Prognostic Models in Growth-Hormone- and Prolactin-Secreting Pituitary Neuroendocrine Tumors: A Systematic Review
Abstract
:1. Introduction
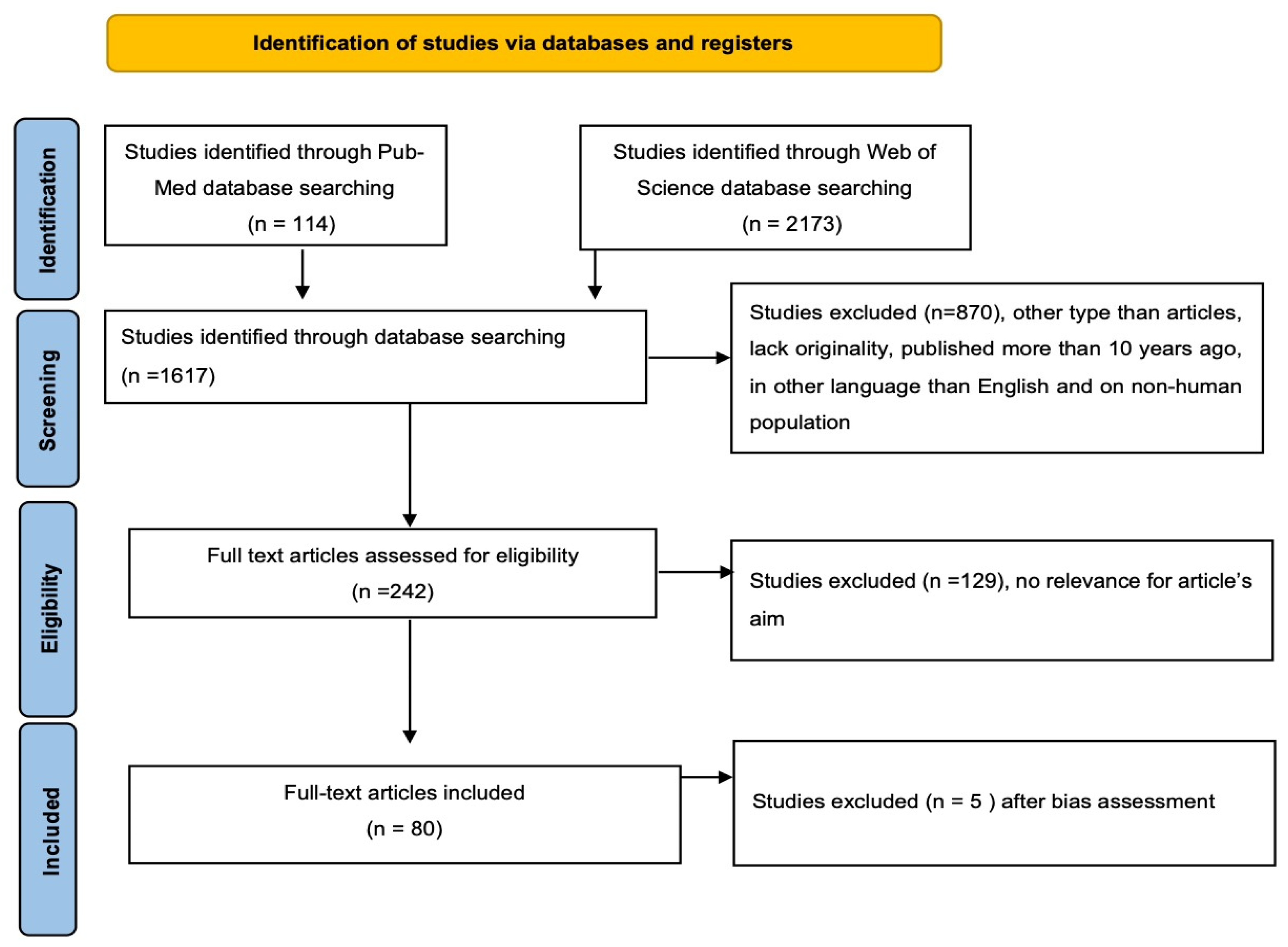
2. Materials and Methods
- Study parameters: authors, title, year, design, and number of patients.
- Clinical parameters: GH and PRL secretion, tumor volume and surgical treatment.
- Imaging parameters: MRI evaluation and invasion.
- Histopathological, immunohistochemical and molecular analysis.
- Surgical outcome and postoperative complications.
- Morbidity and mortality.
