Coronary Angiography Upgraded by Imaging Post-Processing: Present and Future Directions
Abstract
:1. Introduction
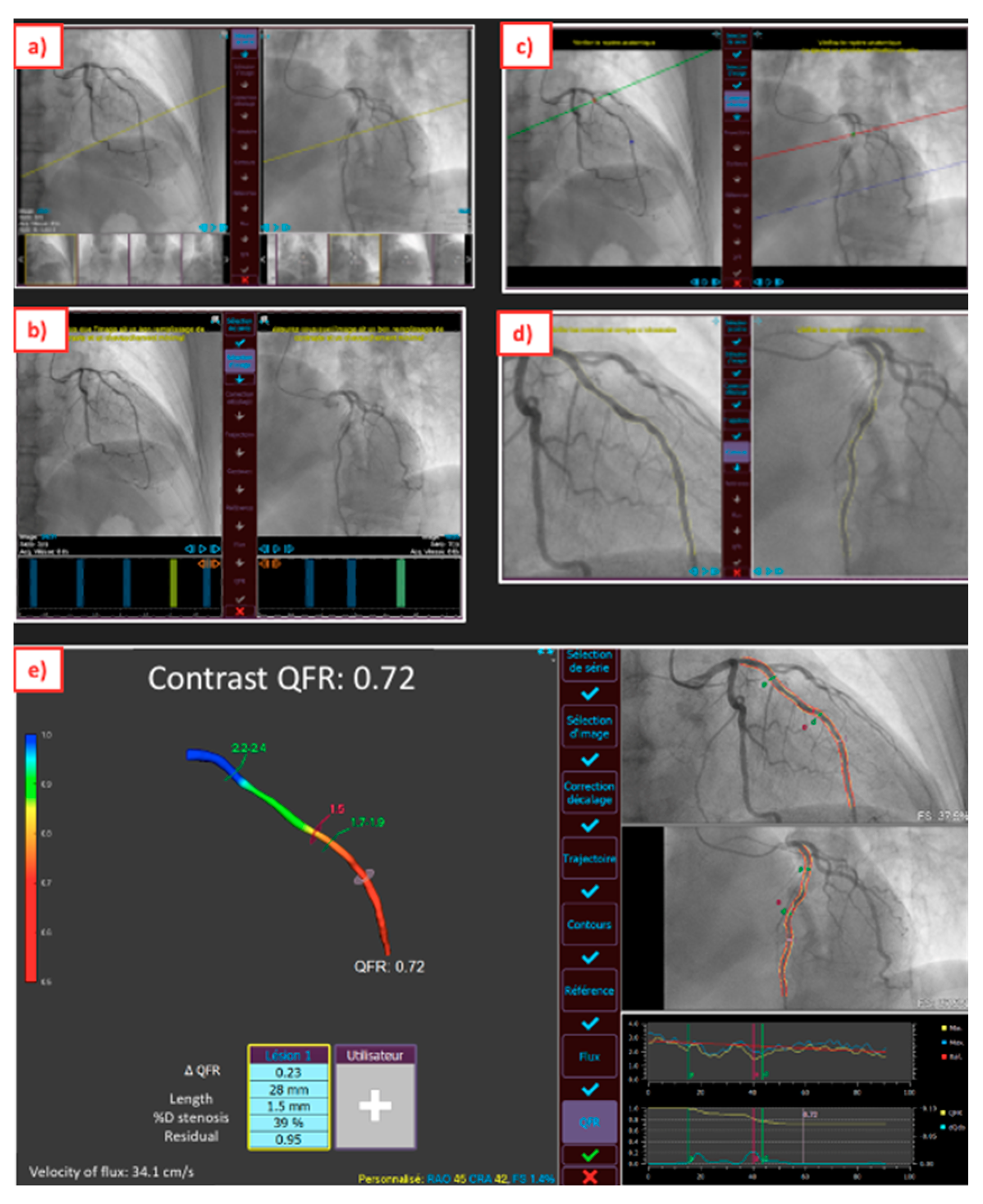
2. Upgraded ICA for Pre-PCI Time
3. Limits of Technology
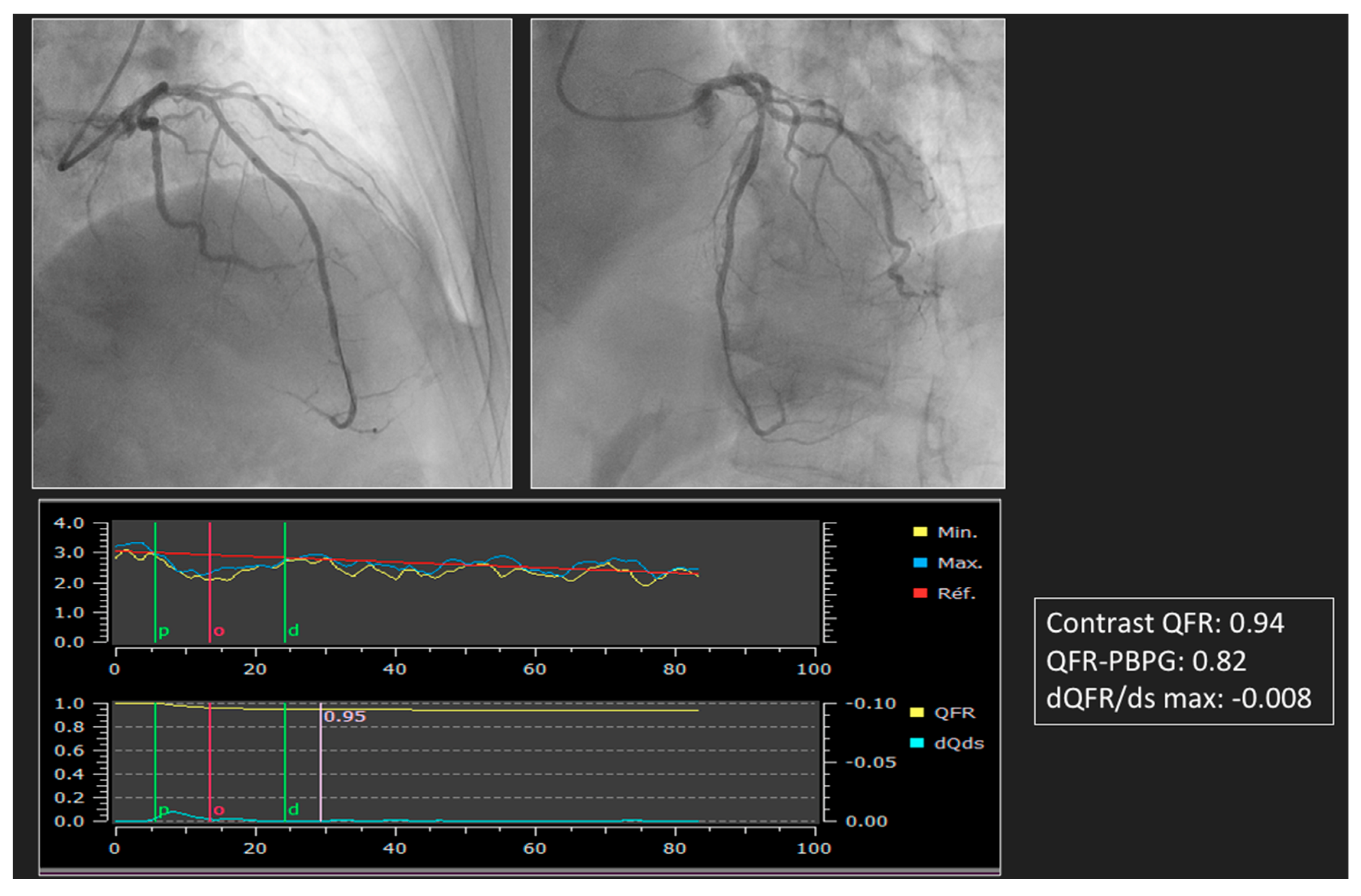
4. Advances in ICA to Assess PCI Results
5. Virtual PCI
6. Future Research
6.1. Application to Bifurcations
6.2. Evaluation of the Coronary Microcirculation Dysfunction
6.3. Non-Invasive Diagnostic Method in Coronary Artery Disease
6.4. Development of Pre-PCI via Coronary Computed Tomography Angiography (CCTA)
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tonino, P.A.L.; Fearon, W.F.; De Bruyne, B.; Oldroyd, K.G.; Leesar, M.A.; Ver Lee, P.N.; Maccarthy, P.A.; Van’t Veer, M.; Pijls, N.H. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J. Am. Coll. Cardiol. 2010, 55, 2816–2821. [Google Scholar] [CrossRef] [Green Version]
- Writing Committee Members; Lawton, J.S.; Tamis-Holland, J.E.; Bangalore, S.; Bates, E.R.; Beckie, T.M.; Bischoff, J.M.; Bittl, J.A.; Cohen, M.G.; DiMaio, J.M.; et al. 2021 ACC/AHA/SCAI Guideline for Coronary Artery Revascularization: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2022, 79, e21–e129. [Google Scholar] [CrossRef]
- Neumann, F.J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.P.; Falk, V.; Head, S.J.; et al. ESC Scientific Document Group. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef] [Green Version]
- Scoccia, A.; Tomaniak, M.; Neleman, T.; Groenland, F.T.W.; Plantes, A.C.Z.D.; Daemen, J. Angiography-Based Fractional Flow Reserve: State of the Art. Curr. Cardiol. Rep. 2022, 24, 667–678. [Google Scholar] [CrossRef]
- Ramasamy, A.; Jin, C.; Tufaro, V.; Bajaj, R.; Kilic, Y.; Safi, H.; Amersey, R.; Jones, D.; Torii, R.; Lansky, A.; et al. Computerised Methodologies for Non-Invasive Angiography- Derived Fractional Flow Reserve Assessment: A Critical Review. J. Interv. Cardiol. 2020, 2020, 6381637. [Google Scholar] [CrossRef] [Green Version]
- Tu, S.; Barbato, E.; Köszegi, Z.; Yang, J.; Sun, Z.; Holm, N.R.; Tar, B.; Li, Y.; Rusinaru, D.; Wijns, W.; et al. Fractional flow reserve calculation from 3-dimensional quantitative coronary angiography and TIMI frame count: A fast computer model to quantify the functional significance of moderately obstructed coronary arteries. JACC Cardiovasc. Interv. 2014, 7, 768–777. [Google Scholar] [CrossRef] [Green Version]
- Tu, S.; Westra, J.; Yang, J.; von Birgelen, C.; Ferrara, A.; Pellicano, M.; Nef, H.; Tebaldi, M.; Murasato, Y.; Lansky, A.; et al. Diagnostic Accuracy of Fast Computational Approaches to Derive Fractional Flow Reserve From Diagnostic Coronary Angiography: The International Multicenter FAVOR Pilot Study. JACC Cardiovasc. Interv. 2016, 9, 2024–2035. [Google Scholar] [CrossRef]
- Westra, J.; Tu, S.; Winther, S.; Nissen, L.; Vestergaard, M.B.; Andersen, B.K.; Holck, E.N.; Fox Maule, C.; Johansen, J.K.; Andreasen, L.N.; et al. Evaluation of Coronary Artery Stenosis by Quantitative Flow Ratio During Invasive Coronary Angiography: The WIFI II Study (Wire-Free Functional Imaging II). Circ. Cardiovasc. Imaging 2018, 11, e007107. [Google Scholar] [CrossRef] [Green Version]
- Westra, J.; Andersen, B.K.; Campo, G.; Matsuo, H.; Koltowski, L.; Eftekhari, A.; Liu, T.; Di Serafino, L.; Di Girolamo, D.; Escaned, J.; et al. Diagnostic Performance of In-Procedure Angiography-Derived Quantitative Flow Reserve Compared to Pressure-Derived Fractional Flow Reserve: The FAVOR II Europe-Japan Study. J. Am. Heart Assoc. 2018, 7, e009603. [Google Scholar] [CrossRef] [Green Version]
- Stähli, B.E.; Erbay, A.; Steiner, J.; Klotsche, J.; Mochmann, H.C.; Skurk, C.; Lauten, A.; Landmesser, U.; Leistner, D.M. Comparison of resting distal to aortic coronary pressure with angiography-based quantitative flow ratio. Int. J. Cardiol. 2019, 279, 12–17. [Google Scholar] [CrossRef]
- Masdjedi, K.; van Zandvoort, L.J.C.; Balbi, M.M.; Gijsen, F.J.H.; Ligthart, J.M.R.; Rutten, M.C.M.; Lemmert, M.E.; Wilschut, J.M.; Diletti, R.; de Jaegere, P.; et al. Validation of a threedimensional quantitative coronary angiography-based software to calculate fractional flow reserve: The FAST study. EuroIntervention 2020, 16, 591–599. [Google Scholar] [CrossRef]
- Neleman, T.; Masdjedi, K.; Van Zandvoort, L.J.C.; Tomaniak, M.; Ligthart, J.M.R.; Witberg, K.T.; Vermaire, A.A.; Boersma, E.; Van Mieghem, N.M.; Daemen, J. Extended Validation of Novel 3D Quantitative Coronary Angiography-Based Software to Calculate vFFR: The FAST EXTEND Study. JACC Cardiovasc. Imaging 2021, 14, 504–506. [Google Scholar] [CrossRef]
