Fetal Pancreatic Hamartoma Associated with Hepatoblastoma—An Unusual Tumor Association
Abstract
1. Introduction
2. Case Report
| Type of Exam | Age | Imagistic Findings | |
|---|---|---|---|
| Preoperative | Lung radiograph | 2nd day |
|
| CT of the thorax, abdomen, and pelvis | 6th day |
| |
| Postoperative | CT of abdomen and pelvis | Three weeks |
|
| CT of the brain, thorax, abdomen, and pelvis | Four weeks |
| |
| Abdominal ultrasound | Five weeks |
| |
| Abdominal ultrasound | Seven weeks |
| |
| Abdominal ultrasound | Three months + three weeks |
| |
| Full-body CT | Six months |
| |
| Last CT | Nine months |
|
| Type of Exam | Histopathological Findings |
|---|---|
| The histopathological exam with immunohistochemical tests (surgical specimen) |
|
| Histopathological re-evaluation (Dr. Monique Fabre, Necker Hospital, Paris, France) |
|
| The histopathological exam with immunohistochemical tests (liver tumor biopsy) |
|
| Autopsy |
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cass, D.L. Fetal Abdominal Tumors and Cysts. Transl. Pediatr. 2021, 10, 1530–1541. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, M.R.; Chapman, T.; Dighe, M. Fetal Tumors: Imaging Features. Pediatr. Radiol. 2010, 40, 1263–1273. [Google Scholar] [CrossRef] [PubMed]
- Solt, I.; Lowenstein, L.; Goldstein, I. Prenatal diagnosis of fetal neoplasms. Harefuah 2004, 143, 131–135, 165. [Google Scholar] [PubMed]
- Catanzarite, V.; Hilfiker, M.; Daneshmand, S.; Willert, J. Prenatal Diagnosis of Fetal Hepatoblastoma: Case Report and Review of the Literature. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2008, 27, 1095–1098. [Google Scholar] [CrossRef] [PubMed]
