The Low Expression of Fc-Gamma Receptor III (CD16) and High Expression of Fc-Gamma Receptor I (CD64) on Neutrophil Granulocytes Mark Severe COVID-19 Pneumonia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Statistics
3. Results
3.1. Study Population and Patient Characteristics
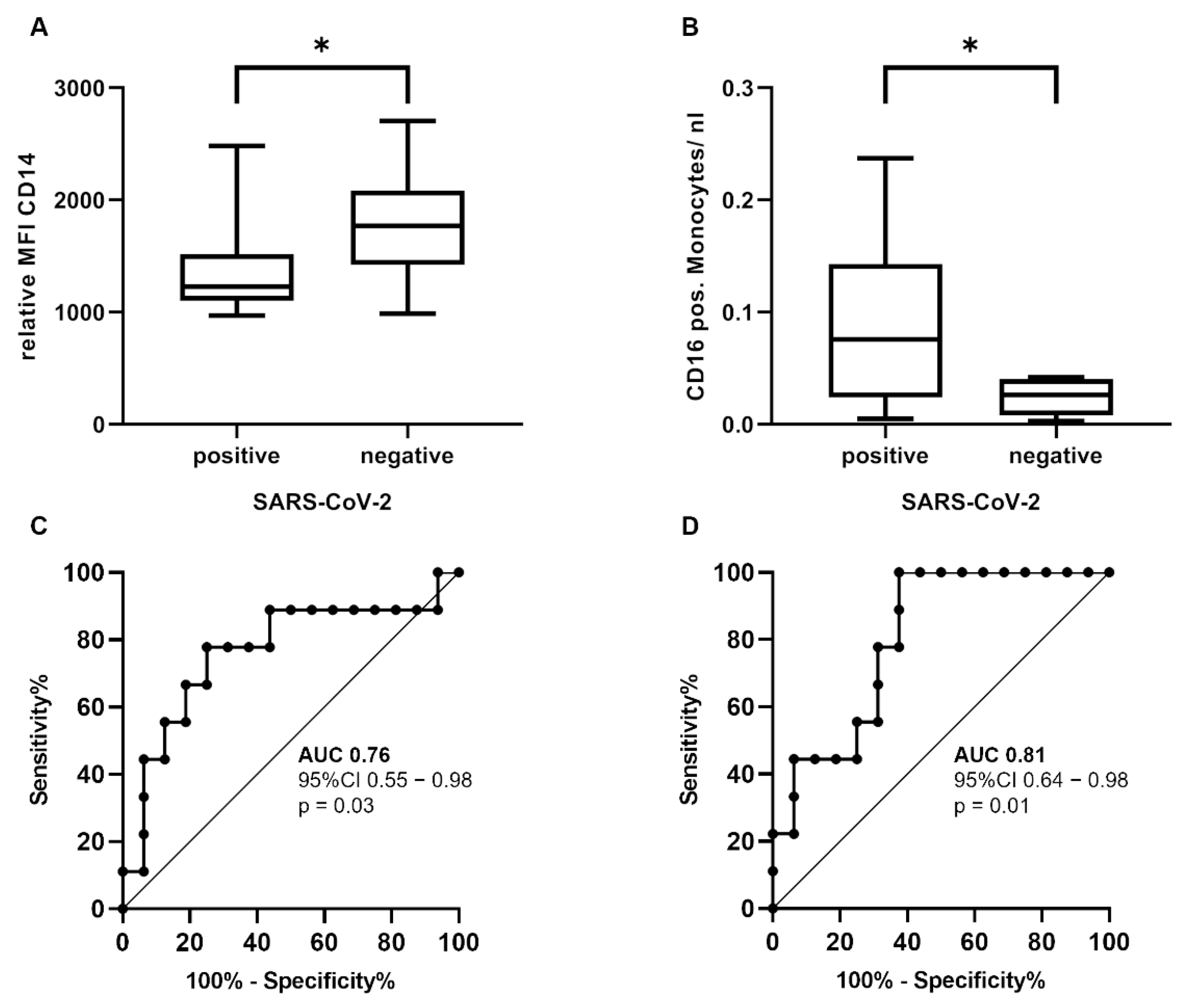
3.2. Immunological Differences between SARS-CoV-2-Positive and SARS-CoV-2-Negative Patients
