Paget’s Disease of the Bone and Lynch Syndrome: An Exceptional Finding
Abstract
:1. Introduction
1.1. Paget’s Disease of Bone
1.2. Lynch Syndrome
1.3. Aim
2. Case Report
2.1. Presentation: On Admission
2.2. Gene Testing: Confirmation of Harbouring Two Pathogenic Variants
2.3. Case Management
3. Discussion
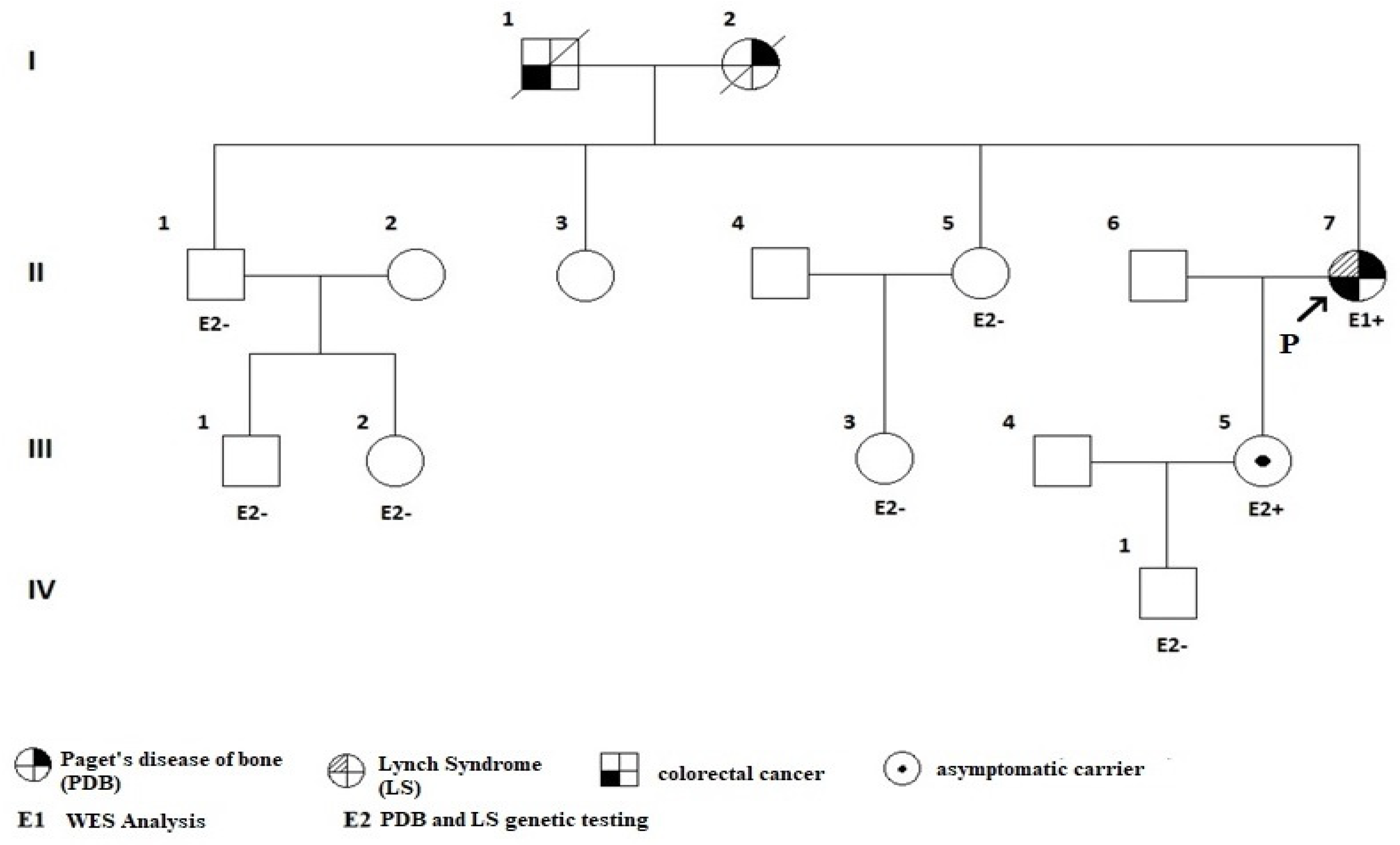
3.1. The First Reported Case of PDB (Maternal Inheritance) and LS (Paternal Inheritance)
3.2. Genetic Considerations
3.3. Bone Turnover Markers’ Profile: From PDB Diagnosis to Outcome
3.4. Denosumab Use in PBD
3.5. Cancer Risk in Patients with PDB: Focus on Osteosarcoma
3.6. MMR Pathogenic Variants and Skeleton Findings: How Far to an Osteosarcoma?
3.7. Other Clinical Observations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ALP | alkaline phosphatase |
| BALP | bone alkaline phosphatase |
| CTX | type I collagen C-telopeptide |
| DXA | dual-energy X-ray absorptiometry |
| GCT | giant cell tumour |
| GWAS | Genome Wide Association Studies |
| LS | Lynch syndrome |
| MMR | mismatch repair |
| NTx | N-telopeptide |
| OPG | osteoprotegerin |
| RANKL | receptor activator of nuclear factor-kB ligand |
| P1NP | procollagen type 1 amino-terminal peptide |
| PCR | polymerase chain reaction |
| PDB | Paget’s disease of bone |
| PTH | parathormone (parathyroid hormone) |
| SQSTM1 | sequestosome 1 |
| TBS | trabecular bone score |
| 99mTc-MDP | technetium-99m methylene diphosphonate |
| WES | whole exome sequencing |
References
- Gennari, L.; Rendina, D.; Merlotti, D.; Cavati, G.; Mingiano, C.; Cosso, R.; Materozzi, M.; Pirrotta, F.; Abate, V.; Calabrese, M.; et al. Update on the Pathogenesis and Genetics of Paget’s Disease of Bone. Front. Cell Dev. Biol. 2022, 10, 932065. [Google Scholar] [CrossRef]
- Paget, J. On a Form of Chronic Inflammation of Bones (Osteitis Deformans). Medico-Chir. Trans. 1877, 60, 37–64.9. [Google Scholar] [CrossRef]
- Seitz, S.; Priemel, M.; von Domarus, C.; Beil, F.T.; Barvencik, F.; Amling, M.; Rueger, J.M. The Second Most Common Bone Disease: A Review on Paget’s Disease of Bone. Eur. J. Trauma. Emerg. Surg. 2008, 34, 549–553. [Google Scholar] [CrossRef] [PubMed]
- Corral-Gudino, L.; Borao-Cengotita-Bengoa, M.; Pino-Montes, J.D.; Ralston, S. Epidemiology of Paget’s Disease of Bone: A Systematic Review and Meta-Analysis of Secular Changes. Bone 2013, 55, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Rogers, J.; Jeffrey, D.R.; Watt, I. Paget’s Disease in an Archeological Population. J. Bone Miner. Res. 2002, 17, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Poór, G.; Donáth, J.; Fornet, B.; Cooper, C. Epidemiology of Paget’s Disease in Europe: The Prevalence Is Decreasing. J. Bone Miner. Res. 2006, 21, 1545–1549. [Google Scholar] [CrossRef] [PubMed]
- Morales-Piga, A.A.; Bachiller-Corral, F.J.; Abraira, V.; Beltrán, J.; Rapado, A. Is Clinical Expressiveness of Paget’s Disease of Bone Decreasing? Bone 2002, 30, 399–403. [Google Scholar] [CrossRef]
- Alonso, N.; Calero-Paniagua, I.; Del Pino-Montes, J. Clinical and Genetic Advances in Paget’s Disease of Bone: A Review. Clin. Rev. Bone Miner. Metab. 2017, 15, 37–48. [Google Scholar] [CrossRef] [Green Version]
- Roodman, G.D.; Windle, J.J. Paget Disease of Bone. J. Clin. Investig. 2005, 115, 200–208. [Google Scholar] [CrossRef]
- Banaganapalli, B.; Fallatah, I.; Alsubhi, F.; Shetty, P.J.; Awan, Z.; Elango, R.; Shaik, N.A. Paget’s Disease: A Review of the Epidemiology, Etiology, Genetics, and Treatment. Front. Genet. 2023, 14, 1131182. [Google Scholar] [CrossRef]
- Mills, B.G.; Singer, F.R.; Weiner, L.P.; Suffin, S.C.; Stabile, E.; Holst, P. Evidence for Both Respiratory Syncytial Virus and Measles Virus Antigens in the Osteoclasts of Patients with Paget’s Disease of Bone. Clin. Orthop. Relat. Res. 1984, 183, 303–311. [Google Scholar] [CrossRef]
- Lever, J.H. Paget’s Disease of Bone in Lancashire and Arsenic Pesticide in Cotton Mill Wastewater: A Speculative Hypothesis. Bone 2002, 31, 434–436. [Google Scholar] [CrossRef]