- Stiller, C.A.; Pritchard, J.; Steliarova-Foucher, E. Liver Cancer in European Children: Incidence and Survival, 1978–1997. Report from the Automated Childhood Cancer Information System Project. Eur. J. Cancer Oxf. Engl. 1990 2006, 42, 2115–2123. [Google Scholar] [CrossRef] [PubMed]
- Bulterys, M.; Goodman, M.T.; Smith, M.A.; Buckley, J.D. Hepatic Tumors. In Cancer Incidence and Survival Among Children and Adolescents: United States SEER Program, 1975–1995; Reis, L.A.G., Smith, M.A., Gurney, J.G., Linet, M., Tamra, T., Young, J.L., Bunin, G.R., Eds.; NIH Pub. No. 99-4649; National Cancer Institute SEER Program: Bethesda, MD, USA, 1999; pp. 91–97. [Google Scholar]
- Delgado, P.I.; Correa-Medina, M.; Rojas, C.P. Pancreatic Hamartoma in a Premature Trisomy 18 Female. Autopsy Case Rep. 2017, 7, 26–29. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hosfield, B.D.; Grayson, B.L.; Nakeeb, A.; Albright, E.A.; Markel, T.A. Multicystic Adenomatoid Hamartoma of the Pancreas. J. Pediatr. Surg. Case Rep. 2019, 48, 101258. [Google Scholar] [CrossRef]
- Cui, H.; Lian, Y.; Chen, F. Imaging Findings for Pancreatic Hamartoma: Two Case Reports and a Review of the Literature. BMC Gastroenterol. 2020, 20, 37. [Google Scholar] [CrossRef]
- Santana Valenciano, Á.; Molina Villar, J.M.; Barranquero, A.G.; Sanjuanbenito Dehesa, A.; Fernández Cebrián, J.M. Pancreatic Hamartoma: A Rare and Benign Cause of Pancreatic Incidentaloma. Cir. Esp. 2021, S0009-739X(21)00071-3. [Google Scholar] [CrossRef]