- Masdjedi, K.; Tanaka, N.; Van Belle, E.; Porouchani, S.; Linke, A.; Woitek, F.J.; Bartorelli, A.L.; Ali, Z.A.; den Dekker, W.K.; Wilschut, J.; et al. Vessel fractional flow reserve (vFFR) for the assessment of stenosis severity: The FAST II study. EuroIntervention 2022, 17, 1498–1505. [Google Scholar] [CrossRef]
- Kornowski, R.; Lavi, I.; Pellicano, M.; Xaplanteris, P.; Vaknin-Assa, H.; Assali, A.; Valtzer, O.; Lotringer, Y.; De Bruyne, B. Fractional flow reserve derived from routine coronary angiograms. J. Am. Coll. Cardiol. 2016, 68, 2235–2237. [Google Scholar] [CrossRef]
- Kornowski, R.; Vaknin-Assa, H.; Assali, A.; Greenberg, G.; Valtzer, O.; Lavi, I. Online angiography image-based FFR assessment during coronary catheterization: A single-center study. J. Invasive Cardiol. 2018, 30, 224–229. [Google Scholar]
- Pellicano, M.; Lavi, I.; De Bruyne, B.; Vaknin-Assa, H.; Assali, A.; Valtzer, O.; Lotringer, Y.; Weisz, G.; Almagor, Y.; Xaplanteris, P.; et al. Validation study of image-based fractional flow reserve during coronary angiography. Circ. Cardiovasc. Interv. 2017, 10, e005259. [Google Scholar] [CrossRef]
- Fearon, W.F.; Achenbach, S.; Engstrom, T.; Assali, A.; Shlofmitz, R.; Jeremias, A.; Fournier, S.; Kirtane, A.J.; Kornowski, R.; Greenberg, G.; et al. FAST-FFR Study Investigators. Accuracy of fractional flow reserve derived from coronary angiography. Circulation 2019, 139, 477–484. [Google Scholar] [CrossRef]
- Omori, H.; Witberg, G.; Kawase, Y. Angiogram based fractional flow reserve in patients with dual/triple vessel coronary artery disease. Int. J. Cardiol. 2019, 283, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Witberg, G.; De Bruyne, B.; Fearon, W.F.; Achenbach, S.; Engstrom, T.; Matsuo, H.; Kornowski, R. Diagnostic Performance of Angiogram-Derived Fractional Flow Reserve: A Pooled Analysis of 5 Prospective Cohort Studies. JACC Cardiovasc. Interv. 2020, 13, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Onuma, Y.; Sonck, J.; Asano, T.; Vandeloo, B.; Kornowski, R.; Tu, S.; Westra, J.; Holm, N.R.; Xu, B.; et al. Diagnostic performance of angiography-derived fractional flow reserve: A systematic review and Bayesian metaanalysis. Eur. Heart J. 2018, 39, 3314–3321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Gong, Y.; Wang, W.; Yang, Q.; Liu, B.; Lu, Y.; Xu, Y.; Huo, Y.; Yi, T.; Liu, J.; et al. Accuracy of computational pressure-fluid dynamics applied to coronary angiography to derive fractional flow reserve: FLASH FFR. Cardiovasc. Res. 2020, 116, 1349–1356. [Google Scholar] [CrossRef]
- Morris, P.D.; Ryan, D.; Morton, A.C.; Lycett, R.; Lawford, P.V.; Hose, D.R.; Gunn, J.P. Virtual fractional flow reserve from coronary angiography: Modeling the significance of coronary lesions: Results from the VIRTU-1 (Virtual Fractional Flow Reserve from Coronary Angiography) study. JACC Cardiovasc. Interv. 2013, 6, 149–157. [Google Scholar] [CrossRef] [Green Version]
- Gosling, R.C.; Morris, P.D.; Silva Soto, D.A.; Lawford, P.V.; Hose, D.R.; Gunn, J.P. Virtual Coronary Intervention: A Treatment Planning Tool Based Upon the Angiogram. JACC Cardiovasc. Imaging 2019, 12, 865–872. [Google Scholar] [CrossRef]
- Gould, K.L.; Kelley, K.O.; Bolson, E.L. Experimental validation of quantitative coronary arteriography for determining pressure-flow characteristics of coronary stenosis. Circulation 1982, 66, 930–937. [Google Scholar] [CrossRef] [Green Version]
- Kirkeeide, R.L.; Gould, K.L.; Parsel, L. Assessment of coronary stenoses by myocardial perfusion imaging during pharmacologic coronary vasodilation. VII. Validation of coronary flow reserve as a single integrated functional measure of stenosis severity reflecting all its geometric dimensions. J. Am. Coll. Cardiol. 1986, 7, 103–113. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Tu, S.; Song, L.; Jin, Z.; Yu, B.; Fu, G.; Zhou, Y.; Wang, J.; Chen, Y.; Pu, J.; et al. Angiographic quantitative flow ratio-guided coronary intervention (FAVOR III China): A multicentre, randomised, sham-controlled trial. Lancet 2021, 398, 2149–2159. [Google Scholar] [CrossRef]
- Scoccia, A.; Byrne, R.A.; Banning, A.P.; Landmesser, U.; Van Belle, E.; Amat-Santos, I.J.; Sabaté, M.; Tijssen, J.G.P.; Spitzer, E.; Daemen, J. Fractional Flow Reserve or 3D-Quantitative-Coronary-Angiography Based Vessel-FFR guided revascularization. Rationale and study design of the prospective randomized FAST III Trial. Am. Heart J. 2023, 260, 1–8. [Google Scholar] [CrossRef]