- Barker, D.J.; Gardner, M.J. Distribution of Paget’s Disease in England, Wales and Scotland and a Possible Relationship with Vitamin D Deficiency in Childhood. Br. J. Prev. Soc. Med. 1974, 28, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Hamdy, R. Trauma and Paget’s Disease of Bone. Br. Med. J. 1979, 1, 1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, N.; Hiruma, Y.; Yamana, K.; Michou, L.; Rousseau, C.; Morissette, J.; Galson, D.L.; Teramachi, J.; Zhou, H.; Dempster, D.W.; et al. Contributions of the Measles Virus Nucleocapsid Gene and the SQSTM1/P62(P392L) Mutation to Paget’s Disease. Cell Metab. 2011, 13, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Hosking, D.J. Paget’s Disease of Bone. Br. Med. J. (Clin. Res. Ed.) 1981, 283, 686–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ralston, S.H.; Corral-Gudino, L.; Cooper, C.; Francis, R.M.; Fraser, W.D.; Gennari, L.; Guañabens, N.; Javaid, M.K.; Layfield, R.; O’Neill, T.W.; et al. Diagnosis and Management of Paget’s Disease of Bone in Adults: A Clinical Guideline. J. Bone Miner. Res. 2019, 34, 579–604. [Google Scholar] [CrossRef] [PubMed]
- Gennari, L.; Rendina, D.; Falchetti, A.; Merlotti, D. Paget’s Disease of Bone. Calcif. Tissue Int. 2019, 104, 483–500. [Google Scholar] [CrossRef]
- Singer, F.R. Bone Quality in Paget’s Disease of Bone. Curr. Osteoporos. Rep. 2016, 14, 39–42. [Google Scholar] [CrossRef]
- Zimmermann, E.A.; Köhne, T.; Bale, H.A.; Panganiban, B.; Gludovatz, B.; Zustin, J.; Hahn, M.; Amling, M.; Ritchie, R.O.; Busse, B. Modifications to Nano- and Microstructural Quality and the Effects on Mechanical Integrity in Paget’s Disease of Bone. J. Bone Miner. Res. 2015, 30, 264–273. [Google Scholar] [CrossRef] [Green Version]
- Viguet-Carrin, S.; Garnero, P.; Delmas, P.D. The Role of Collagen in Bone Strength. Osteoporos. Int. 2006, 17, 319–336. [Google Scholar] [CrossRef]
- Kravets, I. Paget’s Disease of Bone: Diagnosis and Treatment. Am. J. Med. 2018, 131, 1298–1303. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.; Ralston, S.H. Clinical Presentation of Paget’s Disease: Evaluation of a Contemporary Cohort and Systematic Review. Calcif. Tissue Int. 2014, 95, 385–392. [Google Scholar] [CrossRef] [PubMed]
- Makaram, N.; Woods, L.; Beattie, N.; Roberts, S.B.; Macpherson, G.J. Long-Term Outcomes Following Total Hip and Total Knee Arthroplasty in Patients with Paget’s Disease of Bone (PDB)—A National Study. Surgeon 2020, 18, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Evangelatos, G.; Iliopoulos, A. Headache in Patients with Paget’s Disease of Bones. J. Frailty Sarcopenia Falls 2017, 2, 16–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al Nofal, A.A.; Altayar, O.; BenKhadra, K.; Qasim Agha, O.Q.; Asi, N.; Nabhan, M.; Prokop, L.J.; Tebben, P.; Murad, M.H. Bone Turnover Markers in Paget’s Disease of the Bone: A Systematic Review and Meta-Analysis. Osteoporos. Int. 2015, 26, 1875–1891. [Google Scholar] [CrossRef]
- Gillett, M.J.; Vasikaran, S.D.; Inderjeeth, C.A. The Role of PINP in Diagnosis and Management of Metabolic Bone Disease. Clin. Biochem. Rev. 2021, 42, 3–10. [Google Scholar] [CrossRef]
- Singer, F.R.; Bone, H.G.; Hosking, D.J.; Lyles, K.W.; Murad, M.H.; Reid, I.R.; Siris, E.S. Endocrine Society Paget’s Disease of Bone: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 2014, 99, 4408–4422. [Google Scholar] [CrossRef] [Green Version]
- Brandi, M.L.; Falchetti, A. What Is the Relationship Between Paget’s Disease of Bone and Hyperparathyroidism? J. Bone Miner. Res. 2006, 21, P69–P74. [Google Scholar] [CrossRef]
- Kannan, S.; Mahadevan, S.; Sathya, A.; Sriram, U. A Tale of Three Diseases of the Bone. Singapore Med. J. 2008, 49, e263–e265. [Google Scholar]
- Teramachi, J.; Hiruma, Y.; Ishizuka, S.; Ishizuka, H.; Brown, J.P.; Michou, L.; Cao, H.; Galson, D.L.; Subler, M.A.; Zhou, H.; et al. Role of ATF7-TAF12 Interactions in the VDR Hyper-Sensitivity of Osteoclast Precursors in Paget’s Disease. J. Bone Miner. Res. 2013, 28, 1489–1500. [Google Scholar] [CrossRef] [Green Version]