- Rohde, R.A.; Hodgman, J.E.; Cleland, R.S. Multiple Congenital Anomalies in the E1-Trisomy (Group 16-18) Syndrome. Pediatrics 1964, 33, 258–270. [Google Scholar] [CrossRef]
- Smith, D.W.; Patau, K.; Therman, E.; Inhorn, S.L. A New Autosomal Trisomy Syndrome: Multiple Congenital Anomalies Caused by an Extra Chromosome. J. Pediatr. 1960, 57, 338–345. [Google Scholar] [CrossRef]
- Burt, T.B.; Condon, V.R.; Matlak, M.E. Fetal Pancreatic Hamartoma. Pediatr. Radiol. 1983, 13, 287–289. [Google Scholar] [CrossRef] [PubMed]
- Flaherty, M.J.; Benjamin, D.R. Multicystic Pancreatic Hamartoma: A Distinctive Lesion with Immunohistochemical and Ultrastructural Study. Hum. Pathol. 1992, 23, 1309–1312. [Google Scholar] [CrossRef]
- Sepulveda, W.; Carstens, E.; Sanchez, J.; Gutierrez, J. Prenatal Diagnosis of Congenital Pancreatic Cyst: Case Report and Review of the Literature. J. Ultrasound Med. Off. J. Am. Inst. Ultrasound Med. 2000, 19, 349–352. [Google Scholar] [CrossRef]
- Thrall, M.; Jessurun, J.; Stelow, E.B.; Adsay, N.V.; Vickers, S.M.; Whitson, A.K.; Saltzman, D.A.; Pambuccian, S.E. Multicystic Adenomatoid Hamartoma of the Pancreas: A Hitherto Undescribed Pancreatic Tumor Occurring in a 3-Year-Old Boy. Pediatr. Dev. Pathol. Off. J. Soc. Pediatr. Pathol. Paediatr. Pathol. Soc. 2008, 11, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Sueyoshi, R.; Okazaki, T.; Lane, G.J.; Arakawa, A.; Yao, T.; Yamataka, A. Multicystic Adenomatoid Pancreatic Hamartoma in a Child: Case Report and Literature Review. Int. J. Surg. Case Rep. 2012, 4, 98–100. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.Y.; Lee, Y.H. Fetal Tumors: Prenatal Ultrasonographic Findings and Clinical Characteristics. Ultrasonography 2014, 33, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Birkemeier, K.L. Imaging of Solid Congenital Abdominal Masses: A Review of the Literature and Practical Approach to Image Interpretation. Pediatr. Radiol. 2020, 50, 1907–1920. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, J.Y.; Song, M.J.; Min, J.Y.; Han, B.H.; Lee, Y.H.; Cho, B.J.; Kim, S.H. Prenatal Ultrasound Findings of Fetal Neoplasms. Korean J. Radiol. 2002, 3, 64–73. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sebire, N.J.; Jauniaux, E. Fetal and Placental Malignancies: Prenatal Diagnosis and Management. Ultrasound Obstet. Gynecol. 2009, 33, 235–244. [Google Scholar] [CrossRef] [PubMed]
- Kamil, D.; Tepelmann, J.; Berg, C.; Heep, A.; Axt-Fliedner, R.; Gembruch, U.; Geipel, A. Spectrum and Outcome of Prenatally Diagnosed Fetal Tumors. Ultrasound Obstet. Gynecol. Off. J. Int. Soc. Ultrasound Obstet. Gynecol. 2008, 31, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Amari, F.; Beyer, D.A.; Diedrich, K.; Weichert, J. Fetal Intra-Abdominal Tumors: Assessment of Spectrum, Accuracy of Prenatal Diagnosis, Perinatal Outcome and Therapy at a Tertiary Referral Center. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013, 167, 160–166. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh-Lotrario, A.D.; Chaiyachati, B.H.; Meyers, R.L.; Häberle, B.; Tomlinson, G.E.; Katzenstein, H.M.; Malogolowkin, M.H.; von Schweinitz, D.; Krailo, M.; Feusner, J.H. Outcomes for Patients with Congenital Hepatoblastoma. Pediatr. Blood Cancer 2013, 60, 1817–1825. [Google Scholar] [CrossRef] [PubMed]