3. Results
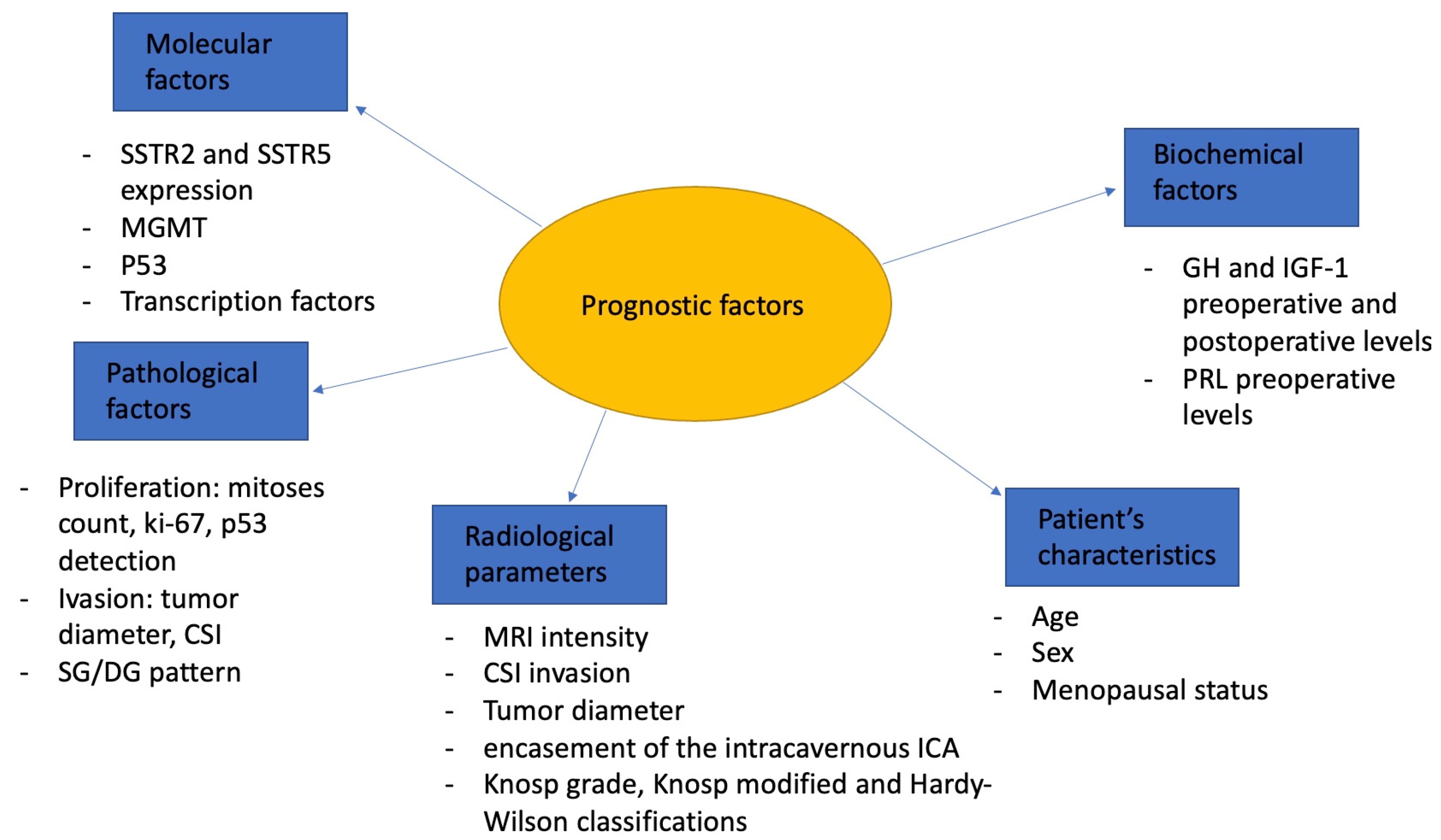
4. Discussion
4.1. Biochemical Factors
4.2. Radiological Parameters
4.3. Invasion and Proliferation
4.4. Pathological and Molecular Factors
4.5. Age and Sex
4.6. Clinical Prediction Models
4.7. Artificial Intelligence and Machine Learning for Prognosis
4.8. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chin, S.O. Epidemiology of Functioning Pituitary Adenomas. Endocrinol. Metab. 2020, 35, 237–242. [Google Scholar] [CrossRef]
- Villa, C.; Vasiljevic, A.; Jaffrain-Rea, M.L.; Ansorge, O.; Asioli, S.; Barresi, V.; Chinezu, L.; Gardiman, M.P.; Lania, A.; Lapshina, A.M.; et al. A standardised diagnostic approach to pituitary neuroendocrine tumours (PitNETs): A European Pituitary Pathology Group (EPPG) proposal. Virchows Arch. 2019, 475, 687–692. [Google Scholar] [CrossRef]
- Trouillas, J.; Jaffrain-Rea, M.-L.; Vasiljevic, A.; Raverot, G.; Roncaroli, F.; Villa, C. How to Classify the Pituitary Neuroendocrine Tumors (PitNET)s in 2020. Cancers 2020, 12, 514. [Google Scholar] [CrossRef] [Green Version]
- Raverot, G.; Vasiljevic, A.; Jouanneau, E.; Trouillas, J. A prognostic clinicopathologic classification of pituitary endocrine tumors. Endocrinol. Metab. Clin. North. Am. 2015, 44, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Raverot, G.; Dantony, E.; Beauvy, J.; Vasiljevic, A.; Mikolasek, S.; Borson-Chazot, F.; Jouanneau, E.; Roy, P.; Trouillas, J. Risk of Recurrence in Pituitary Neuroendocrine Tumors: A Prospective Study Using a Five-Tiered Classification. J. Clin. Endocrinol. Metab. 2017, 102, 3368–3374. [Google Scholar] [CrossRef] [PubMed]
- Lelotte, J.; Jouret-Mourin, A.; Fomekong, E.; Michotte, A.; Raftopoulos, C.; Maiter, D. Both invasiveness and proliferation criteria predict recurrence of non-functioning pituitary macroadenomas after surgery: A retrospective analysis of a monocentric cohort of 120 patients. Eur. J. Endocrinol. 2018, 178, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Asioli, S.; Righi, A.; Iommi, M.; Baldovini, C.; Ambrosi, F.; Guaraldi, F.; Zoli, M.; Mazzatenta, D.; Faustini-Fustini, M.; Rucci, P.; et al. Validation of a clinicopathological score for the prediction of post-surgical evolution of pituitary adenoma: Retrospective analysis on 566 patients from a tertiary care centre. Eur. J. Endocrinol. 2019, 180, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Sahakian, N.; Appay, R.; Resseguier, N.; Graillon, T.; Piazzola, C.; Laure, C.; Figarella-Branger, D.; Régis, J.; Castinetti, F.; Brue, T.; et al. Real-life clinical impact of a five-tiered classification of pituitary tumors. Eur. J. Endocrinol. 2022, 187, 893–904. [Google Scholar] [CrossRef] [PubMed]
- Asa, S.L.; Mete, O.; Perry, A.; Osamura, R.Y. Overview of the 2022 WHO Classification of Pituitary Tumors. Endocr. Pathol. 2022, 33, 6–26. [Google Scholar] [CrossRef]
- Arya, S.; Kaji, A.H.; Boermeester, M.A. PRISMA reporting guidelines for meta-analyses and systematic reviews. JAMA Surg. 2021, 156, 789–790. [Google Scholar] [CrossRef]
- Whiting, P.; Savović, J.; Higgins, J.P.T.; Caldwell, D.M.; Reeves, B.C.; Shea, B.; Davies, P.; Kleijnen, J.; Churchill, R.; ROBIS group. A new tool to assess risk of bias in systematic reviews was developed. J. Clin. Epidemiol. 2016, 69, 225–234. [Google Scholar] [CrossRef] [Green Version]
- Alhambra-Expósito, M.R.; Ibáñez-Costa, A.; Moreno-Moreno, P.; Rivero-Cortés, E.; Vázquez-Borrego, M.C.; Blanco-Acevedo, C.; Toledano-Delgado, Á.; Lombardo-Galera, M.S.; Vallejo-Casas, J.A.; Gahete, M.D.; et al. Association between radiological parameters and clinical and molecular characteristics in human somatotropinomas. Sci. Rep. 2018, 8, 6173. [Google Scholar] [CrossRef] [Green Version]
- Donegan, D.M.; Iñiguez-Ariza, N.; Sharma, A.; Nippoldt, T.; Young, W.; Van Gompel, J.; Atkinson, J.; Meyer, F.; Pollock, B.; Natt, N.; et al. Necessity of Multimodal Treatment of Acromegaly and Outcomes. Endocr. Pract. 2018, 24, 668–676. [Google Scholar] [CrossRef]