- Merlotti, D.; Rendina, D.; Cavati, G.; Abate, V.; Falchetti, A.; Mingiano, C.; Nuti, R.; Gennari, L. Drug Treatment Strategies for Paget’s Disease: Relieving Pain and Preventing Progression. Expert. Opin. Pharmacother. 2023, 24, 715–727. [Google Scholar] [CrossRef]
- Russell, R.; Graham, G. Bisphosphonates: From Bench to Bedside. Ann. N. Y. Acad. Sci. 2006, 1068, 367–401. [Google Scholar] [CrossRef] [Green Version]
- Tsoumpra, M.K.; Muniz, J.R.; Barnett, B.L.; Kwaasi, A.A.; Pilka, E.S.; Kavanagh, K.L.; Evdokimov, A.; Walter, R.L.; Von Delft, F.; Ebetino, F.H.; et al. The inhibition of human farnesyl pyrophosphate synthase by nitrogen-containing bisphosphonates. Elucidating the role of active site threonine 201 and tyrosine 204 residues using enzyme mutants. Bone 2015, 81, 478–486. [Google Scholar] [CrossRef] [Green Version]
- Garnero, P.; Fledelius, C.; Gineyts, E.; Serre, C.M.; Vignot, E.; Delmas, P.D. Decreased Beta-Isomerization of the C-Terminal Telopeptide of Type I Collagen Alpha 1 Chain in Paget’s Disease of Bone. J. Bone Miner. Res. 1997, 12, 1407–1415. [Google Scholar] [CrossRef]
- Langston, A.L.; Campbell, M.K.; Fraser, W.D.; MacLennan, G.S.; Selby, P.L.; Ralston, S.H. PRISM Trial Group Randomized Trial of Intensive Bisphosphonate Treatment versus Symptomatic Management in Paget’s Disease of Bone. J. Bone Miner. Res. 2010, 25, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Garnero, P.; Gineyts, E.; Schaffer, A.V.; Seaman, J.; Delmas, P.D. Measurement of Urinary Excretion of Nonisomerized and Beta-Isomerized Forms of Type I Collagen Breakdown Products to Monitor the Effects of the Bisphosphonate Zoledronate in Paget’s Disease. Arthritis Rheum. 1998, 41, 354–360. [Google Scholar] [CrossRef] [PubMed]
- Paul Tuck, S.; Layfield, R.; Walker, J.; Mekkayil, B.; Francis, R. Adult Paget’s Disease of Bone: A Review. Rheumatology 2017, 56, 2050–2059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cremers, S.C.; Eekhoff, M.E.; Den Hartigh, J.; Hamdy, N.A.; Vermeij, P.; Papapoulos, S.E. Relationships Between Pharmacokinetics and Rate of Bone Turnover After Intravenous Bisphosphonate (Olpadronate) in Patients with Paget’s Disease of Bone. J. Bone Miner. Res. 2003, 18, 868–875. [Google Scholar] [CrossRef]
- Win, A.K.; Jenkins, M.A.; Dowty, J.G.; Antoniou, A.C.; Lee, A.; Giles, G.G.; Buchanan, D.D.; Clendenning, M.; Rosty, C.; Ahnen, D.J.; et al. Prevalence and Penetrance of Major Genes and Polygenes for Colorectal Cancer. Cancer Epidemiol. Biomark. Prev. 2017, 26, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.H.; Hadjinicolaou, A.V.; Norton, B.C.; Kader, R.; Lovat, L.B. Lynch Syndrome: From Detection to Treatment. Front. Oncol. 2023, 13, 1166238. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.A.; Pritchard, C.C.; Jarvik, G.P. Lynch Syndrome: From Screening to Diagnosis to Treatment in the Era of Modern Molecular Oncology. Annu. Rev. Genom. Hum. Genet. 2019, 20, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Gray, P.N.; Tsai, P.; Chen, D.; Wu, S.; Hoo, J.; Mu, W.; Li, B.; Vuong, H.; Lu, H.-M.; Batth, N.; et al. Tumor Next-Lynch-MMR: A Comprehensive next Generation Sequencing Assay for the Detection of Germline and Somatic Mutations in Genes Associated with Mismatch Repair Deficiency and Lynch Syndrome. Oncotarget 2018, 9, 20304–20322. [Google Scholar] [CrossRef] [Green Version]
- Duraturo, F.; Liccardo, R.; De Rosa, M.; Izzo, P. Genetics, Diagnosis and Treatment of Lynch Syndrome: Old Lessons and Current Challenges. Oncol. Lett. 2019, 17, 3048–3054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinicrope, F.A. Lynch Syndrome-Associated Colorectal Cancer. N. Engl. J. Med. 2018, 379, 764–773. [Google Scholar] [CrossRef]
- Kansikas, M.; Vähätalo, L.; Kantelinen, J.; Kasela, M.; Putula, J.; Døhlen, A.; Paloviita, P.; Kärkkäinen, E.; Lahti, N.; Arnez, P.; et al. Tumor-Independent Detection of Inherited Mismatch Repair Deficiency for the Diagnosis of Lynch Syndrome with High Specificity and Sensitivity. Cancer Res. Commun. 2023, 3, 361–370. [Google Scholar] [CrossRef]