- Kogame, N.; Takahashi, K.; Tomaniak, M.; Chichareon, P.; Modolo, R.; Chang, C.C.; Komiyama, H.; Katagiri, Y.; Asano, T.; Stables, R.; et al. Clinical Implication of Quantitative Flow Ratio After Percutaneous Coronary Intervention for 3-Vessel Disease. JACC Cardiovasc. Interv. 2019, 12, 2064–2075. [Google Scholar] [CrossRef]
- Biscaglia, S.; Tebaldi, M.; Brugaletta, S.; Cerrato, E.; Erriquez, A.; Passarini, G.; Ielasi, A.; Spitaleri, G.; Di Girolamo, D.; Mezzapelle, G.; et al. Prognostic value of QFR measured immediately after successful stent implantation: The international ulticenterp Prospective HAWKEYE study. JACC Cardiovasc. Interv. 2019, 12, 2079–2088. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, R.; Yuan, S.; Hu, N.; Guan, C.; Zou, T.; Qiao, Z.; He, J.; Duan, S.; Xie, L.; et al. Prognostic Implications of Quantitative Flow Ratio-Derived Physiological 2-Dimensional Residual Disease Patterns After Stenting. JACC Cardiovasc. Interv. 2022, 15, 1624–1634. [Google Scholar] [CrossRef]
- Masdjedi, K.; van Zandvoort, L.J.; Balbi, M.M.; Nuis, R.J.; Wilschut, J.; Diletti, R.; de Jaegere, P.P.T.; Zijlstra, F.; Van Mieghem, N.M.; Daemen, J. Validation of novel 3-dimensional quantitative coronary angiography based software to calculate fractional flow reserve post stenting. Catheter. Cardiovasc. Interv. 2021, 98, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Ely Pizzato, P.; Samdani, A.J.; Vergara-Martel, A.; Palma Dallan, L.A.; Tensol Rodrigues Pereira, G.; Zago, E.; Zimin, V.; Grando Bezerra, H. Feasibility of coronary angiogram-derived vessel fractional flow reserve in the setting of standard of care percutaneous coronary intervention and its correlation with invasive FFR. Int. J. Cardiol. 2020, 301, 45–49. [Google Scholar] [CrossRef] [PubMed]
- Rubimbura, V.; Guillon, B.; Fournier, S.; Amabile, N.; Chi Pan, C.; Combaret, N.; Eeckhout, E.; Kibler, M.; Silvain, J.; Wijns, W.; et al. Quantitative flow ratio virtual stenting and post stenting correlations to post stenting fractional flow reserve measurements from the DOCTORS (Does Optical Coherence Tomography Optimize Results of Stenting) study population. Catheter. Cardiovasc. Interv. 2020, 96, 1145–1153. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Mejía-Rentería, H.; Escaned, J.; Doh, J.H.; Lee, J.M.; Hwang, D.; Yuasa, S.; Choi, K.H.; Jang, H.J.; Jeon, K.H.; et al. Prediction of functional results of percutaneous coronary interventions with virtual stenting and quantitative flow ratio. Catheter. Cardiovasc. Interv. 2022, 100, 1208–1217. [Google Scholar] [CrossRef]
- Tomaniak, M.; Neleman, T.; Ziedses des Plantes, A.; Masdjedi, K.; van Zandvoort, L.J.C.; Kochman, J.; den Dekker, W.K.; Wilschut, J.M.; Diletti, R.; Kardys, I.; et al. Diagnostic Accuracy of Coronary Angiography-Based Vessel Fractional Flow Reserve (vFFR) Virtual Stenting. J. Clin. Med. 2022, 11, 1397. [Google Scholar] [CrossRef]
- Zhang, R.; Xu, B.; Dou, K.; Guan, C.; Zhao, Y.; Wang, X.; Zou, T.; Qiao, Z.; Xie, L.; Wang, H.; et al. Post-PCI outcomes predicted by pre-intervention simulation of residual quantitative flow ratio using augmented reality. Int. J. Cardiol. 2022, 352, 33–39. [Google Scholar] [CrossRef]
- Biscaglia, S.; Uretsky, B.F.; Tebaldi, M.; Erriquez, A.; Brugaletta, S.; Cerrato, E.; Quadri, G.; Spitaleri, G.; Colaiori, I.; Di Girolamo, D.; et al. Angio-Based Fractional Flow Reserve, Functional Pattern of Coronary Artery Disease, and Prediction of Percutaneous Coronary Intervention Result: A Proof-of-Concept Study. Cardiovasc. Drugs Ther. 2022, 36, 645–653. [Google Scholar] [CrossRef]
- Dai, N.; Zhang, R.; Hu, N.; Guan, C.; Zou, T.; Qiao, Z.; Zhang, M.; Duan, S.; Xie, L.; Dou, K.; et al. Integrated coronary disease burden and patterns to discriminate vessels benefiting from percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2022, 99, E12–E21. [Google Scholar] [CrossRef]
- Dai, N.; Yuan, S.; Dou, K.; Zhang, R.; Hu, N.; He, J.; Guan, C.; Zou, T.; Qiao, Z.; Duan, S.; et al. Prognostic Implications of Prestent Pullback Pressure Gradient and Poststent Quantitative Flow Ratio in Patients Undergoing Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2022, 11, e024903. [Google Scholar] [CrossRef]