- Parkes, S.E.; Muir, K.R.; Southern, L.; Cameron, A.H.; Darbyshire, P.J.; Stevens, M.C. Neonatal Tumours: A Thirty-Year Population-Based Study. Med. Pediatr. Oncol. 1994, 22, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Matsushita, D.; Kurahara, H.; Mataki, Y.; Maemura, K.; Higashi, M.; Iino, S.; Sakoda, M.; Shinchi, H.; Ueno, S.; Natsugoe, S. Pancreatic Hamartoma: A Case Report and Literature Review. BMC Gastroenterol. 2016, 16, 3. [Google Scholar] [CrossRef] [PubMed]
- Spector, L.G.; Puumala, S.E.; Carozza, S.E.; Chow, E.J.; Fox, E.E.; Horel, S.; Johnson, K.J.; McLaughlin, C.C.; Reynolds, P.; Behren, J.V.; et al. Cancer Risk among Children with Very Low Birth Weights. Pediatrics 2009, 124, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Evers, C.; Gaspar, H.; Kloor, M.; Bozukova, G.; Kadmon, M.; Keller, M.; Sutter, C.; Moog, U. Hepatoblastoma in Two Siblings and Familial Adenomatous Polyposis: Causal Nexus or Coincidence? Fam. Cancer 2012, 11, 529–533. [Google Scholar] [CrossRef] [PubMed]
- Trobaugh-Lotrario, A.D.; Venkatramani, R.; Feusner, J.H. Hepatoblastoma in Children with Beckwith-Wiedemann Syndrome: Does It Warrant Different Treatment? J. Pediatr. Hematol. Oncol. 2014, 36, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Mussa, A.; Ferrero, G.B. Screening Hepatoblastoma in Beckwith-Wiedemann Syndrome: A Complex Issue. J. Pediatr. Hematol. Oncol. 2015, 37, 627. [Google Scholar] [CrossRef] [PubMed]
