Prognostic Nomograms for Hospital Survival and Transplant-Free Survival of Patients with Hepatorenal Syndrome: A Retrospective Cohort Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cohort Construction
2.2. Statistical Analysis
3. Results
3.1. Demographic and Clinicopathological Characteristics
3.2. Model Specifications, and Predictors of Hospital Survival and Transplant-Free Survival
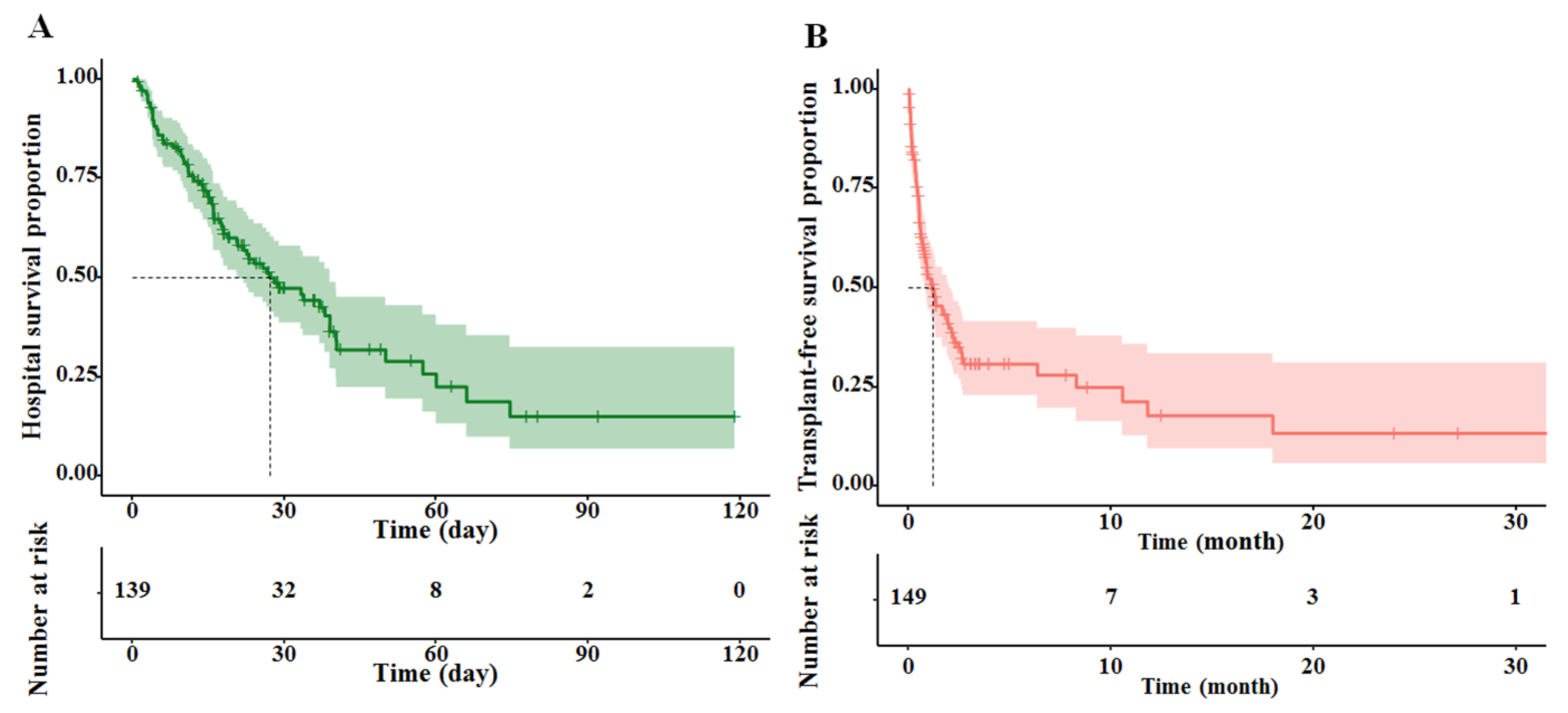
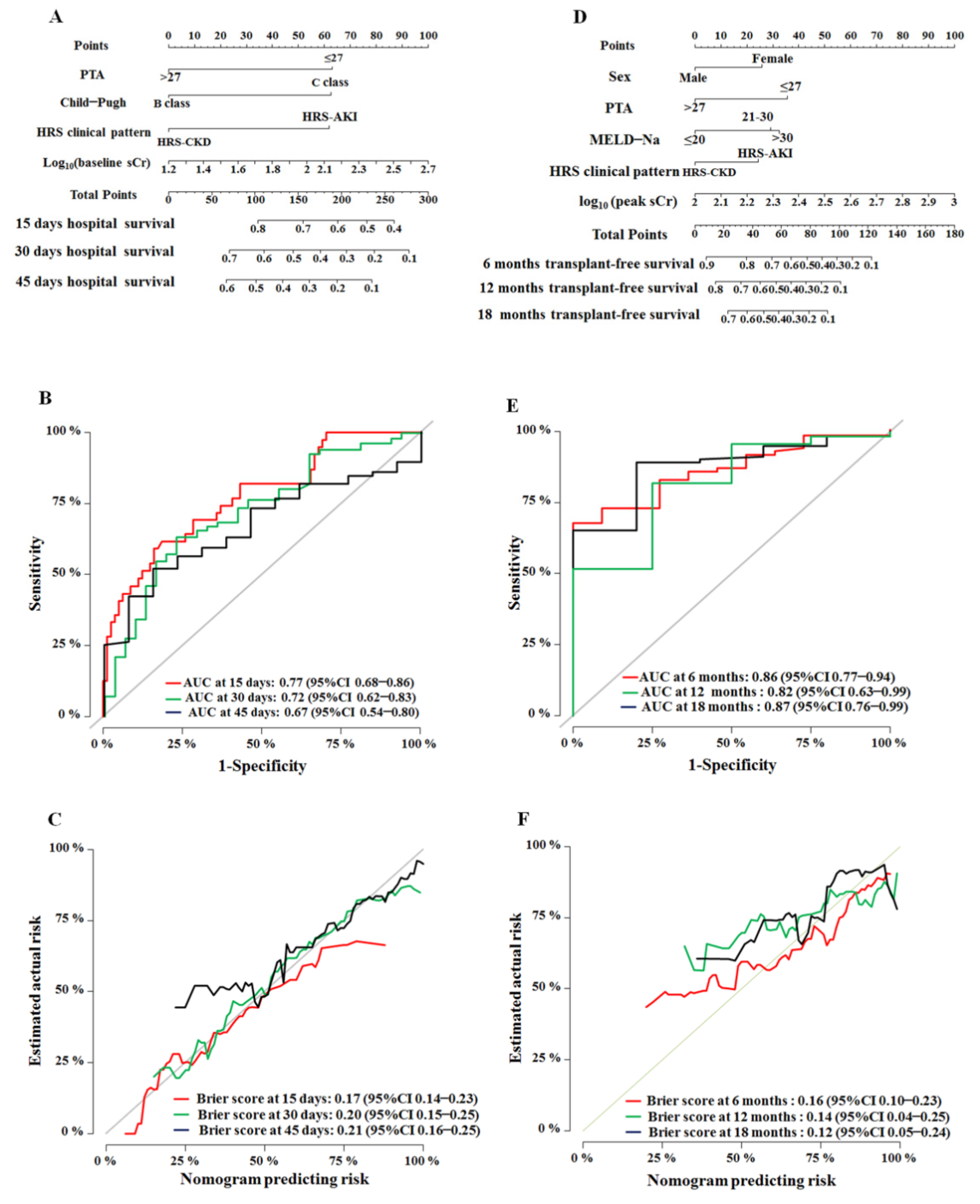
3.3. Development and Internal Validation of the Nomograms
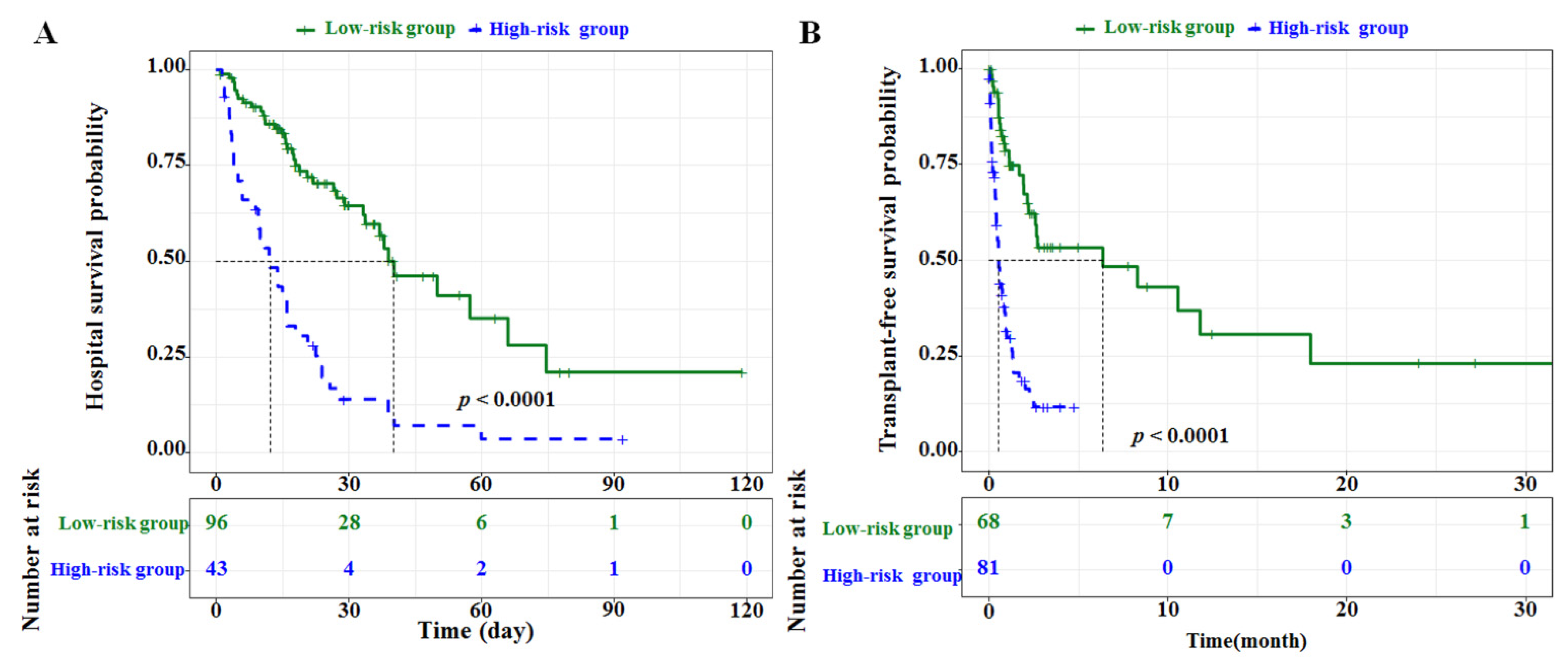
3.4. Performance of Nomograms
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ginès, P.; Solà, E.; Angeli, P.; Wong, F.; Nadim, M.K.; Kamath, P.S. Hepatorenal syndrome. Nat. Rev. Dis. Primers. 2018, 4, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Wong, F.; Pappas, S.C.; Curry, M.P.; Reddy, K.R.; Rubin, R.A.; Porayko, M.K.; Gonzalez, S.A.; Mumtaz, K.; Lim, N.; Simonetto, D.A.; et al. Terlipressin plus albumin for the treatment of type 1 hepatorenal syndrome. N. Engl. J. Med. 2021, 384, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Bashir, M.H.; Iqbal, S.; Miller, R.; Singh, J.; Mubarak, G.; Likhtshteyn, M.; Bigajer, E.; Gallagher, B.; Hurairah, A.; Stefanov, D.; et al. Management and outcomes of hepatorenal syndrome at an urban academic medical center: A retrospective study. Eur. J. Gastroenterol. Hepatol. 2019, 31, 1545–1549. [Google Scholar] [CrossRef]
- Durand, F.; Francoz, C.; Asrani, S.K.; Khemichian, S.; Pham, T.A.; Sung, R.S.; Genyk, Y.S.; Nadim, M.K. Acute kidney injury after liver transplantation. Transplantation 2018, 102, 1636–1649. [Google Scholar] [CrossRef] [PubMed]
- Alessandria, C.; Ozdogan, O.; Guevara, M.; Restuccia, T.; Jiménez, W.; Arroyo, V.; Rodés, J.; Ginès, P. MELD score and clinical type predict prognosis in hepatorenal syndrome: Relevance to liver transplantation. Hepatology 2005, 41, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute kidney injury and chronic kidney disease as interconnected syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [Green Version]