- Shin, D.; Dai, N.; Lee, S.H.; Choi, K.H.; Lefieux, A.; Molony, D.; Hwang, D.; Kim, H.K.; Jeon, K.H.; Lee, H.J.; et al. Physiological Distribution and Local Severity of Coronary Artery Disease and Outcomes After Percutaneous Coronary Intervention. JACC Cardiovasc. Interv. 2021, 14, 1771–1785. [Google Scholar] [CrossRef]
- Dai, N.; Tang, X.; Chen, Z.; Huang, D.; Duan, S.; Qian, J.; Ge, J. Pre-stenting angiography-FFR based physiological map provides virtual intervention and predicts physiological and clinical outcomes. Catheter. Cardiovasc. Interv. 2023, 101, 1053–1061. [Google Scholar] [CrossRef]
- Morris, P.D.; Curzen, N.; Gunn, J.P. Angiography-Derived Fractional Flow Reserve: More or Less Physiology? J. Am. Heart Assoc. 2020, 9, e015586. [Google Scholar] [CrossRef]
- Johnson, N.P. What about All the Recent “Negative” FFR Trials? Interv. Cardiol. Clin. 2023, 12, 31–39. [Google Scholar] [CrossRef]
- Fearon, W.F.; Zimmermann, F.M.; De Bruyne, B.; Piroth, Z.; van Straten, A.H.M.; Szekely, L.; Davidavičius, G.; Kalinauskas, G.; Mansour, S.; Kharbanda, R.; et al. Fractional Flow Reserve-Guided PCI as Compared with Coronary Bypass Surgery. N. Engl. J. Med. 2022, 386, 128–137. [Google Scholar] [CrossRef]
- Piroth, Z.; Otsuki, H.; Zimmermann, F.M.; Ferenci, T.; Keulards, D.C.J.; Yeung, A.C.; Pijls, N.H.J.; De Bruyne, B.; Fearon, W.F. Prognostic Value of Measuring Fractional Flow Reserve After Percutaneous Coronary Intervention in Patients With Complex Coronary Artery Disease: Insights from the FAME 3 Trial. Circ. Cardiovasc. Interv. 2022, 15, 884–891. [Google Scholar] [CrossRef]
- Biscaglia, S.; Uretsky, B.; Barbato, E.; Collet, C.; Onuma, Y.; Jeremias, A.; Tebaldi, M.; Hakeem, A.; Kogame, N.; Sonck, J.; et al. Invasive Coronary Physiology after Stent Implantation: Another Step Toward Precision Medicine. JACC Cardiovasc. Interv. 2021, 14, 237–246. [Google Scholar] [CrossRef]
- Lee, J.M.; Lee, S.H.; Shin, D.; Choi, K.H.; van de Hoef, T.P.; Kim, H.K.; Samady, H.; Kakuta, T.; Matsuo, H.; Koo, B.K.; et al. Physiology-Based Revascularization: A New Approach to Plan and Optimize Percutaneous Coronary Intervention. JACC Asia 2021, 1, 14–36. [Google Scholar] [CrossRef]
- Collet, C.; Sonck, J.; Vandeloo, B.; Mizukami, T.; Roosens, B.; Lochy, S.; Argacha, J.F.; Schoors, D.; Colaiori, I.; Di Gioia, G.; et al. Measurement of Hyperemic Pullback Pressure Gradients to Characterize Patterns of Coronary Atherosclerosis. J. Am. Coll. Cardiol. 2019, 74, 1772–1784. [Google Scholar] [CrossRef]
- Lee, S.H.; Shin, D.; Lee, J.M.; Lefieux, A.; Molony, D.; Choi, K.H.; Hwang, D.; Lee, H.J.; Jang, H.J.; Kim, H.K.; et al. Automated Algorithm Using Pre-Intervention Fractional Flow Reserve Pullback Curve to Predict Post- Intervention Physiological Results. JACC Cardiovasc. Interv. 2020, 13, 2670–2684. [Google Scholar] [CrossRef]
- Fournier, S.; Ciccarelli, G.; Toth, G.G.; Milkas, A.; Xaplanteris, P.; Tonino, P.A.L.; Fearon, W.F.; Pijls, N.H.J.; Barbato, E.; De Bruyne, B. Association of improvement in fractional flow reserve with outcomes, including symptomatic relief, after percutaneous coronary intervention. JAMA Cardiol. 2019, 4, 370–374. [Google Scholar] [CrossRef]
- Murai, T.; Lee, T.; Yonetsu, T.; Isobe, M.; Kakuta, T. Influence of microvascular resistance on fractional flow reserve after successful percutaneous coronary intervention. Catheter. Cardiovasc. Interv. 2015, 85, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Dai, N.; Hwang, D.; Lee, J.M.; Zhang, J.; Jeon, K.H.; Paeng, J.C.; Cheon, G.J.; Koo, B.K.; Ge, J. Feasibility of Quantitative Flow Ratio-Derived Pullback Pressure Gradient Index and Its Impact on Diagnostic Performance. JACC Cardiovasc. Interv. 2021, 14, 353–355. [Google Scholar] [CrossRef] [PubMed]
- Scarsini, R.; Fezzi, S.; Leone, A.M.; De Maria, G.L.; Pighi, M.; Marcoli, M.; Tavella, D.; Pesarini, G.; Banning, A.P.; Barbato, E.; et al. Functional Patterns of Coronary Disease: Diffuse, Focal, and Serial Lesions. JACC Cardiovasc. Interv. 2022, 15, 2174–2191. [Google Scholar] [CrossRef] [PubMed]
- Collison, D.; Didagelos, M.; Aetesam-Ur-Rahman, M.; Copt, S.; McDade, R.; McCartney, P.; Ford, T.J.; McClure, J.; Lindsay, M.; Shaukat, A.; et al. Post-stenting fractional flow reserve vs coronary angiography for optimization of percutaneous coronary intervention (TARGETFFR). Eur. Heart J. 2021, 42, 4656–4668. [Google Scholar] [CrossRef]