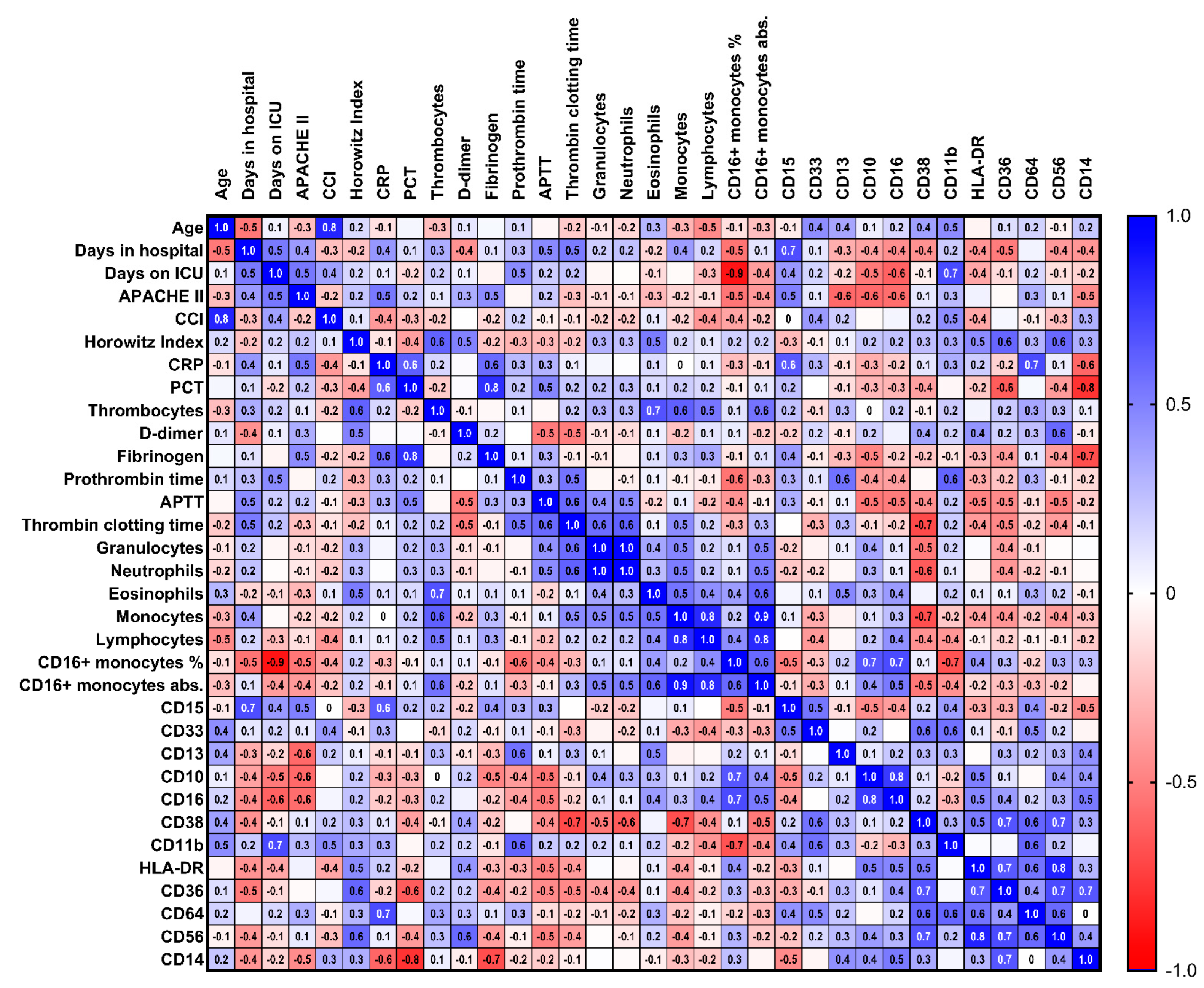
3.3. Immunological Characteristics of Severe COVID-19 Infection
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Weekly Epidemiological Update on COVID-19—3 August 2022. Available online: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---3-august-2022 (accessed on 8 August 2022).