- Hiruma, Y.; Kurihara, N.; Subler, M.A.; Zhou, H.; Boykin, C.S.; Zhang, H.; Ishizuka, S.; Dempster, D.W.; Roodman, G.D.; Windle, J.J. A SQSTM1/P62 Mutation Linked to Paget’s Disease Increases the Osteoclastogenic Potential of the Bone Microenvironment. Hum. Mol. Genet. 2008, 17, 3708–3719. [Google Scholar] [CrossRef] [Green Version]
- Chamoux, E.; Couture, J.; Bisson, M.; Morissette, J.; Brown, J.P.; Roux, S. The P62 P392L Mutation Linked to Paget’s Disease Induces Activation of Human Osteoclasts. Mol. Endocrinol. 2009, 23, 1668–1680. [Google Scholar] [CrossRef] [Green Version]
- Sundaram, K.; Shanmugarajan, S.; Rao, D.S.; Reddy, S.V. Mutant P62P392L Stimulation of Osteoclast Differentiation in Paget’s Disease of Bone. Endocrinology 2011, 152, 4180–4189. [Google Scholar] [CrossRef] [Green Version]
- Visconti, M.R.; Langston, A.L.; Alonso, N.; Goodman, K.; Selby, P.L.; Fraser, W.D.; Ralston, S.H. Mutations of SQSTM1 Are Associated with Severity and Clinical Outcome in Paget Disease of Bone. J. Bone Miner. Res. 2010, 25, 2368–2373. [Google Scholar] [CrossRef]
- Mangold, E.; Pagenstecher, C.; Friedl, W.; Mathiak, M.; Buettner, R.; Engel, C.; Loeffler, M.; Holinski-Feder, E.; Müller-Koch, Y.; Keller, G.; et al. Spectrum and Frequencies of Mutations in MSH2 and MLH1 Identified in 1,721 German Families Suspected of Hereditary Nonpolyposis Colorectal Cancer. Int. J. Cancer 2005, 116, 692–702. [Google Scholar] [CrossRef]
- Bonadona, V.; Bonaïti, B.; Olschwang, S.; Grandjouan, S.; Huiart, L.; Longy, M.; Guimbaud, R.; Buecher, B.; Bignon, Y.-J.; Caron, O.; et al. Cancer Risks Associated with Germline Mutations in MLH1, MSH2, and MSH6 Genes in Lynch Syndrome. JAMA 2011, 305, 2304–2310. [Google Scholar] [CrossRef] [Green Version]
- Miladi, S.; Rouached, L.; Maatallah, K.; Rahmouni, S.; Fazaa, A.; Sellami, M.; Ferjani, H.; Kaffel, D.; Hamdi, W.; Abdelghani, K.B.; et al. Complications of Paget Bone Disease: A Study of 69 Patients. Curr. Rheumatol. Rev. 2021, 17, 390–396. [Google Scholar] [CrossRef] [PubMed]
- Pálla, S.; Anker, P.; Farkas, K.; Plázár, D.; Kiss, S.; Marschalkó, P.; Szalai, Z.; Bene, J.; Hadzsiev, K.; Maróti, Z.; et al. Co-Occurrence of Neurofibromatosis Type 1 and Pseudoachondroplasia—A First Case Report. BMC Pediatr. 2023, 23, 110. [Google Scholar] [CrossRef]
- Ebner, K.; Reintjes, N.; Feldkötter, M.; Körber, F.; Nagel, M.; Dötsch, J.; Hoppe, B.; Weber, L.T.; Beck, B.B.; Liebau, M.C. A Case Report on the Exceptional Coincidence of Two Inherited Renal Disorders: ADPKD and Alport Syndrome. Clin. Nephrol. 2017, 88, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Miller, P.; Lyles, K.; Fraser, W.; Brown, J.P.; Saidi, Y.; Mesenbrink, P.; Su, G.; Pak, J.; Zelenakas, K.; et al. Comparison of a Single Infusion of Zoledronic Acid with Risedronate for Paget’s Disease. N. Engl. J. Med. 2005, 353, 898–908. [Google Scholar] [CrossRef] [Green Version]
- Polyzos, S.A.; Makras, P.; Tournis, S.; Anastasilakis, A.D. Off-Label Uses of Denosumab in Metabolic Bone Diseases. Bone 2019, 129, 115048. [Google Scholar] [CrossRef] [PubMed]
- Michou, L.; Brown, J.P. Emerging Strategies and Therapies for Treatment of Paget’s Disease of Bone. Drug. Des. Devel Ther. 2011, 5, 225–239. [Google Scholar] [CrossRef] [Green Version]
- Menaa, C.; Reddy, S.V.; Kurihara, N.; Maeda, H.; Anderson, D.; Cundy, T.; Cornish, J.; Singer, F.R.; Bruder, J.M.; Roodman, G.D. Enhanced RANK Ligand Expression and Responsivity of Bone Marrow Cells in Paget’s Disease of Bone. J. Clin. Investig. 2000, 105, 1833–1838. [Google Scholar] [CrossRef] [Green Version]
- Kostine, M.; Mehsen-Cetre, N.; Bannwarth, B. Denosumab-Induced Severe Hypocalcemia in a Patient with Paget’s Disease of Bone and Impaired Renal Function. Therapie 2017, 72, 383–385. [Google Scholar] [CrossRef]