- Goyal-Honavar, A.; Sarkar, S.; Asha, H.S.; Kapoor, N.; Thomas, R.; Balakrishnan, R.; Chacko, G.; Chacko, A.G. Impact of Experience on Outcomes After Endoscopic Transsphenoidal Surgery for Acromegaly. World Neurosurg. 2021, 151, e1007–e1015. [Google Scholar] [CrossRef]
- Heng, L.; Liu, X.; Jia, D.; Guo, W.; Zhang, S.; Gao, G.; Gong, L.; Qu, Y. Preoperative prediction of granulation pattern subtypes in GH-secreting pituitary adenomas. Clin. Endocrinol. 2021, 95, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Dai, C.; Bao, X.; Deng, K.; Yao, Y.; Feng, M.; Li, M.; Chen, G.; Wang, R. The Clinical and Pathological Characteristics of Refractory Pituitary Adenomas: A Single Center Experience. Front. Oncol. 2022, 12, 846614. [Google Scholar] [CrossRef]
- Ozturk, S.; Donmez-Altuntas, H.; Ozturk, F.; Kurtsoy, A.; Gokay, F.; Simsek, Y.; Bayram, F. The significance of estrogen receptors in acromegaly: Are they useful as predictors of prognosis and therapy regimen? Growth Horm. IGF Res. 2020, 55, 101337. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Kim, E.H.; Ku, C.R.; Lee, E.J.; Kim, S.H. Outcomes of Aggressive Surgical Resection in Growth Hormone-Secreting Pituitary Adenomas with Cavernous Sinus Invasion. World Neurosurg. 2018, 117, e280–e289. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Ku, C.R.; Moon, J.H.; Kim, E.H.; Kim, S.H.; Lee, E.J. Age- and Sex-Specific Differences as Predictors of Surgical Remission Among Patients With Acromegaly. J. Clin. Endocrinol. Metab. 2018, 103, 909–916. [Google Scholar] [CrossRef]
- Swanson, A.A.; Erickson, D.; Donegan, D.M.; Jenkins, S.M.; Van Gompel, J.J.; Atkinson, J.L.D.; Erickson, B.J.; Giannini, C. Clinical, biological, radiological, and pathological comparison of sparsely and densely granulated somatotroph adenomas: A single center experience from a cohort of 131 patients with acromegaly. Pituitary 2021, 24, 192–206. [Google Scholar] [CrossRef]
- Ferrés, A.; Reyes, L.; Di Somma, A.; Topczewski, T.; Mosteiro, A.; Guizzardi, G.; De Rosa, A.; Halperin, I.; Hanzu, F.; Mora, M.; et al. The Prognostic-Based Approach in Growth Hormone-Secreting Pituitary Neuroendocrine Tumors (PitNET): Tertiary Reference Center, Single Senior Surgeon, and Long-Term Follow-Up. Cancers 2022, 15, 267. [Google Scholar] [CrossRef]
- Coopmans, E.C.; Postma, M.R.; Wolters, T.L.C.; van Meyel, S.W.F.; Netea-Maier, R.; van Beek, A.P.; Neggers, S.J.C.M.M. Predictors for Remission after Transsphenoidal Surgery in Acromegaly: A Dutch Multicenter Study. J. Clin. Endocrinol. Metab. 2021, 106, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Baussart, B.; Villa, C.; Jouinot, A.; Raffin-Sanson, M.; Foubert, L.; Cazabat, L.; Bernier, M.; Bonnet, F.; Dohan, A.; Bertherat, J.; et al. Pituitary surgery as alternative to dopamine agonists treatment for microprolactinomas: A cohort study. Eur. J. Endocrinol. 2021, 185, 783–791. [Google Scholar] [CrossRef] [PubMed]
- Vermeulen, E.; D’Haens, J.; Stadnik, T.; Unuane, D.; Barbe, K.; Van Velthoven, V.; Gläsker, S. Predictors of dopamine agonist resistance in prolactinoma patients. BMC Endocr. Disord. 2020, 20, 68. [Google Scholar] [CrossRef]
- Cander, S.; Gül, Ö.Ö.; Ertürk, E.; Tuncel, E.; Ersoy, C. Prolactin levels and gender are associated with tumour behaviour in prolactinomas but Ki-67 index is not. Endokrynol. Pol. 2014, 65, 210–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.-L.; Chen, D.-M.; Zhang, C.; Pan, M.; Yang, X.-P.; Wu, Y.-G. Retrospective analysis of 52 patients with prolactinomas following endoscopic endonasal transsphenoidal surgery. Medicine 2018, 97, e13198. [Google Scholar] [CrossRef]
- Lv, L.; Hu, Y.; Yin, S.; Zhou, P.; Yang, Y.; Ma, W.; Zhang, S.; Wang, X.; Jiang, S. Giant Prolactinomas: Outcomes of Multimodal Treatments for 42 Cases with Long-Term Follow-Up. Exp. Clin. Endocrinol. Diabetes. 2019, 127, 295–302. [Google Scholar] [CrossRef] [Green Version]