- Stokes, E.K.; Zambrano, L.D.; Anderson, K.N.; Marder, E.P.; Raz, K.M.; El Burai Felix, S.; Tie, Y.; Fullerton, K.E. Coronavirus Disease 2019 Case Surveillance—United States, 22 January–30 May 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; de Villiers, T.; Van der Walt, Z.; et al. Decreased Severity of Disease during the First Global Omicron Variant COVID-19 Outbreak in a Large Hospital in Tshwane, South Africa. Int. J. Infect. Dis. 2022, 116, 38–42. [Google Scholar] [CrossRef]
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological Assessment of Hospitalized Patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L.; et al. Pathological Findings of COVID-19 Associated with Acute Respiratory Distress Syndrome. Lancet Respir. Med. 2020, 8, 420–422. [Google Scholar] [CrossRef]
- Lippi, G.; Mattiuzzi, C.; Sanchis-Gomar, F.; Henry, B.M. Clinical and Demographic Characteristics of Patients Dying from COVID-19 in Italy versus China. J. Med. Virol. 2020, 92, 1759. [Google Scholar] [CrossRef]
- Lighter, J.; Phillips, M.; Hochman, S.; Sterling, S.; Johnson, D.; Francois, F.; Stachel, A. Obesity in Patients Younger than 60 Years Is a Risk Factor for Covid-19 Hospital Admission. Clin. Infect. Dis. 2020, 71, 896–897. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA 2020, 323, 2052–2059. [Google Scholar] [CrossRef]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical Features of Patients Infected with 2019 Novel Coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Buszko, M.; Nita-Lazar, A.; Park, J.-H.; Schwartzberg, P.L.; Verthelyi, D.; Young, H.A.; Rosenberg, A.S. Lessons Learned: New Insights on the Role of Cytokines in COVID-19. Nat. Immunol. 2021, 22, 404–411. [Google Scholar] [CrossRef]
- Blanco-Melo, D.; Nilsson-Payant, B.E.; Liu, W.-C.; Uhl, S.; Hoagland, D.; Møller, R.; Jordan, T.X.; Oishi, K.; Panis, M.; Sachs, D.; et al. Imbalanced Host Response to SARS-CoV-2 Drives Development of COVID-19. Cell 2020, 181, 1036–1045.e9. [Google Scholar] [CrossRef] [PubMed]
- Lucas, C.; Wong, P.; Klein, J.; Castro, T.B.R.; Silva, J.; Sundaram, M.; Ellingson, M.K.; Mao, T.; Oh, J.E.; Israelow, B.; et al. Longitudinal Analyses Reveal Immunological Misfiring in Severe COVID-19. Nature 2020, 584, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, A.; Johow, J.; Mack, E.; Burchert, A.; Meyn, D.; Kadlubiec, A.; Torje, I.; Wulf, H.; Vogelmeier, C.F.; Hoyer, J.; et al. The Janus-Kinase Inhibitor Ruxolitinib in SARS-CoV-2 Induced Acute Respiratory Distress Syndrome (ARDS). Leukemia 2021, 35, 2917–2923. [Google Scholar] [CrossRef] [PubMed]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with COVID-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Gupta, S.; Wang, W.; Hayek, S.S.; Chan, L.; Mathews, K.S.; Melamed, M.L.; Brenner, S.K.; Leonberg-Yoo, A.; Schenck, E.J.; Radbel, J.; et al. Association Between Early Treatment With Tocilizumab and Mortality Among Critically Ill Patients With COVID-19. JAMA Intern. Med. 2021, 181, 41–51. [Google Scholar] [CrossRef]