- Pijls, N.H.; De Bruyne, B.; Bech, G.J.; Liistro, F.; Heyndrickx, G.R.; Bonnier, H.J.; Koolen, J.J. Coronary pressure measurement to assess the hemodynamic significance of serial stenoses within one coronary artery: Validation in humans. Circulation 2000, 102, 2371–2377. [Google Scholar] [CrossRef] [Green Version]
- Biscaglia, S.; Verardi, F.M.; Tebaldi, M.; Guiducci, V.; Caglioni, S.; Campana, R.; Scala, A.; Marrone, A.; Pompei, G.; Marchini, F.; et al. QFR-Based Virtual PCI or Conventional Angiography to Guide PCI: The AQVA Trial. JACC Cardiovasc. Interv. 2023, 16, 783–794. [Google Scholar] [CrossRef]
- Karanasos, A.; Tu, S.; van Ditzhuijzen, N.S.; Ligthart, J.M.; Witberg, K.; Van Mieghem, N.; van Geuns, R.J.; de Jaegere, P.; Zijlstra, F.; Reiber, J.H.; et al. A novel method to assess coronary artery bifurcations by OCT: Cut-plane analysis for side-branch ostial assessment from a main-vessel pullback. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Raphael, C.E.; O’Kane, P.D.; Johnson, T.W.; Prasad, A.; Gulati, R.; Sandoval, Y.; Di Mario, C.; Holmes, D.R., Jr. Evolution of the Crush Technique for Bifurcation Stenting. JACC Cardiovasc. Interv. 2021, 14, 2315–2326. [Google Scholar] [CrossRef]
- Tu, S.; Echavarria-Pinto, M.; von Birgelen, C.; Holm, N.R.; Pyxaras, S.A.; Kumsars, I.; Lam, M.K.; Valkenburg, I.; Toth, G.G.; Li, Y.; et al. Fractional flow reserve and coronary bifurcation anatomy: A novel quantitative model to assess and report the stenosis severity of bifurcation lesions. JACC Cardiovasc. Interv. 2015, 8, 564–574. [Google Scholar] [CrossRef] [Green Version]
- Del Buono, M.G.; Montone, R.A.; Camilli, M.; Carbone, S.; Narula, J.; Lavie, C.J.; Niccoli, G.; Crea, F. Coronary Microvascular Dysfunction Across the Spectrum of Cardiovascular Diseases: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2021, 78, 1352–1371. [Google Scholar] [CrossRef]
- Niccoli, G.; Scalone, G.; Lerman, A.; Crea, F. Coronary microvascular obstruction in acute myocardial infarction. Eur. Heart J. 2016, 37, 1024–1033. [Google Scholar] [CrossRef] [Green Version]
- Fearon, W.F.; Kobayashi, Y. Invasive Assessment of the Coronary Microvasculature: The Index of Microcirculatory Resistance. Circ. Cardiovasc. Interv. 2017, 10, e005361. [Google Scholar] [CrossRef]
- Fernández-Peregrina, E.; Garcia-Garcia, H.M.; Sans-Rosello, J.; Sanz-Sanchez, J.; Kotronias, R.; Scarsini, R.; Echavarria-Pinto, M.; Tebaldi, M.; De Maria, G.L. Angiography-derived versus invasively-determined index of microcirculatory resistance in the assessment of coronary microcirculation: A systematic review and meta-analysis. Catheter. Cardiovasc. Interv. 2022, 99, 2018–2025. [Google Scholar] [CrossRef]
- Choi, K.H.; Dai, N.; Li, Y.; Kim, J.; Shin, D.; Lee, S.H.; Joh, H.S.; Kim, H.K.; Jeon, K.H.; Ha, S.J.; et al. Functional Coronary Angiography-Derived Index of Microcirculatory Resistance in Patients With ST-Segment Elevation Myocardial Infarction. JACC Cardiovasc. Interv. 2021, 14, 1670–1684. [Google Scholar] [CrossRef]
- Maznyczka, A.M.; Oldroyd, K.G.; McCartney, P.; McEntegart, M.; Berry, C. The Potential Use of the Index of Microcirculatory Resistance to Guide Stratification of Patients for Adjunctive Therapy in Acute Myocardial Infarction. JACC Cardiovasc. Interv. 2019, 12, 951–966. [Google Scholar] [CrossRef]
- Bedetti, G.; Pasanisi, E.M.; Pizzi, C.; Turchetti, G.; Loré, C. Economic analysis including long-term risks and costs of alternative diagnostic strategies to evaluate patients with chest pain. Cardiovasc. Ultrasound 2008, 6, 21. [Google Scholar] [CrossRef] [Green Version]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic accuracy of stress myocardial perfusion imaging compared to invasive coronary angiography with fractional flow reserve meta-analysis. Circ. Cardiovasc. Imaging 2015, 8, e002666. [Google Scholar] [CrossRef] [Green Version]
- Danad, I.; Szymonifka, J.; Twisk, J.W.R.; Norgaard, B.L.; Zarins, C.K.; Knaapen, P.; Min, J.K. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: A meta-analysis. Eur. Heart J. 2017, 38, 991–998. [Google Scholar] [CrossRef] [Green Version]
- Mittal, T.K.; Hothi, S.S.; Venugopal, V.; Taleyratne, J.; O’Brien, D.; Adnan, K.; Sehmi, J.; Daskalopoulos, G.; Deshpande, A.; Elfawal, S.; et al. The Use and Efficacy of FFR-CT: Real-World Multicenter Audit of Clinical Data With Cost Analysis. JACC Cardiovasc. Imaging 2023. [Google Scholar] [CrossRef]