- Huelin, P.; Solà, E.; Elia, C.; Solé, C.; Risso, A.; Moreira, R.; Carol, M.; Fabrellas, N.; Bassegoda, O.; Juanola, A.; et al. Neutrophil gelatinase-associated lipocalin for assessment of acute kidney injury in cirrhosis: A prospective study. Hepatology 2019, 70, 319–333. [Google Scholar] [CrossRef]
- Piano, S.; Rosi, S.; Maresio, G.; Fasolato, S.; Cavallin, M.; Romano, A.; Morando, F.; Gola, E.; Frigo, A.C.; Gatta, A.; et al. Evaluation of the acute kidney injury network criteria in hospitalized patients with cirrhosis and ascites. J. Hepatol. 2013, 59, 482–489. [Google Scholar] [CrossRef]
- Fagundes, C.; Barreto, R.; Guevara, M.; Garcia, E.; Solà, E.; Rodríguez, E.; Graupera, I.; Ariza, X.; Pereira, G.; Alfaro, I.; et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J. Hepatol. 2013, 59, 474–481. [Google Scholar] [CrossRef]
- Huelin, P.; Piano, S.; Solà, E.; Stanco, M.; Solé, C.; Moreira, R.; Pose, E.; Fasolato, S.; Fabrellas, N.; de Prada, G.; et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin. Gastroenterol. Hepatol. 2017, 15, 438–445. [Google Scholar] [CrossRef] [Green Version]
- Tsien, C.D.; Rabie, R.; Wong, F. Acute kidney injury in decompensated cirrhosis. Gut 2013, 62, 131–137. [Google Scholar] [CrossRef]
- Mauro, E.; Garcia-Olveira, L.; Gadano, A. End-stage liver disease: Management of hepatorenal syndrome. Liver Int. 2021, 1, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Salerno, F.; Cazzaniga, M.; Merli, M.; Spinzi, G.; Saibeni, S.; Salmi, A.; Fagiuoli, S.; Spadaccini, A.; Trotta, E.; Laffi, G.; et al. Diagnosis, treatment and survival of patients with hepatorenal syndrome: A survey on daily medical practice. J. Hepatol. 2011, 55, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Barreto, R.; Fagundes, C.; Guevara, M.; Solà, E.; Pereira, G.; Rodríguez, E.; Graupera, I.; Martín-Llahí, M.; Ariza, X.; Cárdenas, A.; et al. Type-1 hepatorenal syndrome associated with infections in cirrhosis: Natural history, outcome of kidney function, and survival. Hepatology 2014, 59, 1505–1513. [Google Scholar] [CrossRef]