- Kalil, A.C.; Patterson, T.F.; Mehta, A.K.; Tomashek, K.M.; Wolfe, C.R.; Ghazaryan, V.; Marconi, V.C.; Ruiz-Palacios, G.M.; Hsieh, L.; Kline, S.; et al. Baricitinib plus Remdesivir for Hospitalized Adults with COVID-19. N. Engl. J. Med. 2021, 384, 795–807. [Google Scholar] [CrossRef]
- Stone, J.H.; Frigault, M.J.; Serling-Boyd, N.J.; Fernandes, A.D.; Harvey, L.; Foulkes, A.S.; Horick, N.K.; Healy, B.C.; Shah, R.; Bensaci, A.M.; et al. Efficacy of Tocilizumab in Patients Hospitalized with COVID-19. N. Engl. J. Med. 2020, 383, 2333–2344. [Google Scholar] [CrossRef]
- Ghosn, L.; Chaimani, A.; Evrenoglou, T.; Davidson, M.; Graña, C.; Schmucker, C.; Bollig, C.; Henschke, N.; Sguassero, Y.; Nejstgaard, C.H.; et al. Interleukin-6 Blocking Agents for Treating COVID-19: A Living Systematic Review. Cochrane Database Syst. Rev. 2021, 2021, CD013881. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Y.; Xiang, P.; Pu, L.; Xiong, H.; Li, C.; Zhang, M.; Tan, J.; Xu, Y.; Song, R.; et al. Neutrophil-to-Lymphocyte Ratio Predicts Critical Illness Patients with 2019 Coronavirus Disease in the Early Stage. J. Transl. Med. 2020, 18, 206. [Google Scholar] [CrossRef]
- Zhang, B.; Zhou, X.; Zhu, C.; Song, Y.; Feng, F.; Qiu, Y.; Feng, J.; Jia, Q.; Song, Q.; Zhu, B.; et al. Immune Phenotyping Based on the Neutrophil-to-Lymphocyte Ratio and IgG Level Predicts Disease Severity and Outcome for Patients With COVID-19. Front. Mol. Biosci. 2020, 7, 157. [Google Scholar] [CrossRef]
- Passlick, B.; Flieger, D.; Ziegler-Heitbrock, H.W.L. Identification and Characterization of a Novel Monocyte Subpopulation in Human Peripheral Blood. Blood 1989, 74, 2527–2534. [Google Scholar] [CrossRef] [PubMed]
- Grage-Griebenow, E.; Zawatzky, R.; Kahlert, H.; Brade, L.; Flad, H.-D.; Ernst, M. Identification of a Novel Dendritic Cell-like Subset of CD64+/CD16+ Blood Monocytes. Eur. J. Immunol. 2001, 31, 48–56. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of Monocytes and Dendritic Cells in Blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Zawada, A.M.; Rogacev, K.S.; Rotter, B.; Winter, P.; Marell, R.-R.; Fliser, D.; Heine, G.H. SuperSAGE Evidence for CD14++CD16+ Monocytes as a Third Monocyte Subset. Blood 2011, 118, e50–e61. [Google Scholar] [CrossRef]
- Wong, K.L.; Tai, J.J.-Y.; Wong, W.-C.; Han, H.; Sem, X.; Yeap, W.-H.; Kourilsky, P.; Wong, S.-C. Gene Expression Profiling Reveals the Defining Features of the Classical, Intermediate, and Nonclassical Human Monocyte Subsets. Blood 2011, 118, e16–e31. [Google Scholar] [CrossRef]
- Gren, S.T.; Rasmussen, T.B.; Janciauskiene, S.; Håkansson, K.; Gerwien, J.G.; Grip, O. A Single-Cell Gene-Expression Profile Reveals Inter-Cellular Heterogeneity within Human Monocyte Subsets. PLoS ONE 2015, 10, e0144351. [Google Scholar] [CrossRef]
- Schulte-Schrepping, J.; Reusch, N.; Paclik, D.; Baßler, K.; Schlickeiser, S.; Zhang, B.; Krämer, B.; Krammer, T.; Brumhard, S.; Bonaguro, L.; et al. Severe COVID-19 Is Marked by a Dysregulated Myeloid Cell Compartment. Cell 2020, 182, 1419–1440.e23. [Google Scholar] [CrossRef]
- Knaus, W.A.; Draper, E.A.; Wagner, D.P.; Zimmerman, J.E. APACHE II: A Severity of Disease Classification System. Crit. Care Med. 1985, 13, 818–829. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The Acute Respiratory Distress Syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef]