- Lv, L.; Jiang, Y.; Yin, S.; Hu, Y.; Chen, C.; Ma, W.; Jiang, S.; Zhou, P. Mammosomatotroph and mixed somatotroph-lactotroph adenoma in acromegaly: A retrospective study with long-term follow-up. Endocrine 2019, 66, 310–318. [Google Scholar] [CrossRef]
- Monsalves, E.; Larjani, S.; Godoy, B.L.; Juraschka, K.; Carvalho, F.; Kucharczyk, W.; Kulkarni, A.; Mete, O.; Gentili, F.; Ezzat, S.; et al. Growth patterns of pituitary adenomas and histopathological correlates. J. Clin. Endocrinol. Metab. 2014, 99, 1330–1338. [Google Scholar] [CrossRef]
- Nikitin, P.V.; Ryzhova, M.V.; Shishkina, L.V.; Shugay, S.V.; Zubova, I.V. Study of Simple Immunohistochemical Cytocolorimetric Assay Application for More Accurate Assessment of Prognosis in Patients with Pituitary Adenomas. World Neurosurg. 2019, 122, e1047–e1051. [Google Scholar] [CrossRef]
- Pappy, A.L., 2nd; Savinkina, A.; Bicknese, C.; Neill, S.; Oyesiku, N.M.; Ioachimescu, A.G. Predictive modeling for pituitary adenomas: Single center experience in 501 consecutive patients. Pituitary 2019, 22, 520–531. [Google Scholar] [CrossRef]
- Chen, Y.; Cai, F.; Cao, J.; Gao, F.; Lv, Y.; Tang, Y.; Zhang, A.; Yan, W.; Wang, Y.; Hu, X.; et al. Analysis of Related Factors of Tumor Recurrence or Progression After Transnasal Sphenoidal Surgical Treatment of Large and Giant Pituitary Adenomas and Establish a Nomogram to Predict Tumor Prognosis. Front. Endocrinol. 2021, 12, 793337. [Google Scholar] [CrossRef] [PubMed]
- Qiao, N.; Shen, M.; He, W.; He, M.; Zhang, Z.; Ye, H.; Li, Y.; Shou, X.; Li, S.; Jiang, C.; et al. Machine learning in predicting early remission in patients after surgical treatment of acromegaly: A multicenter study. Pituitary 2021, 2, 53–61. [Google Scholar] [CrossRef]
- Huber, M.; Luedi, M.M.; Schubert, G.A.; Musahl, C.; Tortora, A.; Frey, J.; Beck, J.; Mariani, L.; Christ, E.; Andereggen, L. Machine Learning for Outcome Prediction in First-Line Surgery of Prolactinomas. Front. Endocrinol. 2022, 13, 810219. [Google Scholar] [CrossRef]
- Niu, J.; Zhang, S.; Ma, S.; Diao, J.; Zhou, W.; Tian, J.; Zang, Y.; Jia, W. Preoperative prediction of cavernous sinus invasion by pituitary adenomas using a radiomics method based on magnetic resonance images. Eur. Radiol. 2019, 29, 1625–1634. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Tu, S.; Duan, L.; Fu, W.; Wang, J.; Geng, S. Classification of Pituitary Adenomas Invading the Cavernous Sinus Assisted by Three-Dimensional Multimodal Imaging and Its Clinical Application. J. Neurol. Surg. Part B Skull Base 2021, 82, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Staartjes, V.E.; Serra, C.; Maldaner, N.; Muscas, G.; Tschopp, O.; Soyka, M.B.; Holzmann, D.; Regli, L. The Zurich Pituitary Score predicts utility of intraoperative high-field magnetic resonance imaging in transsphenoidal pituitary adenoma surgery. Acta Neurochir. 2019, 161, 2107–2115. [Google Scholar] [CrossRef] [PubMed]
- Pal’A, A.; Knoll, A.; Brand, C.; Etzrodt-Walter, G.; Coburger, J.; Wirtz, C.R.; Hlaváč, M. The Value of Intraoperative Magnetic Resonance Imaging in Endoscopic and Microsurgical Transsphenoidal Pituitary Adenoma Resection. World Neurosurg. 2017, 102, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Mooney, M.A.; Hardesty, D.A.; Sheehy, J.P.; Bird, C.R.; Chapple, K.; White, W.L.; Little, A.S. Rater Reliability of the Hardy Classification for Pituitary Adenomas in the Magnetic Resonance Imaging Era. J. Neurol. Surg. Part B Skull Base 2017, 78, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Araujo-Castro, M.; Cancela, A.A.; Vior, C.; Pascual-Corrales, E.; Berrocal, V.R. Radiological Knosp, Revised-Knosp, and Hardy-Wilson Classifications for the Prediction of Surgical Outcomes in the Endoscopic Endonasal Surgery of Pituitary Adenomas: Study of 228 Cases. Front. Oncol. 2022, 11, 807040. [Google Scholar] [CrossRef]