- Sheng, X.Y.; Lin, F.Y.; Wu, J.; Cao, H.C. Development and validation of a prognostic model for patients with hepatorenal syndrome: A retrospective cohort study. World J. Gastroenterol. 2021, 27, 2615–2629. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL Clinical practice guidelines for the management of patients with decompensated cirrhosis. J. Hepatol. 2018, 69, 406–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biggins, S.W.; Angeli, P.; Garcia-Tsao, G.; Ginès, P.; Ling, S.C.; Nadim, M.K.; Wong, F.; Kim, W.R. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1014–1048. [Google Scholar] [CrossRef]
- Licata, A.; Maida, M.; Bonaccorso, A.; Macaluso, F.S.; Cappello, M.; Craxì, A.; Almasio, P.L. Clinical course and prognostic factors of hepatorenal syndrome: A retrospective single-center cohort study. World J. Hepatol. 2013, 5, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; He, L.L.; Wang, X.H.; Dang, Z.B.; Liu, X.L.; Li, M.G.; Wang, X.B.; Yang, Z.Y. A novel scoring model for predicting mortality risk in patients with cirrhosis and hepatorenal syndrome. Eur. J. Gastroenterol. Hepatol. 2018, 30, 938–943. [Google Scholar] [CrossRef]
- Yang, Y.W.; Wu, C.H.; Hu, R.H.; Ho, M.C.; Tsai, M.K.; Wu, Y.M.; Lee, P.H. Longitudinal assessment of prognostic factors for patients with hepatorenal syndrome in a tertiary center. Hepatol. Int. 2010, 4, 507–510. [Google Scholar] [CrossRef] [Green Version]
- Rey, R.M.; Delgado, A.F.; De Zubiria, A.; Pinto, R.; De la Hoz-Valle, J.A.; Pérez-Riveros, E.D.; Ardila, G.; Sierra-Arango, F. Prevalence and short-term outcome of hepatorenal syndrome: A 9-year experience in a high-complexity hospital in Colombia. PLoS ONE 2020, 15, e0239834. [Google Scholar] [CrossRef] [PubMed]
- Sasso, R.; Abou Yassine, A.; Deeb, L. Predictors of development of hepatorenal syndrome in hospitalized cirrhotic patients with acute kidney injury. J. Clin. Med. 2021, 10, 5621. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, T.; Zhan, L.; Jia, Z.; Luo, T.; Chen, S.; Zhao, Q.; Guo, Z.; He, X.; Wang, D. Clinical outcomes of liver transplantation in patients with hepatorenal syndrome: A single center study in China. Front. Surg. 2022, 8, 781648. [Google Scholar] [CrossRef] [PubMed]