- Horovitz, J.H.; Carrico, C.J.; Shires, G.T. Pulmonary Response to Major Injury. Arch. Surg. 1974, 108, 349–355. [Google Scholar] [CrossRef]
- Silvin, A.; Chapuis, N.; Dunsmore, G.; Goubet, A.-G.; Dubuisson, A.; Derosa, L.; Almire, C.; Hénon, C.; Kosmider, O.; Droin, N.; et al. Elevated Calprotectin and Abnormal Myeloid Cell Subsets Discriminate Severe from Mild COVID-19. Cell 2020, 182, 1401–1418.e18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fu, B.; Zheng, X.; Wang, D.; Zhao, C.; Qi, Y.; Sun, R.; Tian, Z.; Xu, X.; Wei, H. Pathogenic T-Cells and Inflammatory Monocytes Incite Inflammatory Storms in Severe COVID-19 Patients. Natl. Sci. Rev. 2020, 7, 998–1002. [Google Scholar] [CrossRef] [PubMed]
- Patterson, B.K.; Francisco, E.B.; Yogendra, R.; Long, E.; Pise, A.; Rodrigues, H.; Hall, E.; Herrera, M.; Parikh, P.; Guevara-Coto, J.; et al. Persistence of SARS CoV-2 S1 Protein in CD16+ Monocytes in Post-Acute Sequelae of COVID-19 (PASC) up to 15 Months Post-Infection. Front. Immunol. 2022, 12, 5526. [Google Scholar] [CrossRef]
- Mairpady Shambat, S.; Gómez-Mejia, A.; Schweizer, T.A.; Huemer, M.; Chang, C.-C.; Acevedo, C.; Bergada-Pijuan, J.; Vulin, C.; Hofmaenner, D.A.; Scheier, T.C.; et al. Hyperinflammatory Environment Drives Dysfunctional Myeloid Cell Effector Response to Bacterial Challenge in COVID-19. PLoS Pathog. 2022, 18, e1010176. [Google Scholar] [CrossRef] [PubMed]
- Karawajczyk, M.; Douhan Håkansson, L.; Lipcsey, M.; Hultström, M.; Pauksens, K.; Frithiof, R.; Larsson, A. High Expression of Neutrophil and Monocyte CD64 with Simultaneous Lack of Upregulation of Adhesion Receptors CD11b, CD162, CD15, CD65 on Neutrophils in Severe COVID-19. Ther. Adv. Infect. 2021, 8, 20499361211034064. [Google Scholar] [CrossRef] [PubMed]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-Selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 10, 1068. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Schultz, J.B.; Knauf, P.A.; King, M.R. Mechanical Shedding of L-Selectin from the Neutrophil Surface during Rolling on Sialyl Lewis x under Flow. J. Biol. Chem. 2007, 282, 4812–4820. [Google Scholar] [CrossRef]
- Jin, Y.; Ji, W.; Yang, H.; Chen, S.; Zhang, W.; Duan, G. Endothelial Activation and Dysfunction in COVID-19: From Basic Mechanisms to Potential Therapeutic Approaches. Sig. Transduct. Target. Ther. 2020, 5, 293. [Google Scholar] [CrossRef]
- Middleton, E.A.; He, X.-Y.; Denorme, F.; Campbell, R.A.; Ng, D.; Salvatore, S.P.; Mostyka, M.; Baxter-Stoltzfus, A.; Borczuk, A.C.; Loda, M.; et al. Neutrophil Extracellular Traps Contribute to Immunothrombosis in COVID-19 Acute Respiratory Distress Syndrome. Blood 2020, 136, 1169–1179. [Google Scholar] [CrossRef]
- Riemer, C.; Mack, E.; Burchert, A.; Gündisch, M.; Greib, S.; Etati, R.; Tarawneh, T.; Karim, I.; Keller, C.; Renz, H.; et al. Dysgranulopoiesis in Patients with Coronavirus Disease 2019. Acta Haematol. Pol. 2021, 52, 6. [Google Scholar] [CrossRef]
- Lacy, P. Mechanisms of Degranulation in Neutrophils. Allergy Asthma Clin. Immunol. 2006, 2, 98. [Google Scholar] [CrossRef]