- Braileanu, M.; Hu, R.; Hoch, M.J.; Mullins, M.E.; Ioachimescu, A.G.; Oyesiku, N.M.; Pappy, A., 2nd; Saindane, A.M. Pre-operative MRI predictors of hormonal remission status post pituitary adenoma resection. Clin. Imaging. 2019, 55, 29–34. [Google Scholar] [CrossRef]
- Foltran, R.K.; Amorim, P.V.G.H.; Duarte, F.H.; Grande, I.P.P.; Freire, A.C.T.B.; Frassetto, F.P.; Dettoni, J.B.; Alves, V.A.; Castro, I.; Trarbach, E.B.; et al. Study of major genetic factors involved in pituitary tumorigenesis and their impact on clinical and biological characteristics of sporadic somatotropinomas and non-functioning pituitary adenomas. Braz. J. Med. Biol. Res. 2018, 51, e7427. [Google Scholar] [CrossRef]
- Burcea, I.F.; Năstase, V.N.; Cîmpean, A.M.; Ceaușu, A.R.; Baciu, I.; Căpățînă, C.; Dusceac, R.; Găloiu, S.; Niculescu, D.; Radian, Ș.; et al. Clinicopathological Features of Growth Hormone-producing Pituitary Adenomas and Correlation with Preoperative Laboratory Findings. Anticancer Res. 2021, 41, 2669–2680. [Google Scholar] [CrossRef]
- Petrossians, P.; Daly, A.; Natchev, E.; Maione, L.; Blijdorp, K.; Sahnoun-Fathallah, M.; Auriemma, R.; Diallo, A.M.; Hulting, A.-L.; Ferone, D.; et al. Acromegaly at diagnosis in 3173 patients from the Liège Acromegaly Survey (LAS) Database. Endocr.-Relat. Cancer 2017, 24, 505–518. [Google Scholar] [CrossRef]
- Das, C.; Mondal, P.; Mukhopadhyay, M.; Mukhopadhyay, S.; Ghosh, I.; Handral, A. Evaluation of prognostic utility of Ki-67, P53, and O-6-methylguanine-DNA methyltransferase expression in pituitary tumors. J. Lab. Physicians 2019, 11, 323–329. [Google Scholar] [CrossRef] [Green Version]
- Asa, S.L.; Mete, O. Cytokeratin Profiles in Pituitary Neuroendocrine Tumors. Hum. Pathol. 2021, 107, 87–95. [Google Scholar] [CrossRef]
- Diri, H.; Ozaslan, E.; Kurtsoy, A.; Bayram, F. A single-center observational study assessing the predictive factors associated with the prognosis of acromegaly. Growth Horm. IGF Res. 2020, 55, 101342. [Google Scholar] [CrossRef] [PubMed]
- Antunes, X.; Ventura, N.; Camilo, G.B.; Wildemberg, L.E.; Guasti, A.; Pereira, P.J.M.; Camacho, A.H.S.; Chimelli, L.; Niemeyer, P.; Gadelha, M.R.; et al. Predictors of surgical outcome and early criteria of remission in acromegaly. Endocrine 2018, 60, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Buchy, M.; Lapras, V.; Rabilloud, M.; Vasiljevic, A.; Borson-Chazot, F.; Jouanneau, E.; Raverot, G. Predicting early post-operative remission in pituitary adenomas: Evaluation of the modified knosp classification. Pituitary 2019, 22, 467–475. [Google Scholar] [CrossRef]
- Peixe, C.; Alexandre, M.I.; Gomes, A.R.; Nobre, E.; Silva, A.L.; Oliveira, T.; López-Presa, D.; Faria, C.C.; Miguens, J.; Bugalho, M.J.; et al. Usefulness of a clinicopathological classification in predicting treatment-related outcomes and multimodal therapeutic approaches in pituitary adenoma patients: Retrospective analysis on a Portuguese cohort of 129 patients from a tertiary pituitary center. Pituitary. 2023, volume, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Melmed, S.; Bronstein, M.D.; Chanson, P.; Klibanski, A.; Casanueva, F.F.; Wass, J.A.H.; Strasburger, C.J.; Luger, A.; Clemmons, D.R.; Giustina, A. A Consensus Statement on acromegaly therapeutic outcomes. Nat. Rev. Endocrinol. 2018, 14, 552–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogiwara, T.; Hori, T.; Fujii, Y.; Nakamura, T.; Suzuki, Y.; Watanabe, G.; Hanaoka, Y.; Goto, T.; Hongo, K.; Horiuchi, T. Effectiveness of the intraoperative magnetic resonance imaging during endoscopic endonasal approach for acromegaly. Pituitary 2021, 24, 690–697. [Google Scholar] [CrossRef]
- Ouyang, T.; Zhang, N.; Xie, S.; Tang, B.; Li, J.; Xiao, L.; Zhang, F.; Wu, B.; Zhou, D.; Li, M.; et al. Outcomes and Complications of Aggressive Resection Strategy for Pituitary Adenomas in Knosp Grade 4 With Transsphenoidal Endoscopy. Front. Oncol. 2021, 11, 693063. [Google Scholar] [CrossRef] [PubMed]