- Ziogas, I.A.; Benedetti, D.J.; Wu, W.K.; Matsuoka, L.K.; Izzy, M.; Rauf, M.A.; Pai, A.K.; Bailey, C.E.; Alexopoulos, S.P. Management of Hepatoblastoma in the United States: Can We Do Better? Surgery 2021, 170, 579–586. [Google Scholar] [CrossRef]
- Hyett, J. Intra-Abdominal Masses: Prenatal Differential Diagnosis and Management. Prenat. Diagn. 2008, 28, 645–655. [Google Scholar] [CrossRef] [PubMed]


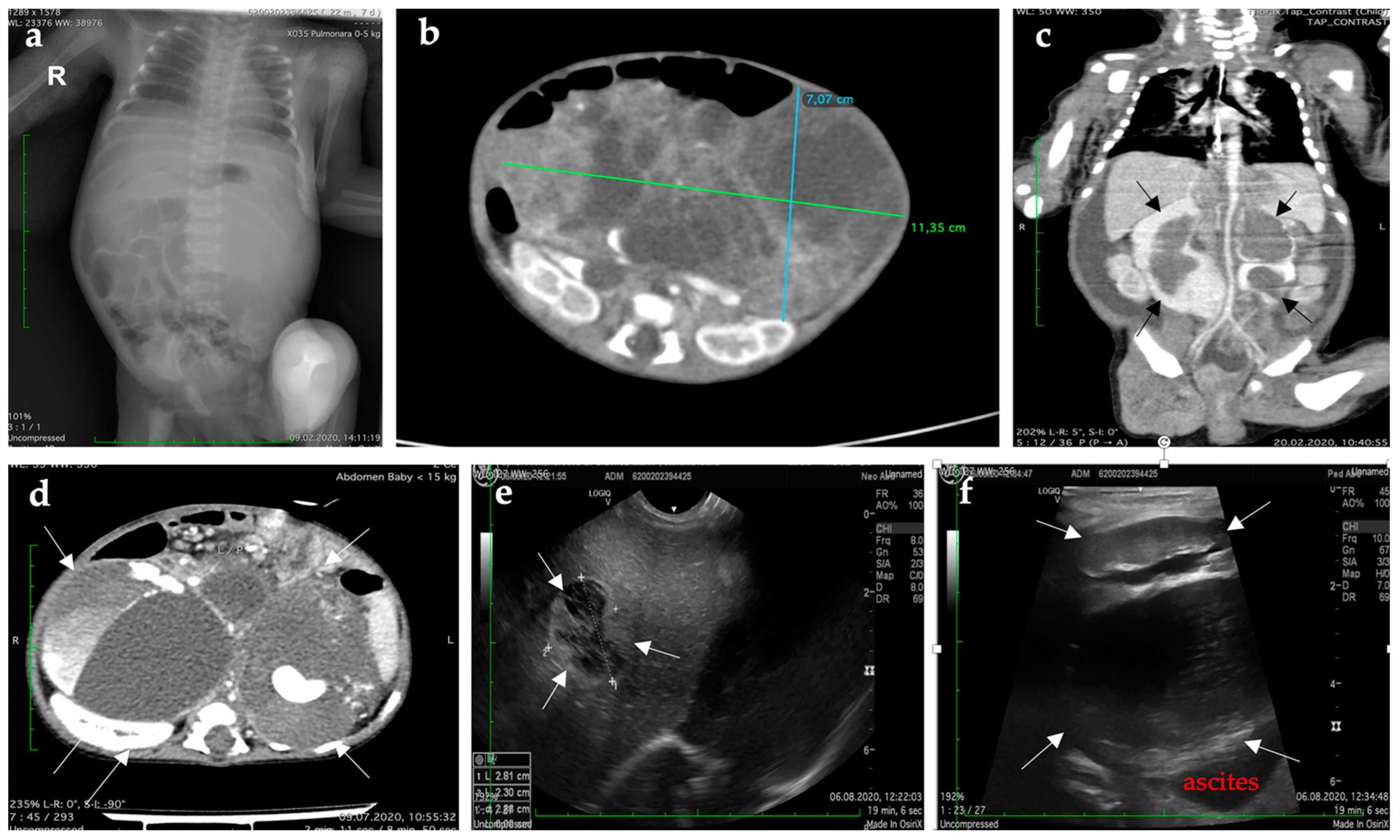
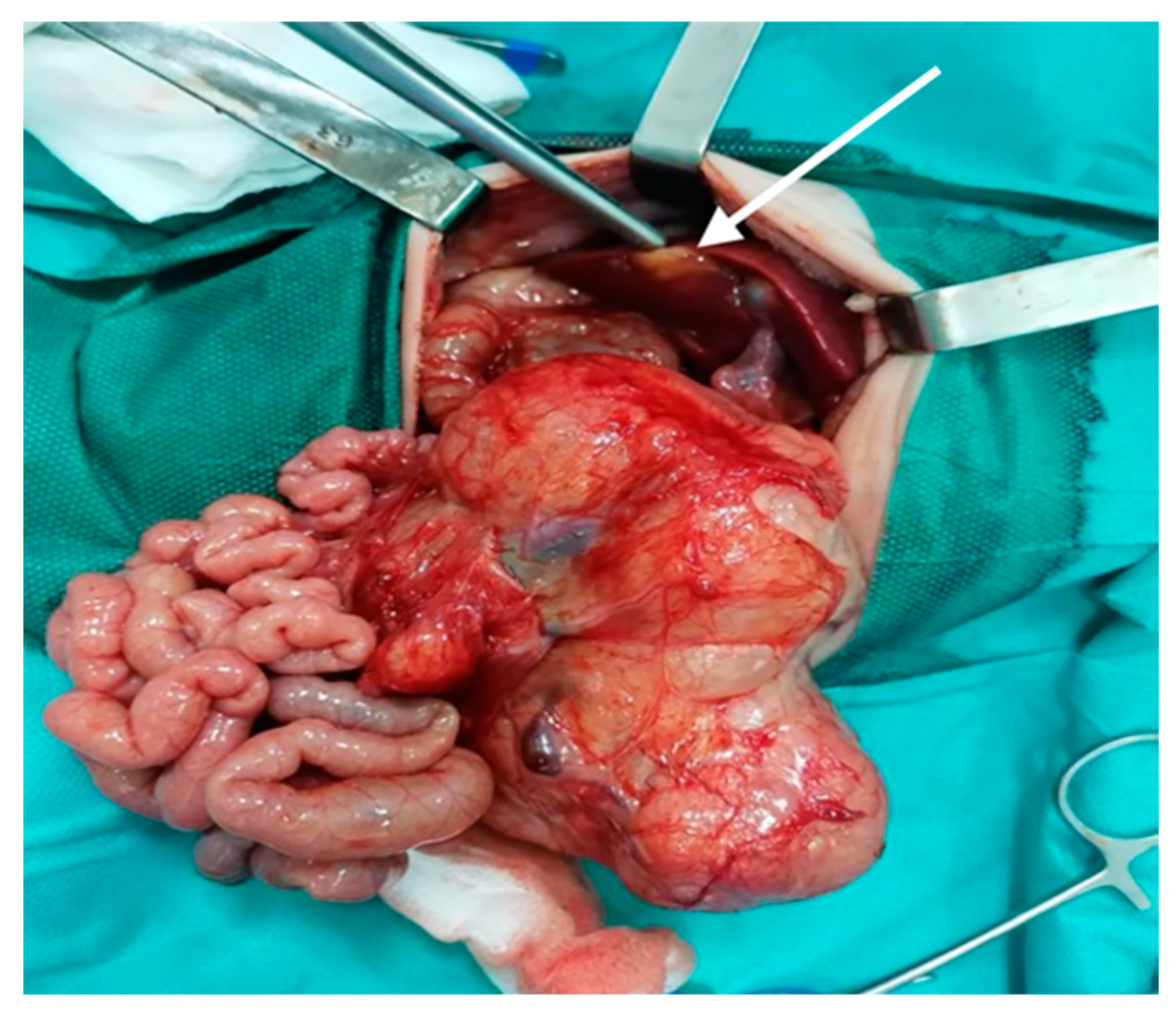

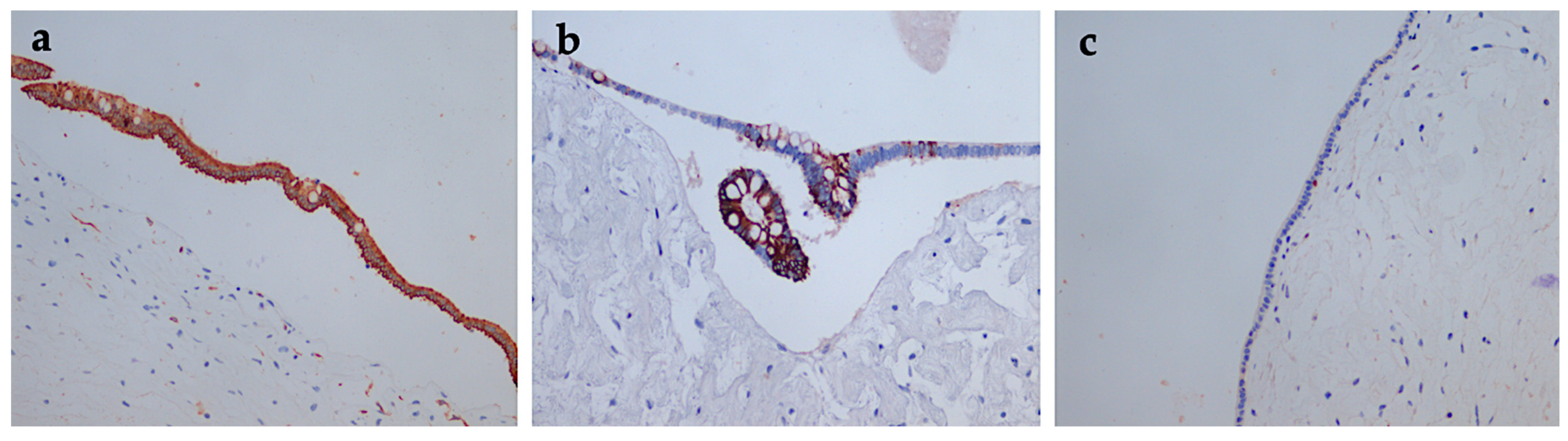
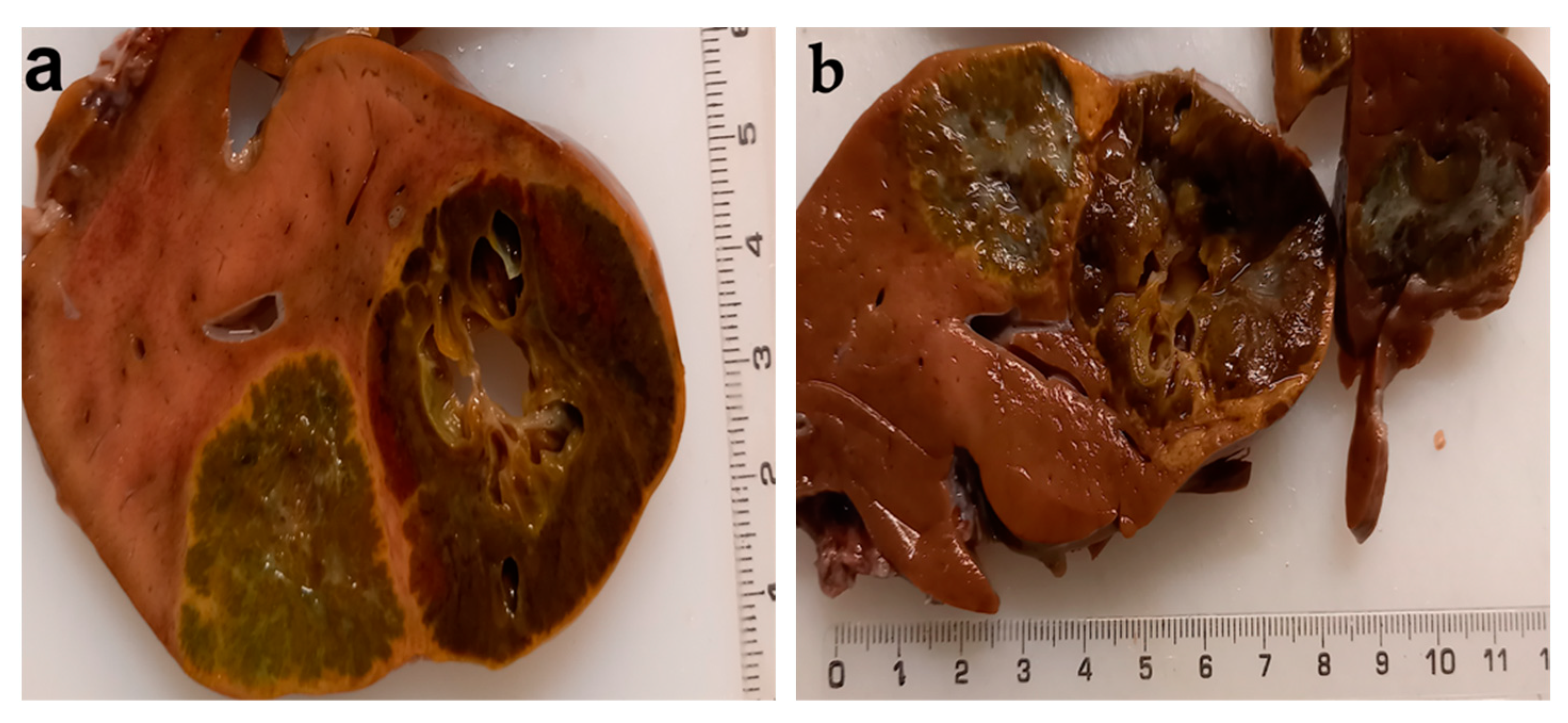
| Type of Exam | Age | Imagistic Findings |
|---|---|---|
| Abdominal ultrasound | 25th week |
|
| Abdominal ultrasound | 29th week |
|
| Author (Year) [Ref] | Age | Sex | Clinical Features of the Tumor | Management and Prognosis | |
|---|---|---|---|---|---|
| 1 | Smith (1960) [12] | 2 m | F | Solid, tail | Trisomy 18 |
| 2 | Rohde (1964) [11] | 2 m | F | Cysts | Trisomy 18 |
| 3 | Burt (1983) [13] | 34 w | F | Solid and cystic, 11.5 cm, diffuse | PD and splenectomy/alive 3 m |
| 4 | Flaherthy (1992) [14] | 20 m | F | Solid and cystic, 9 cm, head | Local resection/alive 9 m |
| 5 | Sepulveda (2000) [15] | 27 w | M | Large multicystic, 12 cm, diffuse | Excision of the tumor and partial duodenectomy/at 1 year of age, he remains symptom-free |
| 6 | Thrall (2007) [16] | 3 y | M | Multicystic adenomatoid, 3 cm, head | PD |
| 7 | Sueyoshi (2009) [17] | 14 m | M | Multicystic adenomatoid, 14 cm, tail | Local resection/alive 26 m |
| 8 | Delgado (2017) [7] | 33 w | F | Cysts, 1.2 cm | Trisomy 18/alive 1 h |
| 9 | Hosfield (2019) [8] | 4 y | M | Multicystic adenomatoid, 9.5 cm, head | PD/alive 3 m |