- Colle, I.; Durand, F.; Pessione, F.; Rassiat, E.; Bernuau, J.; Barrière, E.; Lebrec, D.; Valla, D.C.; Moreau, R. Clinical course, predictive factors and prognosis in patients with cirrhosis and type 1 hepatorenal syndrome treated with terlipressin: A retrospective analysis. J. Gastroenterol. Hepatol. 2002, 17, 882–888. [Google Scholar] [CrossRef]
- Zhang, X.F.; Lv, Y. Liver Transplant for Patients with Hepato-cellular carcinoma and Child–Pugh C liver function should be mentioned in guidelines. Liver Cancer 2019, 8, 522–523. [Google Scholar] [CrossRef]
- Marroni, C.P.; de Mello Brandão, A.B.; Hennigen, A.W.; Marroni, C.; Zanotelli, M.L.; Cantisani, G.; Fuchs, S.C. MELD scores with incorporation of serum sodium and death prediction in cirrhotic patients on the waiting list for liver transplantation: A single center experience in southern Brazil. Clin. Transplant. 2012, 26, E395–E401. [Google Scholar] [CrossRef]
- Maassel, N.L.; Fleming, M.M.; Luo, J.; Zhang, Y.; Pei, K.Y. Model for end-stage liver disease sodium as a predictor of surgical risk in cirrhotic patients with ascites. J. Surg. Res. 2020, 250, 45–52. [Google Scholar] [CrossRef]
- Goudsmit, B.F.J.; Putter, H.; Tushuizen, M.E.; de Boer, J.; Vogelaar, S.; Alwayn, I.P.J.; van Hoek, B.; Braat, A.E. Validation of the model for end-stage liver disease sodium (MELD–Na) score in the Eurotransplant region. Am. J. Transplant. 2021, 21, 229–240. [Google Scholar] [CrossRef]
- Lai, J.C.; Covinsky, K.E.; Dodge, J.L.; Boscardin, W.J.; Segev, D.L.; Roberts, J.P.; Feng, S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017, 66, 564–574. [Google Scholar] [CrossRef]
- Bucsics, T.; Krones, E. Renal dysfunction in cirrhosis: Acute kidney injury and the hepatorenal syndrome. Gastroenterol. Rep. 2017, 5, 127–137. [Google Scholar] [CrossRef]