- Kuthiah, N.; Er, C. Effective Treatment of Paget’s Disease of the Bone in a Chinese Woman. Ann. Acad. Med. Singap. 2018, 47, 528–530. [Google Scholar] [CrossRef]
- Schwarz, P.; Rasmussen, A.Q.; Kvist, T.M.; Andersen, U.B.; Jørgensen, N.R. Paget’s Disease of the Bone after Treatment with Denosumab: A Case Report. Bone 2012, 50, 1023–1025. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Puri, A.; Shah, S.; Rekhi, B.; Gulia, A. Giant Cell Tumor Developing in Paget’s Disease of Bone: A Case Report with Review of Literature. J. Orthop. Case Rep. 2016, 6, 103–107. [Google Scholar] [PubMed]
- Tanaka, T.; Slavin, J.; McLachlan, S.-A.; Choong, P. Anti-Osteoclastic Agent, Denosumab, for a Giant Cell Tumor of the Bone with Concurrent Paget’s Disease: A Case Report. Oncol. Lett. 2017, 13, 2105–2108. [Google Scholar] [CrossRef]
- Reid, I.R.; Sharma, S.; Kalluru, R.; Eagleton, C. Treatment of Paget’s Disease of Bone with Denosumab: Case Report and Literature Review. Calcif. Tissue Int. 2016, 99, 322–325. [Google Scholar] [CrossRef]
- Hansen, M.F.; Seton, M.; Merchant, A. Osteosarcoma in Paget’s Disease of Bone. J. Bone Miner. Res. 2006, 21, 58–63. [Google Scholar] [CrossRef]
- Tilden, W.; Saifuddin, A. An Update on Imaging of Paget’s Sarcoma. Skeletal Radiol. 2021, 50, 1275–1290. [Google Scholar] [CrossRef]
- Shoaib, Z.; Fan, T.M.; Irudayaraj, J.M.K. Osteosarcoma Mechanobiology and Therapeutic Targets. Br. J. Pharmacol. 2022, 179, 201–217. [Google Scholar] [CrossRef]
- Ferreira, R.M.; Vieira, L.; Pimenta, S.; Pinto, J.; Costa, L. Chondrosarcoma as Inaugural Manifestation of Monostotic Paget’s Disease of Bone. Acta Reumatol. Port. 2019, 44, 163–164. [Google Scholar]
- Dominguez-Valentin, M.; Sampson, J.R.; Møller, P.; Seppälä, T.T. PLSD Collaborators Analysis in the Prospective Lynch Syndrome Database Identifies Sarcoma as Part of the Lynch Syndrome Tumor Spectrum. Int. J. Cancer 2021, 148, 512–513. [Google Scholar] [CrossRef] [PubMed]
- De Angelis de Carvalho, N.; Niitsuma, B.N.; Kozak, V.N.; Costa, F.D.; de Macedo, M.P.; Kupper, B.E.C.; Silva, M.L.G.; Formiga, M.N.; Volc, S.M.; Aguiar Junior, S.; et al. Clinical and Molecular Assessment of Patients with Lynch Syndrome and Sarcomas Underpinning the Association with MSH2 Germline Pathogenic Variants. Cancers 2020, 12, 1848. [Google Scholar] [CrossRef] [PubMed]
- Lynch, H.T.; Deters, C.A.; Hogg, D.; Lynch, J.F.; Kinarsky, Y.; Gatalica, Z. Familial Sarcoma. Cancer 2003, 98, 1947–1957. [Google Scholar] [CrossRef]
- Nilbert, M.; Therkildsen, C.; Nissen, A.; Akerman, M.; Bernstein, I. Sarcomas Associated with Hereditary Nonpolyposis Colorectal Cancer: Broad Anatomical and Morphological Spectrum. Fam. Cancer 2009, 8, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Valentin, M.; Sampson, J.R.; Seppälä, T.T.; Ten Broeke, S.W.; Plazzer, J.-P.; Nakken, S.; Engel, C.; Aretz, S.; Jenkins, M.A.; Sunde, L.; et al. Cancer Risks by Gene, Age, and Gender in 6350 Carriers of Pathogenic Mismatch Repair Variants: Findings from the Prospective Lynch Syndrome Database. Genet. Med. 2020, 22, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Baglietto, L.; Lindor, N.M.; Dowty, J.G.; White, D.M.; Wagner, A.; Gomez Garcia, E.B.; Vriends, A.H.J.T.; Dutch Lynch Syndrome Study Group; Cartwright, N.R.; Barnetson, R.A.; et al. Risks of Lynch Syndrome Cancers for MSH6 Mutation Carriers. JNCI J. Natl. Cancer Inst. 2010, 102, 193–201. [Google Scholar] [CrossRef]
- Mirabello, L.; Troisi, R.J.; Savage, S.A. International Osteosarcoma Incidence Patterns in Children and Adolescents, Middle Ages and Elderly Persons. Int. J. Cancer 2009, 125, 229–234. [Google Scholar] [CrossRef] [Green Version]
- Mirabello, L.; Zhu, B.; Koster, R.; Karlins, E.; Dean, M.; Yeager, M.; Gianferante, M.; Spector, L.G.; Morton, L.M.; Karyadi, D.; et al. Frequency of Pathogenic Germline Variants in Cancer-Susceptibility Genes in Patients with Osteosarcoma. JAMA Oncol. 2020, 6, 724–734. [Google Scholar] [CrossRef]
