.5T |
| Contrast Gadolinium Enhanced | ||||||
| 3D Rapid Acquisition Spoiled Gradient Echo Post-contrast (40 s, 80 s, 3′, 4:30′, 5′) | VIBE (SIEMENS) | T1-weighted (Fat saturated) | Expiration Breath-Hold ≈ 26 s | FOV = 390 mm Thickness = 2.5 mm Plane = Axial | TR = 4.55 ms TE = 2 ms FA = 10 deg Matrix = (153 × 224) | 1.5T |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Volpi, F.; D’Amore, C.A.; Colligiani, L.; Milazzo, A.; Cavaliere, S.; De Liperi, A.; Neri, E.; Romei, C. The Use of Chest Magnetic Resonance Imaging in Malignant Pleural Mesothelioma Diagnosis. Diagnostics 2022, 12, 750. https://doi.org/10.3390/diagnostics12030750
Volpi F, D’Amore CA, Colligiani L, Milazzo A, Cavaliere S, De Liperi A, Neri E, Romei C. The Use of Chest Magnetic Resonance Imaging in Malignant Pleural Mesothelioma Diagnosis. Diagnostics. 2022; 12(3):750. https://doi.org/10.3390/diagnostics12030750
Chicago/Turabian StyleVolpi, Federica, Caterina A. D’Amore, Leonardo Colligiani, Alessio Milazzo, Silvia Cavaliere, Annalisa De Liperi, Emanuele Neri, and Chiara Romei. 2022. "The Use of Chest Magnetic Resonance Imaging in Malignant Pleural Mesothelioma Diagnosis" Diagnostics 12, no. 3: 750. https://doi.org/10.3390/diagnostics12030750