- Tosi, M.F.; Zakem, H. Surface Expression of Fc Gamma Receptor III (CD16) on Chemoattractant-Stimulated Neutrophils Is Determined by Both Surface Shedding and Translocation from Intracellular Storage Compartments. J. Clin. Investig. 1992, 90, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Huizinga, T.W.; de Haas, M.; Kleijer, M.; Nuijens, J.H.; Roos, D.; von dem Borne, A.E. Soluble Fc Gamma Receptor III in Human Plasma Originates from Release by Neutrophils. J. Clin. Investig. 1990, 86, 416–423. [Google Scholar] [CrossRef] [PubMed]
- Middelhoven, P.J.; Buul, J.D.V.; Hordijk, P.L.; Roos, D. Different Proteolytic Mechanisms Involved in FcγRIIIb Shedding from Human Neutrophils. Clin. Exp. Immunol. 2001, 125, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Perussia, B.; Dayton, E.T.; Lazarus, R.; Fanning, V.; Trinchieri, G. Immune Interferon Induces the Receptor for Monomeric IgG1 on Human Monocytic and Myeloid Cells. J. Exp. Med. 1983, 158, 1092–1113. [Google Scholar] [CrossRef]
- Repp, R.; Valerius, T.; Sendler, A.; Gramatzki, M.; Iro, H.; Kalden, J.; Platzer, E. Neutrophils Express the High Affinity Receptor for IgG (Fc Gamma RI, CD64) after in Vivo Application of Recombinant Human Granulocyte Colony- Stimulating Factor. Blood 1991, 78, 885–889. [Google Scholar] [CrossRef]
- Zhang, Y.; Boesen, C.C.; Radaev, S.; Brooks, A.G.; Fridman, W.-H.; Sautes-Fridman, C.; Sun, P.D. Crystal Structure of the Extracellular Domain of a Human FcγRIII. Immunity 2000, 13, 387–395. [Google Scholar] [CrossRef]
- Kimberly, R.; Ahlstrom, J.; Click, M.; Edberg, J. The Glycosyl Phosphatidylinositol-Linked FcγRIII(PMN) Mediates Transmembrane Signaling Events Distinct from FcγRII. J. Exp. Med. 1990, 171, 1239–1255. [Google Scholar] [CrossRef]
- Unkeless, J.C.; Shen, Z.; Lin, C.-W.; DeBeus, E. Function of Human FcγRIIA and FcγRIIIB. Semin. Immunol. 1995, 7, 37–44. [Google Scholar] [CrossRef]
- Ankerhold, J.; Giese, S.; Kolb, P.; Maul-Pavicic, A.; Voll, R.E.; Göppert, N.; Ciminski, K.; Kreutz, C.; Lother, A.; Salzer, U.; et al. Circulating Multimeric Immune Complexes Drive Immunopathology in COVID-19. 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.06.25.449893v4 (accessed on 2 July 2022). [CrossRef]
- Davis, B.H.; Olsen, S.H.; Ahmad, E.; Bigelow, N.C. Neutrophil CD64 Is an Improved Indicator of Infection or Sepsis in Emergency Department Patients. Arch. Pathol. Lab. Med. 2006, 130, 654–661. [Google Scholar] [CrossRef]
- Muller Kobold, A.C.; Tulleken, J.E.; Zijlstra, J.G.; Sluiter, W.; Hermans, J.; Kallenberg, C.G.; Tervaert, J.W. Leukocyte Activation in Sepsis; Correlations with Disease State and Mortality. Intensive Care Med. 2000, 26, 883–892. [Google Scholar] [CrossRef]
- Cid, J.; García-Pardo, G.; Aguinaco, R.; Sánchez, R.; Llorente, A. Neutrophil CD64: Diagnostic Accuracy and Prognostic Value in Patients Presenting to the Emergency Department. Eur. J. Clin. Microbiol. Infect. Dis 2011, 30, 845–852. [Google Scholar] [CrossRef] [PubMed]