- Yang, J.; Wang, N. Analysis of the Molecular Mechanism of Osteosarcoma Using a Bioinformatics Approach. Oncol. Lett. 2016, 12, 3075–3080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, C.; Wang, Y.; Luo, Y.; Pang, Z.; Zhou, Y.; Min, L.; Tu, C. Synchronous Lung and Multiple Soft Tissue Metastases Developed from Osteosarcoma of Tibia: A Rare Case Report and Genetic Profile Analysis. BMC Musculoskelet. Disord. 2022, 23, 74. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, Y.; Gong, Z.; Li, J.; Zhao, X.; Yang, Q.; Yu, H.; Ye, J.; Liang, J.; Jiang, L.; et al. FAT1 and MSH2 Are Predictive Prognostic Markers for Chinese Osteosarcoma Patients Following Chemotherapeutic Treatment. J. Bone Miner. Res. 2022, 37, 885–895. [Google Scholar] [CrossRef]
- Erol, R.S.; Sen, E.C.; Ozturk, F.Y.; Alayci, B.; Altuntas, Y. Frequency of Metabolic Syndrome in Paget’s Disease of Bone. Sisli Etfal Hastan. Tip. Bul. 2022, 56, 360–364. [Google Scholar] [CrossRef]
- Cianferotti, L.; Cipriani, C.; Corbetta, S.; Corona, G.; Defeudis, G.; Lania, A.G.; Messina, C.; Napoli, N.; Mazziotti, G. Bone quality in endocrine diseases: Determinants and clinical relevance. J. Endocrinol. Investig. 2023, 46, 1283–1304. [Google Scholar] [CrossRef]
- Sandru, F.; Carsote, M.; Dumitrascu, M.C.; Albu, S.E.; Valea, A. Glucocorticoids and Trabecular Bone Score. J. Med. Life 2020, 13, 449–453. [Google Scholar] [CrossRef]
- Conte, C.; Pelligra, S.; Sarpietro, G.; Montana, G.D.; Della Corte, L.; Bifulco, G.; Martinelli, C.; Ercoli, A.; Palumbo, M.; Cianci, S. Hereditary Women’s Cancer: Management and Risk-Reducing Surgery. Medicina 2023, 59, 300. [Google Scholar] [CrossRef] [PubMed]
- Verrienti, A.; Carbone, A.; Sponziello, M.; Pecce, V.; Cito, D.S.; Bruno, R. Papillary thyroid carcinoma as first and isolated neoplastic disease in a Lynch syndrome family member with a germline MLH1 mutation. Endocrine 2022, 77, 199–202. [Google Scholar] [CrossRef]
- Aswath, K.; Welch, J.; Gubbi, S.; Veeraraghavan, P.; Avadhanula, S.; Gara, S.K.; Dikoglu, E.; Merino, M.; Raffeld, M.; Xi, L.; et al. Co-Occurrence of Familial Non-Medullary Thyroid Cancer (FNMTC) and Hereditary Non-Polyposis Colorectal Cancer (HNPCC) Associated Tumors-A Cohort Study. Front. Endocrinol. 2021, 12, 653401. [Google Scholar] [CrossRef]
- Punzi, L.; Avossa, M.; De Zambiasi, P.; Volpe, A.; Cesaro, G.; Schiavon, F.; Todesco, S. Paget’s disease of bone associated with autoimmune thyroiditis and joint chondrocalcinosis. Rev. Rhum. Ed. Fr. 1994, 61, 354–356. [Google Scholar] [PubMed]
- Sandru, F.; Carsote, M.; Albu, S.E.; Dumitrascu, M.C.; Valea, A. Vitiligo and chronic autoimmune thyroiditis. J. Med. Life 2021, 14, 127–130. [Google Scholar] [CrossRef]
- Lee, H.J.; Stefan-Lifshitz, M.; Li, C.W.; Tomer, Y. Genetics and epigenetics of autoimmune thyroid diseases: Translational implications. Best Pract. Res. Clin. Endocrinol. Metab. 2023, 37, 101661. [Google Scholar] [CrossRef]
- Ragusa, F.; Fallahi, P.; Elia, G.; Gonnella, D.; Paparo, S.R.; Giusti, C.; Churilov, L.P.; Ferrari, S.M.; Antonelli, A. Hashimotos’ thyroiditis: Epidemiology, pathogenesis, clinic and therapy. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101367. [Google Scholar] [CrossRef] [PubMed]
- Hegde, G.; Azzopardi, C.; Patel, A.; Davies, A.M.; James, S.L.; Botchu, R. “Do-not-touch” lesions of bone revisited. Clin. Radiol. 2022, 77, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.F.; Aihara, A.Y.; Fernandes, A.D.R.C.; Cardoso, F.N. Imaging of Paget’s Disease of Bone. Radiol. Clin. N. Am. 2022, 60, 561–573. [Google Scholar] [CrossRef] [PubMed]
- Aiba, H.; Nakazato, T.; Matsuo, H.; Kimura, H.; Saito, S.; Sakai, T.; Murakami, H.; Kawai, J.; Kawasaki, S.; Imamura, Y. Bone Metastases from Gastric Cancer Resembling Paget’s Disease: A Case Report. J. Clin. Med. 2022, 11, 7306. [Google Scholar] [CrossRef] [PubMed]
- Schini, M.; Vilaca, T.; Gossiel, F.; Salam, S.; Eastell, R. Bone Turnover Markers: Basic Biology to Clinical Applications. Endocr. Rev. 2023, 44, 417–473. [Google Scholar] [CrossRef] [PubMed]