- Sonck, J.; Nagumo, S.; Norgaard, B.L.; Otake, H.; Ko, B.; Zhang, J.; Mizukami, T.; Maeng, M.; Andreini, D.; Takahashi, Y.; et al. Clinical Validation of a Virtual Planner for Coronary Interventions Based on Coronary CT Angiography. JACC Cardiovasc. Imaging 2022, 15, 1242–1255. [Google Scholar] [CrossRef]
- Andreini, D.; Collet, C.; Leipsic, J.; Nieman, K.; Bittencurt, M.; De Mey, J.; Buls, N.; Onuma, Y.; Mushtaq, S.; Conte, E.; et al. Pre-procedural planning of coronary revascularization by cardiac computed tomography: An expert consensus document of the Society of Cardiovascular Computed Tomography. EuroIntervention 2022, 18, e872–e887. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Sonck, J.; Leipsic, J.; Monizzi, G.; Buytaert, D.; Kitslaar, P.; Andreini, D.; De Bruyne, B. Implementing Coronary Computed Tomography Angiography in the Catheterization Laboratory. JACC Cardiovasc. Imaging 2021, 14, 1846–1855. [Google Scholar] [CrossRef] [PubMed]
- Poletti, E.; Ohashi, H.; Sonck, J.; Castaldi, G.; Benedetti, A.; Collet, C.; Agostoni, P.; Zivelonghi, C. Coronary CT-Guided Minimalistic Hybrid Approach for Percutaneous Chronic Total Occlusion Recanalization. JACC Cardiovasc. Interv. 2023, 16, 1107–1108. [Google Scholar] [CrossRef] [PubMed]



| Index | ||||
|---|---|---|---|---|
| QFR | FFRangio | CASS vFFR | FlashAngio caFFR | |
| Vendors | Medis Medical Imaging | CathWorks | Pie Medical Imaging | RainMed |
| Angiography | 2 projections > 25° apart | ≥2 projections 30° apart | 2 projections > 30° apart | 2 projections > 30° apart |
| Data inputs | -3D coronary model -TIMI frame counting | -3D coronary model -Aortic pressure | -3D coronary model -Empiric hyperemic flow -Aortic pressure | -3D coronary model -TIMI frame counting -Dynamic aortic pressure |
| Post processing | Integrated mathematical approach (Bernoulli and Poiseuille) | Flow resistance analysis | Integrated mathematical approach (Bernoulli and Poiseuille) | Computational pressure-flow dynamics |
| Number of vessels analyzed | 1 | Multi-vessels | 1 | 1 |
| Cut-off value | 0.8 | 0.8 | 0.8 | 0.8 |
| Processing time | 5 min | 3.41 min | NA | 4.5 min |
| Limitations | -Correct angiogram is necessary | -Correct angiogram is necessary -Model is mainly based on anatomical features | -Correct angiogram is necessary but even with that high rate of excluded images | -Correct angiogram is necessary with use of auto-injector -Few proofs |
| First Authors, Year, (Ref. #) | N of Patients | Index | Study Methods | Results |
|---|---|---|---|---|
| Pre PCI | ||||
| Masdjedi et al. [11], 2020 | 100 | CASS vFFR | Correlation ofCASS vFFR and FFR in a retrospective study | CASS vFFR correlated with FFR (r = 0.89, p < 0.001), with 95% limit of agreement ± 0.05 |
| Neleman et al. [12], 2021 | 912 | CASS vFFR | Correlation of CASS vFFR and FFR in a retrospective study | CASS vFFR correlated with FFR (r = 0.89, p < 0.001) |
| Masdjedi et al. [13], 2022 | 334 | CASS vFFR | Correlation of CASS vFFR and FFR in a prospective study | CASS vFFR correlated with FFR (r = 0.74, p < 0.001), with 95% limit of agreement ± 0.12 |
| Kornowski et al. [14], 2016 | 88 | FFRangio | Correlation of FFRangio and FFR in a prospective study | FFRangio correlated with FFR (r = 0.90, p < 0.001) |
| Kornowski et al. [15], 2018 | 53 | FFRangio | Correlation of FFRangio and FFR in a prospective study | FFRangio correlated with FFR (r = 0.91, p < 0.001), with 95% limit of agreement ± 0.07 |
| Pellicano et al. [16], 2017 | 184 | FFRangio | Correlation of FFRangio and FFR in a prospective study | FFRangio correlated with FFR (r = 0.88, p < 0.001), with 95% limit of agreement ± 0.10 |
| Fearon et al. [17], 2019 | 301 | FFRangio | Correlation of FFRangio and FFR in a prospective study | FFRangio correlated with FFR (r = 0.80, p < 0.001), with 95% limit of agreement ± 0.13 |
| Omori et al. [18], 2019 | 50 | FFRangio | Correlation of FFRangio and FFR in a prospective study | FFRangio correlated with FFR (r = 0.83, p < 0.001), with 95% limit of agreement ± 0.13 |
| Witberg et al. [19], 2020 | 588 | FFRangio | Correlation of FFRangio and FFR; a pooled analysis of 5 prospective cohort studies | FFRangio correlated with FFR (r = 0.83, p < 0.001), with 95% limit of agreement ± 0.11 |
| Tu et al. [6], 2014 | 68 | QFR | Correlation of QFR and FFR in a retrospective study | QFR correlated with FFR (r = 0.81, p < 0.001), with 95% limit of agreement ± 0.11 |
| Tu et al. [7], 2016 | 73 | QFR | Correlation of QFR and FFR in a prospective study | QFR correlated with FFR (r = 0.77, p < 0.001), with 95% limit of agreement ± 0.12 |