- Zanier, O.; Zoli, M.; Staartjes, V.E.; Guaraldi, F.; Asioli, S.; Rustici, A.; Picciola, V.M.; Pasquini, E.; Faustini-Fustini, M.; Erlic, Z.; et al. Machine learning-based clinical outcome prediction in surgery for acromegaly. Endocrine. 2022, 75, 508–515. [Google Scholar] [CrossRef]
- Ugga, L.; Cuocolo, R.; Solari, D.; Guadagno, E.; D’Amico, A.; Somma, T.; Cappabianca, P.; del Basso de Caro, M.L.D.; Cavallo, L.M.; Brunetti, A. Prediction of high proliferative index in pituitary macroadenomas using MRI-based radiomics and machine learning. Neuroradiology 2019, 61, 1365–1373. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.-Q.; Liang, C.-H.; He, L.; Tian, J.; Liang, C.-S.; Chen, X.; Ma, Z.-L.; Liu, Z.-Y. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J. Clin. Oncol. 2016, 34, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Oliveira, A., Jr.; Faje, A.T.; Barkan, A.L. Limited utility of oral glucose tolerance test in biochemically active acromegaly. Eur. J. Endocrinol. 2011, 164, 17–22. [Google Scholar] [CrossRef] [Green Version]
- Fleseriu, M.; Biller, B.M.K.; Freda, P.U.; Gadelha, M.R.; Giustina, A.; Katznelson, L.; Molitch, M.E.; Samson, S.L.; Strasburger, C.J.; van der Lely, A.J.; et al. A Pituitary Society update to acromegaly management guidelines. Pituitary 2021, 24, 53. [Google Scholar] [CrossRef]
- Dai, C.; Sun, B.; Wang, R.; Kang, J. The Application of Artificial Intelligence and Machine Learning in Pituitary Adenomas. Front. Oncol. 2021, 11, 784–819. [Google Scholar] [CrossRef]
- Valenzuela, F.; Villanueva, P.; Rojas, Z.D.; Gejman, R.; Huete, I.; Zunino, R.; Díaz, R.E.; Wohllk, N.; Tissera, C.; Carrasco, C.A. Caracterización de tumores secretores de hormona de crecimiento de acuerdo al patrón granular y su rol en el pronóstico [Prognostic value of granular pattern of growth hormone secreting tumors]. Rev. Med. Chil. 2019, 147, 852–859. [Google Scholar] [CrossRef]
- Bonneville, J.F.; Potorac, J.; Beckers, A. Neuroimaging of aggressive pituitary tumors. Rev. Endocr. Metab. Disord. 2020, 21, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Y.; Waqar, M.; Abou-Zeid, A.; Kearney, T.; Caputo, C.; Davis, J.; Trainer, P.; Higham, C.; Roncaroli, F.; Gnanalingham, K.K. Value of Early Post-Operative Growth Hormone Testing in Predicting Long-Term Remission and Residual Disease after Transsphenoidal Surgery for Acromegaly. Neuroendocrinology 2022, 112, 345–357. [Google Scholar] [CrossRef] [PubMed]
- Thommen, R.; Kazim, S.F.; Cole, K.L.; Olson, G.T.; Shama, L.; Lovato, C.M.; Gonzales, K.M.; Dicpinigaitis, A.J.; Couldwell, W.T.; Mckee, R.G.; et al. Worse Pituitary Adenoma Surgical Outcomes Predicted by Increasing Frailty, Not Age. World Neurosurg. 2022, 161, e347–e354. [Google Scholar] [CrossRef]
- Yao, A.; Rutland, J.W.; Verma, G.; Banihashemi, A.; Padormo, F.; Tsankova, N.M.; Delman, B.N.; Shrivastava, R.K.; Balchandani, P. Pituitary adenoma consistency: Direct correlation of ultrahigh field 7T MRI with histopathological analysis. Eur. J. Radiol. 2020, 126, 108931. [Google Scholar] [CrossRef]
- Boling, C.C.; Karnezis, T.T.; Baker, A.B.; Lawrence, L.A.; Soler, Z.M.; Vandergrift, W.A., 3rd; Wise, S.K.; Del Gaudio, J.M.; Patel, Z.M.; Rereddy, S.K.; et al. Multi-institutional study of risk factors for perioperative morbidity following transnasal endoscopic pituitary adenoma surgery. Int. Forum Allergy Rhinol. 2016, 6, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.D.; Broder, M.S.; Cherepanov, D.; Chang, E.; Mamelak, A.; Said, Q.; Neary, M.P.; Bonert, V. Long-term treatment outcomes of acromegaly patients presenting biochemically-uncontrolled at a tertiary pituitary center. BMC Endocr. Disord. 2017, 17, 49. [Google Scholar] [CrossRef] [PubMed]