- Steyerberg, E.W.; Bleeker, S.E.; Moll, H.A.; Grobbee, D.E.; Moons, K.G. Internal and external validation of predictive models: A simulation study of bias and precision in small samples. J. Clin. Epidemiol. 2003, 56, 441–447. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How to build and interpret a nomogram for cancer prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Balachandran, V.P.; Gonen, M.; Smith, J.J.; DeMatteo, R.P. Nomograms in oncology: More than meets the eye. Lancet Oncol. 2015, 16, 71116–71117. [Google Scholar] [CrossRef] [Green Version]

| Characteristics | Total (n = 149) |
|---|---|
| Age (year), mean ± SD | 55.01 ± 12.71 |
| Sex (male/female) | 109/40 |
| Causes of cirrhosis | |
| Viral hepatitis | 65 (43.62%) |
| Alcoholic | 48 (32.22%) |
| Others | 36 (24.16%) |
| Mean arterial pressure (mmHg), median (MIN, MAX) | 83 (52–121) |
| Albumin (g/L), mean ± SD | 25.94 ± 4.75 |
| Serum total bilirubin (μmol/L), median (MIN, MAX) | 140.0 (9.5–815.0) |
| Serum sodium (mmol/L), median (MIN, MAX) | 133.0 (110.7–150.1) |
| Prothrombin activity (%), mean ± SD | 38.95 ± 18.68 |
| Serum NH3 (μmol/L), median (MIN, MAX) | 79 (16–483) |
| Spontaneous bacterial peritonitis | 17 (11.40%) |
| Hemoglobin (g/L), median (MIN, MAX) | 83 (50–145) |
| Clinical pattern of HRS (HRS-AKI/CKD) | 128/21 |
| Gastrointestinal bleeding | 43 (28.86%) |
| Child–Pugh score (B class/C class) | 20/129 |
| MELD–Na score, median (MIN, MAX) | 28 (11–51) |
| Baseline serum creatinine (μmol/L), median (MIN, MAX) | 90.5 (34.5–354.2) |
| Peak serum creatinine (μmol/L), median (MIN, MAX) | 211.5 (136.2–680.6) |
| Treatment strategy | |
| Terlipressin in combination with albumin | 55 (36.91%) |
| Norepinephrine in combination with albumin | 27 (18.12%) |
| Midodrine plus octreotide in combination with albumin | 28 (18.79%) |
| Renal replacement therapy | 26 (17.45%) |
| Liver transplantation | 13 (8.72%) |
| Variables | Hospital Survival | Transplant-Free Survival | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |||||
| HR (95%CI) | p Value | HR (95%CI) | p Value | HR (95%CI) | p Value | HR (95%CI) | p Value | |
| Age | 0.99 (0.98–1.01) | 0.722 | 1.00 (0.98–1.02) | 0.931 | ||||
| Sex (female/male) | 1.08 (0.66–1.77) | 0.782 | 1.29 (0.82–2.03) | 0.027 | 1.54 (0.95–2.50) | 0.077 | ||