| Westra et al. [8], 2018 | 191 | QFR | Correlation of QFR and FFR in a prospective study | QFR correlated with FFR (r = 0.70, p < 0.0001), with 95% limit of agreement ± 0.16 |
| Westra et al. [9], 2018 | 272 | QFR | Correlation of QFR and FFR in a prospective study | QFR correlated with FFR (r = 0.83, p < 0.001), with 95% limit of agreement ± 0.12 |
| Stähli et al. [10], 2019 | 436 | QFR | Correlation of QFR and FFR in a retrospective study | QFR correlated with FFR (r = 0.8, p < 0.001), with 95% limit of agreement ± 0.07 |
| Xu et al. [26], 2021 | 3847 | QFR | Multicenter, blinded randomized between a QFR-guided strategy or an angiography-guided strategy; the primary endpoint was the 1-year rate of major adverse cardiac events, a composite of death from any cause, myocardial infarction, or ischemia-driven revascularization | The 1-year primary endpoint occurred in 5.8% in the QFR-guided group and in 8.8% participants in the angiography-guided group (p = 0·0004) |
| Li et al. [21], 2020 | 328 | FlashAngio caFFR | Correlation of FlashAngio caFFR and FFR in a prospective multicenter study | FlashAngio caFFR correlated with FFR (r = 0.89, p < 0.001), with a 95% limit of agreement ± 0.09 |
| Post PCI | ||||
| Kogame et al. [28], 2019 | 393 | QFR | Prognostic value of QFR measured immediately after PCI in patients with a de novo 3-vessel disease-retrospective study by VOCO at 2 years | The incidence of 2-year VOCO in the vessels with post-PCI QFR < 0.91 was significantly higher compared with vessels with post-PCI QFR ≥ 0.91 (12.0% vs. 3.7%; HR: 3.37; 95% confidence interval: 1.91 to 5.97; p < 0.001). |
| Biscaglia et al. [29], 2019 | 602 | QFR | Prognostic value of QFR measured immediately after a PCI-prospective study | ROC analysis identified a post-PCI QFR best cutoff of ≤ 0.89 (AUC 0.77; 95% confidence interval: 0.74 to 0.80; p < 0.001). Post-PCI QFR ≤ 0.89 was independent associated with VOCO (hazard ratio: 2.91; 95% confidence interval: 1.63 to 5.19; p < 0.001). |
| Dai et al. [30], 2022 | 1335 | QFR-PBPG, dQFR/ds | Prognosis of 4 patient groups defined by predominant focal disease (QFR-PBPG > 0.78) with (dQFR/dt ≥ 0.005/mm) and without major gradient and predominant diffuse disease (QFR-PBPG ≤ 0.78) with and without major gradient by VOCO in a 2-year retrospective study | At 2 years, VOCO was lowest in patients with predominant focal without major gradient (1.4% vs. 5.4% in predominant focal with major gradient patients vs. 4.8% in predominant diffuse without major gradient patients vs. 8.5% in predominant diffuse with major gradient patients, all p < 0.05), whereas there was no prognostic value for classifications by visual assessment. Physiological residual disease patterns were independently associated with VOCO and showed increased prognostic value when introduced to a model with clinical risk factors only (C index: 0.77 vs. 0.68, p < 0.008; NRI: 0.65, p < 0.001; IDI: 0.020, p < 0.001). |
| Masdjedi et al. [31], 2020 | 100 | CASS vFFR | Correlation CASS vFFR and a FFR-retrospective study | CASS vFFR correlated with FFR (r = 0.88, p < 0.001), with 95% limit of agreement ± 0.06 |
| Pizzato et al. [32], 2020 | 115 | CASS vFFR | Correlation CASS vFFR and FFR pre and post PCI-retrospective study | -CASS vFFR could be analyzed in about one-third of previously completed angiographies. -Pearson’s correlation coefficient between pre-PCI FFR and CASS vFFR was 0.449 (p = 0.0001). -Pearson’s correlation coefficient between post-PCI FFR and CASS vFRR was 0.115 (p = 0.2703). |
| Virtual PCI | ||||
| Rubimbura et al. [33] 2020 | 93 | QFR | Residual QFR and post-PCI QFR were compared to a post-PCI FFR-retrospective study | The correlation coefficient of residual QFR with post-PCI FFR was 0.68 (95% CI: 0.53–0.78) and the correlation coefficient of post-PCI-QFR with post-PCI FFR was 0.79 (95% CI: 0.70–0.86). |
| Lee et al. [34], 2022 | 274 | QFR | QFR and residual QFR were compered to pre-PCI FFR and post-PCI FFR. Prognosis of rQFR was analyzed by a VOCO-retrospective study. | -Pre-PCI QFR and FFR were correlated (r = 0.756, p < 0.001). -rQFR and FFR post PCI were correlated (r = 0.528, p < 0.001). -rQFR predicted incidence of 2-year VOCO after index PCI (AUC: 0.712 [0.555–0.869], p = 0.041). |