- Song, S.H.; Kim, H.K.; Park, M.H.; Cho, H.-I. Neutrophil CD64 Expression Is Associated with Severity and Prognosis of Disseminated Intravascular Coagulation. Thromb. Res. 2008, 121, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Bradley, P.; Frost, F.; Tharmaratnam, K.; Wootton, D.G. Utility of Established Prognostic Scores in COVID-19 Hospital Admissions: Multicentre Prospective Evaluation of CURB-65, NEWS2 and QSOFA. BMJ Open Resp. Res. 2020, 7, e000729. [Google Scholar] [CrossRef] [PubMed]
- Bradley, J.; Sbaih, N.; Chandler, T.R.; Furmanek, S.; Ramirez, J.A.; Cavallazzi, R. Pneumonia Severity Index and CURB-65 Score Are Good Predictors of Mortality in Hospitalized Patients With SARS-CoV-2 Community-Acquired Pneumonia. Chest 2022, 161, 927–936. [Google Scholar] [CrossRef]
- Artero, A.; Madrazo, M.; Fernández-Garcés, M.; Muiño Miguez, A.; González García, A.; Crestelo Vieitez, A.; García Guijarro, E.; Fonseca Aizpuru, E.M.; García Gómez, M.; Areses Manrique, M.; et al. Severity Scores in COVID-19 Pneumonia: A Multicenter, Retrospective, Cohort Study. J. Gen. Intern. Med. 2021, 36, 1338–1345. [Google Scholar] [CrossRef]



| Characteristic | COVID-19 Positive (n = 16) | COVID-19 Negative (n = 9) |
|---|---|---|
| Median age (range)—years | 66.5 (23–82) | 70 (28–88) |
| Male sex—no. (%) | 13 (81%) | 5 (56%) |
| Female sex—no. (%) | 3 (19%) | 4 (44%) |
| Underlying Health Condition—No./Total No. (%) | ||
| Diabetes | 6 (38%) | 1 (11%) |
| Hypertension | 11 (69%) | 5 (56%) |
| Obesity (BMI ≥ 30) | 4 (25%) | 2 (22%) |
| Hyperlipidemia | 3 (19%) | 0 (0%) |
| Chronic heart disease | 5 (31%) | 1 (11%) |
| Cardiovascular disease | 3 (19%) | 4 (44%) |
| Respiratory disease | 1 (6%) | 3 (33%) |
| Chronic kidney disease | 1 (6%) | 3 (33%) |
| Cancer | 3 (19% | 3 (33%) |
| Immunosuppression | 3 (19%) | 1 (11%) |
| Chronic liver disease | 2 (13%) | 1 (11%) |
| Charlson Comorbidity Index -/median (range) | 4 (1–7) | 5 (0–11) |
| Complications—No./Total No. (%) | ||
| Acute respiratory distress syndrome | 11 (69%) | 4 (44%) |
| Non-invasive ventilation | 0 (0%) | 2 (22%) |
| Invasive mechanical ventilation | 11 (69%) | 4 (44%) |
| Acute kidney failure | 8 (50%) | 2 (22%) |
| Thromboembolic events | 3 (19%) | 0 (0%) |
| ICU | 12 (75%) | 6 (67%) |
| Death | 3 (19%) | 1 (11%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoffmann, J.; Etati, R.; Brendel, C.; Neubauer, A.; Mack, E. The Low Expression of Fc-Gamma Receptor III (CD16) and High Expression of Fc-Gamma Receptor I (CD64) on Neutrophil Granulocytes Mark Severe COVID-19 Pneumonia. Diagnostics 2022, 12, 2010. https://doi.org/10.3390/diagnostics12082010
Hoffmann J, Etati R, Brendel C, Neubauer A, Mack E. The Low Expression of Fc-Gamma Receptor III (CD16) and High Expression of Fc-Gamma Receptor I (CD64) on Neutrophil Granulocytes Mark Severe COVID-19 Pneumonia. Diagnostics. 2022; 12(8):2010. https://doi.org/10.3390/diagnostics12082010
Chicago/Turabian StyleHoffmann, Joerg, Rojin Etati, Cornelia Brendel, Andreas Neubauer, and Elisabeth Mack. 2022. "The Low Expression of Fc-Gamma Receptor III (CD16) and High Expression of Fc-Gamma Receptor I (CD64) on Neutrophil Granulocytes Mark Severe COVID-19 Pneumonia" Diagnostics 12, no. 8: 2010. https://doi.org/10.3390/diagnostics12082010