- Treurniet, S.; Eekhoff, E.M.W.; Schmidt, F.N.; Micha, D.; Busse, B.; Bravenboer, N. A Clinical Perspective on Advanced Developments in Bone Biopsy Assessment in Rare Bone Disorders. Front. Endocrinol. 2020, 11, 399. [Google Scholar] [CrossRef]
- Barale, M.; Sigrist, S.; Bioletto, F.; Maiorino, F.; Ghigo, E.; Mazzetti, R.; Procopio, M. Long-Term Efficacy of Intensive Zoledronate Therapy and Predictors of Retreatment in Paget’s Disease of Bone. Calcif. Tissue Int. 2021, 109, 383–392. [Google Scholar] [CrossRef]
- Nistor, C.E.; Staden, R.S.; Dumitru, A.V.; Găvan, C.S. A Screening Test for Early Diagnosis of Microcellular Bronchopulmonary Cancer-Pilot Study. J. Clin. Med. 2019, 9, 76. [Google Scholar] [CrossRef] [Green Version]
- Cakmak, S.; Umur, L.; Keklikci, K.; Rodop, O. Monostotic Paget’s disease of the second metacarpal. BMJ Case Rep. 2015, 2015, 206877. [Google Scholar] [CrossRef] [Green Version]
- Calif, E.; Vlodavsky, E.; Stahl, S. Ivory fingers: Monostotic Paget’s disease of the phalanges. J. Clin. Endocrinol. Metab. 2007, 92, 1590–1591. [Google Scholar] [CrossRef] [Green Version]
- Cavestro, G.M.; Mannucci, A.; Balaguer, F.; Hampel, H.; Kupfer, S.S.; Repici, A.; Sartore-Bianchi, A.; Seppälä, T.T.; Valentini, V.; Boland, C.R.; et al. Delphi Initiative for Early-Onset Colorectal Cancer (DIRECt) International Management Guidelines. Clin. Gastroenterol. Hepatol. 2023, 21, 581–603.e33. [Google Scholar] [CrossRef]
- Daca Alvarez, M.; Quintana, I.; Terradas, M.; Mur, P.; Balaguer, F.; Valle, L. The Inherited and Familial Component of Early-Onset Colorectal Cancer. Cells 2021, 10, 710. [Google Scholar] [CrossRef] [PubMed]
- Engel, C.; Vasen, H.F.; Seppälä, T.; Aretz, S.; Bigirwamungu-Bargeman, M.; de Boer, S.Y.; Bucksch, K.; Büttner, R.; Holinski-Feder, E.; Holzapfel, S.; et al. No Difference in Colorectal Cancer Incidence or Stage at Detection by Colonoscopy among 3 Countries with Different Lynch Syndrome Surveillance Policies. Gastroenterology 2018, 155, 1400–1409.e2. [Google Scholar] [CrossRef]
- Seppälä, T.T.; Latchford, A.; Negoi, I.; Sampaio Soares, A.; Jimenez-Rodriguez, R.; Sánchez-Guillén, L.; Evans, D.G.; Ryan, N.; Crosbie, E.J.; Dominguez-Valentin, M.; et al. European guidelines from the EHTG and ESCP for Lynch syndrome: An updated third edition of the Mallorca guidelines based on gene and gender. Br. J. Surg. 2021, 108, 484–498. [Google Scholar] [CrossRef] [PubMed]
- Underkofler, K.A.; Ring, K.L. Updates in gynecologic care for individuals with lynch syndrome. Front. Oncol. 2023, 13, 1127683. [Google Scholar] [CrossRef] [PubMed]
- Capasso, I.; Santoro, A.; Lucci Cordisco, E.; Perrone, E.; Tronconi, F.; Catena, U.; Zannoni, G.F.; Scambia, G.; Fanfani, F.; Lorusso, D.; et al. Lynch Syndrome and Gynecologic Tumors: Incidence, Prophylaxis, and Management of Patients with Cancer. Cancers 2023, 15, 1400. [Google Scholar] [CrossRef] [PubMed]




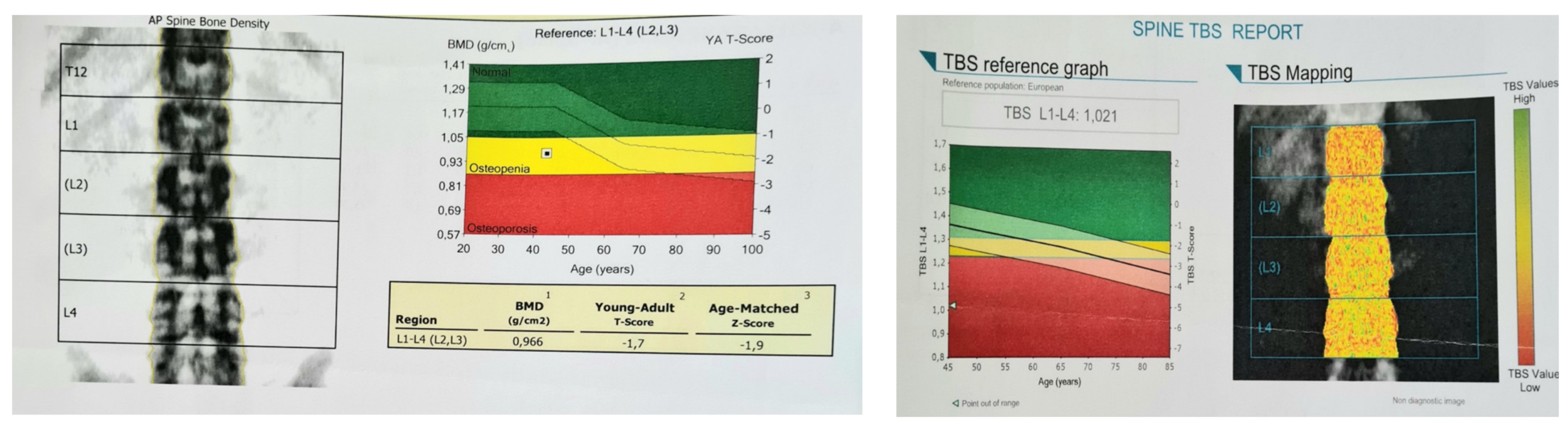

| Parameter (Unit) | Value | Normal |
|---|---|---|
| AST (U/L) | 22 | 5–34 |
| ALT (U/L) | 43 | 0–55 |
| GGT (U/L) | 40 | 6–42 |
| Serum creatinine (mg/dL) | 0.67 | 0.5–1.2 |
| Serum uric acid (mg/dL) | 4.6 | 2.5–5.7 |
| Serum urea (mg/dL) | 28.6 | 15–50 |
| Total cholesterol (mg/dL) | 254 | 0–200 |
| LDL cholesterol (mg/dL) | 189 | 60–160 |
| HDL cholesterol (mg/dL) | 42.7 | 40–65 |
| Triglycerides (mg/dL) | 119 | 0–149 |
| Fasting glycaemia (mg/dL) | 123 | 70–105 |
| Glycated haemoglobin A1c (%) | 6.5 | <5.7 |
| Total proteins (g/dL) | 7.7 | 6.4–8.3 |
| Serum sodium (mmol/L) | 137 | 136–145 |
| Serum potassium (mmol/L) | 4.3 | 3.5–5.1 |
| Serum magnesium (mg/dL) | 1.9 | 1.6–2.6 |
| PTH (pg/mL) | 37.48 | 15–65 |
| 25-hydroxyvitamin D (ng/mL) | 11.8 | 30–100 |
| ALP (U/L) | 347 | 40–150 |
| P1NP (ng/mL) | 203.4 | 20.25–76.31 |
| ßCTX (ng/mL) | 0.67 | 0.162–0.436 |
| Serum total calcium (mg/dL) | 10.3 | 8.4–10.2 |
| Serum phosphorus (mg/dL) | 3.2 | 2.3–4.7 |
| 24 h urinary calcium (g/24-h) | 0.33 | 0.07–0.3 |