| MAP (mmHg) | ||||||||
| >70 vs. ≤70 | 0.81 (0.42–1.54) | 0.523 | 0.72 (0.39–1.32) | 0.288 | ||||
| Albumin (g/L) | ||||||||
| >30 vs. ≤30 | 1.76 (1.00–3.08) | 0.048 | 1.68 (0.99–2.87) | 0.057 | ||||
| Total bilirubin (µmol/L) | ||||||||
| >110 vs. ≤110 | 2.05 (1.26–3.36) | 0.004 | 2.24 (1.44–3.48) | <0.001 | ||||
| Serum sodium (mmol/L) | ||||||||
| >130 vs. ≤130 | 1.07 (0.66–1.74) | 0.79 | 0.89 (0.572–1.38) | 0.604 | ||||
| Prothrombin activity (%) | ||||||||
| >27 vs. ≤27 | 0.34 (0.22–0.55) | <0.001 | 0.44 (0.27–0.73) | 0.001 | 0.29 (0.18–0.45) | <0.001 | 0.41 (0.22–0.77) | 0.006 |
| Serum NH3 (µmol/L) | ||||||||
| >100 vs. ≤100 | 1.56 (0.96–2.51) | 0.071 | 1.71 (1.09–2.68) | 0.020 | ||||
| Spontaneous bacterial peritonitis | ||||||||
| Yes vs. no | 0.77 (0.37–1.62) | 0.492 | 0.77 (0.40–1.50) | 0.451 | ||||
| Hemoglobin (g/L) | ||||||||
| >60 vs. ≤60 | 1.11 (0.62–1.99) | 0.721 | 0.94 (0.56–1.58) | 0.821 | ||||
| HRS clinical pattern | ||||||||
| HRS-CKD vs. HRS-AKI | 0.52 (0.24–1.13) | 0.009 | 0.45 (0.202–1.023) | 0.056 | 0.55 (0.36–1.22) | 0.002 | 0.51 (0.26–0.98) | 0.045 |
| Gastrointestinal bleeding | ||||||||
| Yes vs. no | 1.27 (0.79–2.02) | 0.310 | 1.52 (0.98–2.34) | 0.039 | ||||
| Child–Pugh class | ||||||||
| C vs. B | 1.17 (0.58–2.35) | 0.0001 | 1.49 (0.83–2.68) | 0.045 | 1.31 (0.697–2.47) | 0.0801 | ||
| MELD–Na score | ||||||||
| 21–30 vs. ≤20 | 1.45 (0.69–3.05) | 0.033 | 1.67 (0.87–3.19) | 0.001 | 1.78 (0.92–3.44) | 0.078 | ||
| >30 vs. ≤20 | 3.56 (1.70–7.45) | 0.024 | 4.46 (2.30–8.63) | <0.001 | 2.27 (1.02–5.07) | 0.005 | ||
| Baseline serum creatinine (µmol/L), log10 | 1.70 (0.584–4.96) | 0.0034 | 2.18(0.72–6.62) | 0.050 | 1.31 (0.49–3.53) | 0.019 | ||
| Peak serum creatinine (µmol/L), log10 | NA | NA | NA | NA | 8.32 (3.02–22.97) | <0.001 | 14.72 (4.66–46.51) | <0.001 |
| Vasoconstrictor treatment | ||||||||
| Norepinephrine with albumin vs. terlipressin with albumin | 1.35 (0.76–2.66) | 0.407 | 1.00 (0.54–1.85) | 0.997 | ||||
| Midodrine plus octreotide with albumin vs. terlipressin with albumin | 2.11 (1.09–4.09) | 0.527 | 1.61 (0.88–2.93) | 0.124 | ||||