- Almalki, M.H.; Ahmad, M.M.; Buhary, B.M.; Aljawair, R.; Alyamani, A.; Alhozali, A.; Alshahrani, A.; Alzahrani, S.; Nasser, T.; Alzahrani, W.; et al. Clinical features and therapeutic outcomes of patients with acromegaly in Saudi Arabia: A retrospective analysis. Hormones 2020, 19, 377–383. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, D.M.; Wang, Y.; Mai, R.K.; Zhu, Z.F. Surgical management of growth hormone-secreting pituitary adenomas: A retrospective analysis of 33 patients. Medicine 2020, 99, e1985. [Google Scholar] [CrossRef]
- Haddad, A.F.; Young, J.S.; Oh, T.; Pereira, M.P.; Joshi, R.S.; Pereira, K.M.; Osorio, R.C.; Donohue, K.C.; Peeran, Z.; Sudhir, S.; et al. Clinical characteristics and outcomes of null-cell versus silent gonadotroph adenomas in a series of 1166 pituitary adenomas from a single institution. Neurosurg. Focus 2020, 48, E13. [Google Scholar] [CrossRef]
- Elshazly, K.; Kshettry, V.R.; Farrell, C.J.; Nyquist, G.; Rosen, M.; Evans, J.J. Clinical Outcomes After Endoscopic Endonasal Resection of Giant Pituitary Adenomas. World Neurosurg. 2018, 114, e447–e456. [Google Scholar] [CrossRef]
- Shirvani, M.; Motiei-Langroudi, R. Transsphenoidal surgery for growth hormone-secreting pituitary adenomas in 130 patients. World Neurosurg. 2014, 81, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Taweesomboonyat, C.; Oearsakul, T. Prognostic Factors of Acromegalic Patients with Growth Hormone-Secreting Pituitary Adenoma After Transsphenoidal Surgery. World Neurosurg. 2021, 146, e1360–e1366. [Google Scholar] [CrossRef] [PubMed]
- Erkan, B.; Barut, O.; Akbas, A.; Akpinar, E.; Akdeniz, Y.S.; Tanriverdi, O.; Gunaldi, O. Results of Endoscopic Surgery in Patients with Pituitary Adenomas: Association of Tumor Classification Grades with Resection, Remission, and Complication Rates. J. Korean Neurosurg. Soc. 2021, 64, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Anik, I.; Cabuk, B.; Gokbel, A.; Selek, A.; Cetinarslan, B.; Anik, Y.; Ceylan, S. Endoscopic Transsphenoidal Approach for Acromegaly with Remission Rates in 401 Patients: 2010 Consensus Criteria. World Neurosurg. 2017, 108, 278–290. [Google Scholar] [CrossRef]
- Dutta, P.; Korbonits, M.; Sachdeva, N.; Gupta, P.; Srinivasan, A.; Devgun, J.S.; Bajaj, A.; Mukherjee, K.K. Can immediate postoperative random growth hormone levels predict long-term cure in patients with acromegaly? Neurol. India 2016, 64, 252–258. [Google Scholar]
- Anthony, J.R.; Alwahab, U.A.; Kanakiya, N.K.; Pontell, D.M.; Veledar, E.; Oyesiku, N.M.; Ioachimescu, A.G. Significant Elevation of Growth Hormone Level Impacts Surgical Outcomes in Acromegaly. Endocr. Pract. 2015, 21, 1001–1009. [Google Scholar] [CrossRef]
- Akkus, G.; Odabaş, F.; Sözütok, S.; Sert, M.; Ak, N.E.; Evran, M.; Tetiker, T. Novel Classification of Acromegaly in Accordance with Immunohistochemical Subtypes: Is There Really a Clinical Relevance? Horm. Metab. Res. 2022, 54, 37–41. [Google Scholar] [CrossRef]
- Huan, C.; Cui, G.; Ren, Z. The characteristics of acromegalic patients with hyperprolactinemia and the differences with hyperprolactinemia patients. Pak. J. Pharm. Sci. 2015, 28, 713–718. [Google Scholar]
- Hamidi, O.; Van Gompel, J.; Gruber, L.; Kittah, N.E.; Donegan, D.; Philbrick, K.A.; Koeller, K.K.; Erickson, D.; Natt, N.; Nippoldt, T.B.; et al. Management and Outcomes of Giant Prolactinoma: A Series of 71 Patients. Endocr. Pract. 2019, 25, 340–352. [Google Scholar] [CrossRef]
- Andujar-Plata, P.; Villar-Taibo, R.; Ballesteros-Pomar, M.D.; Vidal-Casariego, A.; Pérez-Corral, B.; Cabezas-Agrícola, J.M.; Álvarez-Vázquez, P.; Serramito, R.; Bernabeu, I. Long-term outcome of multimodal therapy for giant prolactinomas. Endocrine 2017, 55, 231–238. [Google Scholar] [CrossRef]