| BMD (g/cm2) | t-Score (SD) | Z-Score (SD) | |
|---|---|---|---|
| Lumbar L1–L4 | 0.966 | −1.7 | −0.6 |
| Total hip | 0.979 | −0.2 | −0.2 |
| Distal radius | 0.67 | −0.6 | −0.6 |
| First Author Reference Year of Publication | Study Type | Studied Population | PDB Type | Treatment | Biochemical and Scintigraphic Outcome | Clinical Outcome | Side Effects | Reason for Prescribing Denosumab | Other Observations |
|---|---|---|---|---|---|---|---|---|---|
| Schwarz [62] 2012 | Case report | 86-year-old male | polyostotic | denosumab 60 mg subcutaneously re-treated at months 6, 9, 12, and 15 with denosumab 60 mg | normalisation of plasma ALP and BALP; suppression of the other bone markers; less activity on bone scintigraphy (after 15 months of treatment) | improvement of pain | No | BP were CI due to impaired renal function | initially treated with calcitonin without effect |
| Verma [63] 2016 | Case report | 40-year-old male | polyostotic | 6 doses of denosumab | 15 months follow-up: asymptomatic and disease-free | No | 3 years following treatment (pamidronate 60 mg every 3 weeks, calcium, and vitamin D supplements), the patient developed GCT | the patient also underwent surgery | |
| Tanaka [64] 2016 | Case report | 60-year-old male | polyostotic | denosumab 120 mg on days 1, 8, and 15, and then once every 4 weeks | minimal symptoms | No | GCT | ||
| Reid [65] 2016 | Case report | 75-year-old female | monostotic (skull) | denosumab 60 mg and a second dose after two years | ALP levels normalised for 4–6 months after each dose; less marked uptake than at baseline on bone scintigraphy | improvement of headaches and hearing | No | severe musculoskeletal pain after alendronate 40 mg orally | |
| Kostine [60] 2016 | Case report | 79-year-old male | polyostotic | Single-dose of denosumab 60 mg, oral calcium (1 g/day), and vitamin D (800 IU/day) | ALP levels normalised (ALP = 91 IU/L, BALP = 19 IU/L) within 4 months and remained low (BALP: 27 IU/L) after 18 months | rapid and marked pain relief | hypocalcaemia (a nadir of 1.1 mmol/L, ionised calcium: 0.54 mmol/L) | BP were CI due to impaired renal function | the patient did not take the prescribed calcium supplementation |
| Kuthiah [61] 2018 | Case report | 63-year-old female | polyostotic | denosumab 60 mg six-monthly | ALP levels normalised (118 U/L) within 3 months | pain resolved | No | BP were CI due to impaired renal function |
| First author Reference Year | Population | Pathogenic Variant | Sarcoma Type | Other Tumours (Outside LS Panel) |
|---|---|---|---|---|
| Case series | ||||
| Carvalho [71] 2020 | 40-year-old female | MSH2 c.1661+1G>A-LP | osteosarcoma | sebaceoma |
| 20-year-old female | MSH2 c.2152C>T; p.Gln718Ter-P | osteosarcoma | liposarcoma | |
| Dominguez-Valentin [74] 2020 | 5 males and 7 females with a mean age of 64.4 | NA | osteosarcoma | - |
| Nilbert [73] 2009 | 15-year-old female | MLH1 c.1276C>T; p.(Gln426Ter) | osteosarcoma | - |
| 28-year-old male | MLH1 c.1204A>T; p.(Lys402Ter) | chondrosarcoma | - | |
| Baglietto [75] 2009 | 2 patients | NA | bone cancer | - |
| Case reports | ||||
| Lynch [72] 2003 | 25-year-old male | MSH2 exon 4 splice site mutation | osteosarcoma | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gheorghe, A.-M.; Stanescu, L.-S.; Petrova, E.; Carsote, M.; Nistor, C.; Ghemigian, A. Paget’s Disease of the Bone and Lynch Syndrome: An Exceptional Finding. Diagnostics 2023, 13, 2101. https://doi.org/10.3390/diagnostics13122101
Gheorghe A-M, Stanescu L-S, Petrova E, Carsote M, Nistor C, Ghemigian A. Paget’s Disease of the Bone and Lynch Syndrome: An Exceptional Finding. Diagnostics. 2023; 13(12):2101. https://doi.org/10.3390/diagnostics13122101
Chicago/Turabian StyleGheorghe, Ana-Maria, Laura-Semonia Stanescu, Eugenia Petrova, Mara Carsote, Claudiu Nistor, and Adina Ghemigian. 2023. "Paget’s Disease of the Bone and Lynch Syndrome: An Exceptional Finding" Diagnostics 13, no. 12: 2101. https://doi.org/10.3390/diagnostics13122101