| Actual Survival Probability Based on Kaplan–Meier Curves | Predicted Survival Probability Calculated from Nomograms | ||
|---|---|---|---|
| Hospital survival | |||
| 15d | High-risk group | 40.70% (95% CI, 28.03–59.20%) | 41.25% (95% CI, 33.57–48.89%) |
| Low-risk group | 83.40% (95% CI, 76.06–91.50%) | 82.71% (95% CI, 80.93–85.33%) | |
| 30d | High-risk group | 14.00% (95% CI, 6.32–31.00%) | 14.63% (95% CI, 9.41–19.84%) |
| Low-risk group | 64.60% (95% CI, 54.23–76.90%) | 63.70% (95% CI, 59.06–68.34%) | |
| 45d | High-risk group | 7.00% (95% CI, 1.98–24.7%) | 5.74% (95% CI, 2.74–8.74%) |
| Low-risk group | 46.30% (95% CI, 33.37–64.20%) | 49.36% (95% CI, 44.24–52.23%) | |
| Transplant-free survival | |||
| 6m | High-risk group | NA † | 11.33% (95% CI, 6.61–16.06%) |
| Low-risk group | 53.4% (95% CI, 40.33–70.70%) | 56.50% (95% CI, 48.67–64.32%) | |
| 12m | High-risk group | NA | 2.04% (95% CI, 0.09–3.98%) |
| Low-risk group | 30.80% (95% CI, 16.55–57.40%) | 31.69% (95% CI, 22.93–40.52%) | |
| 18m | High-risk group | NA | 0.73% (95% CI, 0.00–1.88%) |
| Low-risk group | 23.10% (95% CI, 9.97–53.60%) | 27.59% (95% CI, 19.03–36.16%) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.; Wang, Y.; Zang, C.; Yang, X.; Li, Z.; Wu, L.; Li, K. Prognostic Nomograms for Hospital Survival and Transplant-Free Survival of Patients with Hepatorenal Syndrome: A Retrospective Cohort Study. Diagnostics 2022, 12, 1417. https://doi.org/10.3390/diagnostics12061417
Song Y, Wang Y, Zang C, Yang X, Li Z, Wu L, Li K. Prognostic Nomograms for Hospital Survival and Transplant-Free Survival of Patients with Hepatorenal Syndrome: A Retrospective Cohort Study. Diagnostics. 2022; 12(6):1417. https://doi.org/10.3390/diagnostics12061417
Chicago/Turabian StyleSong, Yi, Yu Wang, Chaoran Zang, Xiaoxi Yang, Zhenkun Li, Lina Wu, and Kang Li. 2022. "Prognostic Nomograms for Hospital Survival and Transplant-Free Survival of Patients with Hepatorenal Syndrome: A Retrospective Cohort Study" Diagnostics 12, no. 6: 1417. https://doi.org/10.3390/diagnostics12061417