| 10 | Present case (2020) | 36 w | F | Multicystic adenomatoid, 11 cm, diffuse | Local resection/alive 11 m |
| Imagistic Features | Prenatal Diagnosis | |
|---|---|---|
| Pancreas | ||
| Hamartoma |
| 2nd and 3rd trimesters |
| Adenocarcinoma |
| NA |
| Neuroendocrine tumor |
| NA |
| LIVER | ||
| Hemangioma |
| 3rd trimester |
| Mesenchymal hamartoma |
| 2nd and 3rd trimesters |
| Hepatoblastoma |
| late 3rd trimester |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Varlas, V.; Neagu, O.; Moga, A.; Bălănescu, R.; Bohiltea, R.; Vladareanu, R.; Balanescu, L. Fetal Pancreatic Hamartoma Associated with Hepatoblastoma—An Unusual Tumor Association. Diagnostics 2022, 12, 758. https://doi.org/10.3390/diagnostics12030758
Varlas V, Neagu O, Moga A, Bălănescu R, Bohiltea R, Vladareanu R, Balanescu L. Fetal Pancreatic Hamartoma Associated with Hepatoblastoma—An Unusual Tumor Association. Diagnostics. 2022; 12(3):758. https://doi.org/10.3390/diagnostics12030758
Chicago/Turabian StyleVarlas, Valentin, Oana Neagu, Andreea Moga, Radu Bălănescu, Roxana Bohiltea, Radu Vladareanu, and Laura Balanescu. 2022. "Fetal Pancreatic Hamartoma Associated with Hepatoblastoma—An Unusual Tumor Association" Diagnostics 12, no. 3: 758. https://doi.org/10.3390/diagnostics12030758
APA StyleVarlas, V., Neagu, O., Moga, A., Bălănescu, R., Bohiltea, R., Vladareanu, R., & Balanescu, L. (2022). Fetal Pancreatic Hamartoma Associated with Hepatoblastoma—An Unusual Tumor Association. Diagnostics, 12(3), 758. https://doi.org/10.3390/diagnostics12030758