| Tomaniak et al. [35], 2022 | 81 | CASS vFFR | Residual CASS vFFR and post-PCI CASS vFFR were compared to a post-PCI FFR-retrospective study. | -Residual vFFR and post-PCI FFR were correlated (r = 0.84, p < 0.001). -Residual CASS vFFR and post-PCI CASS vFFR were correlated (r = 0.77, p < 0.001). |
| Zhang et al. [36], 2022 | 2348 | QFR | Concordance between residual QFR and post-PCI QFR; prognostic value of residual QFR (VOCO); and forecast of outcomes by virtual randomized controlled trials between residual QFR and an angiographic guidance- retrospective study. | Residual QFR and post-PCI QFR were correlated (r =0.976, p < 0.0001). Low residual QFR (≤0.92) was independently associated with higher risk of 2-year VOCO (adjusted hazard ratio: 5.50; 95% confidence interval: 3.03 to 10.0). Simulated residual QFR-guided strategy had a 2.6% absolute reduction of 2-year incidence of VOCO compared with the angiography-guided strategy. |
| Biscaglia et al. [37], 2022 | 111 | Pullback QFR, QFR-PBPG | Analyze the link between focal, serial lesions; diffuse disease; combination defined by pullback QFR or QFR PBPG prePCI with suboptimal PCI result (post-PCI QFR value ≤ 0.89) in post hoc analysis of the HAWKEYE study. | Suboptimal PCI result occurrences differed across functional patterns of CAD (focal 8% vs. serial lesions 15% vs. diffuse disease 33% vs. combination 29%, p = 0.03). Similarly, QFR-PBPG was correlated with post-PCI QFR value (r = 0.62, 95% CI 0.50–0.72). |
| Dai et al. [38], 2022 | 1003 | QFR-PBPG | Prognostic value QFR-PBPG prePCI by VOCO in a 2-year retrospective study. | After multiple adjustment, QFR-PBPG was an independent predictor for VOCO (HR 1.30, 95% CI 1.05–1.62). The addition of QFR-PBPG to the model of clinical risk factors substantially improved the predictions of VOCO (C-index 0.67 vs. 0.62, net reclassification index 0.42). |
| Dai et al., [39], 2022 | 1744 | QFR-PBPG | Prognostic value of classification according to dichotomous pre-PCI QFR-PBPG and post-PCI QFR by VOCO in a 2-year retrospective study. | Vessels with low pre-PCI QFR-PBPG (3.9% versus 2.0%, HR 1.93; 95% CI, 1.08–3.44; p = 0.02) or low post-PCI QFR-PBPG (9.8% versus 2.7%, HR, 3.78; 95% CI, 1.61–8.87; p = 0.001) demonstrated higher VOCO risk after PCI. Despite high post-PCI QFR-PBPG being achieved, vessels with low pre-PCI QFR-PBPG presented a higher risk of VOCO than those with high pre-PCI QFR-PBPG (3.7% versus 1.8%, HR, 2.03; 95% CI, 1.09–3.76; p = 0.03). |
| Shin et al. [40], 2021 | 341 | QFR-PBPG, qQFR/ds | Prognosis of 4 patient groups defined by predominant focal disease (QFR-PBPG ≥ 0.78) with (dQFR/dt ≥ 0.025/mm) and without major gradient and predominant diffuse disease (QFR-PBPG < 0.78) with and without major gradient by VOCO in a 2-year retrospective study. | Cumulative incidence of VOCO after PCI was significantly higher in patients with predominant diffuse disease (8.1% predominant diffuse disease with major gradient and 9.9% in predominant diffuse disease without major gradient vs. 1.4% predominant focal disease with major gradient and 0.0% in predominant focal disease without major gradient; overall p = 0.024). |
| Dai et al. [41], 2023 | 286 | Flashangio ca FFR | Residual flashangio caFFR and post-PCI flashangio caFFR were compared to post-PCI FFR, and the prognosis value of residual flashangio caFFR was analyzed in a retrospective study (VOCO). | -Pre-PCI flashangio caFFR (r = 0.88, p < 0.001) and post-PCI flashangio caFFR (r = 0.76, p < 0.001) showed a close correlation with FFR. -Residual flashangio caFFR and post PCI FFR were correlated (r = 0.88, p < 0.001). Residual flashangio caFFR and post-PCI flashangio caFFR were correlated (r = 0.82, p < 0.001). -Suboptimal residual flashangio caFFR (≤0.89) was associated with increased risk of 2-year VOCO (adjusted HR: 3.71; 95% confidence interval: 1.50−9.17). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caullery, B.; Riou, L.; Barone-Rochette, G. Coronary Angiography Upgraded by Imaging Post-Processing: Present and Future Directions. Diagnostics 2023, 13, 1978. https://doi.org/10.3390/diagnostics13111978
Caullery B, Riou L, Barone-Rochette G. Coronary Angiography Upgraded by Imaging Post-Processing: Present and Future Directions. Diagnostics. 2023; 13(11):1978. https://doi.org/10.3390/diagnostics13111978
Chicago/Turabian StyleCaullery, Benoit, Laurent Riou, and Gilles Barone-Rochette. 2023. "Coronary Angiography Upgraded by Imaging Post-Processing: Present and Future Directions" Diagnostics 13, no. 11: 1978. https://doi.org/10.3390/diagnostics13111978