- Almalki, M.H.; Aljohani, N.; Alzahrani, S.; Almohareb, O.; Ahmad, M.M.; A Alrashed, A.; Alshahrani, F.; Buhary, B.M. Clinical Features, Therapeutic Trends, and Outcome of Giant Prolactinomas: A Single-Center Experience Over a 12-Year Period. Clin. Med. Insights Endocrinol. Diabetes 2020, 13, 1179551420926181. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Wei, X. Outcomes of transsphenoidal surgery in dopamine agonist-resistant prolactinomas: A retrospective study. Hormones 2021, 20, 745–752. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, P.; Arcano, K.; Berrocal, V.R.; Bernal, C.; Villabona, C.; Díez, J.J. Giant Prolactinoma in Men: Clinical Features and Therapeutic Outcomes. Horm. Metab. Res. 2018, 50, 791–796. [Google Scholar] [CrossRef] [Green Version]
- Smith, T.R.; Hulou, M.M.; Huang, K.T.; Gokoglu, A.; Cote, D.J.; Woodmansee, W.W.; Laws, E.R., Jr. Current indications for the surgical treatment of prolactinomas. J. Clin. Neurosci. 2015, 22, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Li, X.; Ge, A.; Gu, J. Correlation Analysis of Magnetic Resonance Imaging Characteristics and Prognosis of Invasive Pituitary Adenomas in Neurosurgery Hospitals. J. Healthc. Eng. 2022, 2022, 8280540. [Google Scholar] [CrossRef]
- Olarescu, N.C.; Perez-Rivas, L.G.; Gatto, F.; Cuny, T.; Tichomirowa, M.A.; Tamagno, G.; Gahete, M.D.; EYRC (ENEA Young Researcher Committee). Aggressive and Malignant Prolactinomas. Neuroendocrinology 2019, 109, 57–69. [Google Scholar] [CrossRef] [PubMed]
| Author | Date of Publishing | No. of Patients | Study Type | Main Results |
|---|---|---|---|---|
| Alhambra-Expósito et al. [12] | 2018 | 22 | Retrospective Single Center | Hyperintensity on MRI correlates with invasion in the CS and extrasellar growth (p = 0.023) |
| Donegan D. et al. [13] | 2022 | 106 | Retrospective Single Center | Early postoperative IGF-1 concentration, index or % change from diagnosis (at 6 weeks) were the best predictors of surgical outcome (p < 0.001) |
| Goyal-Honavar A et al. [14] | 2021 | 203 | Retrospective Single Center | Preoperative basal GH value < 40 ng/mL (p < 0.001) and tumor size correlate with better rates of surgical remission |
| Heng L. et al. [15] | 2021 | 83 | Retrospective Single Center | SG subtype presented with tumors significantly larger compared with the DG group (p < 0.001) and had a higher Knosp grade (p = 0.0012) The grading scale for predicting SG GH-secreting pituitary adenomas with AUC = 0.84 (DKGO score) |
| Liu X. et al. [16] | 2022 | 44 | Retrospective Single Center | Ki-67 index was higher in the refractory tumors group (mean 8.6%) vs. refractory tumors group (p < 0.001); EGFR increased in refractory tumors (p < 0.01) |
| Ozturk et al. [17] | 2020 | 67 | Retrospective Single Center | Pre-operative IGF-1 correlates with ESR1 Ct (r = 0.373, p = 0.30) and ESR2 Ct (r = 0.48, p = 0.017). High ER expression in acromegalic patients associated with a decrease in pre-operative IGF-1 only in male patients |
| Park HH et al. [18] | 2018 | 132 | Retrospective Single Center | Tumors with far-lateral TSA showed significant reductions in postoperative nadir GH at 1 week (p = 0.014), 6 months (p < 0.01), and 1 year (p = 0.018), and in IGF-I at 1 year (p < 0.01) |
| Park SH et al. [19] | 2017 | 463 | Retrospective Single Center | Premenopausal females had a higher proportion CSI compared to males aged < 50 years (35.3% vs. 21.7%, p = 0.007) and had significantly lower long-term remission rates than males aged < 50 years (69.3% vs. 88.0%, p < 0.001) |
| Swanson et al. [20] | 2021 | 131 | Retrospective Single Center | SG-As were larger (p = 0.03), more frequently invasive (p = 0.004), associated with higher MRI, T2-weighted signal ratio (p = 0.01), and showed lower SSTR2 expression (p < 0.0001) |
| Ferrés et al. [21] | 2023 | 44 | Retrospective Single Center | SG was related to a reduced probability for IGF-1 normalization (p = 0.01), augmented recurrence risk (RR = 34.5, p = 0.01), and a significant need for reintervention (p = 0.014) |
| Coopmans EC. et al. [22] | 2021 | 282 | Retrospective Multicenter | Higher random GH concentration at diagnosis and a larger maximum tumor diameter associated with a lower change in long-term remission (OR = 0.93, 95% CI 0.89–0.97, p = 0.0022, respectively; OR = 0.98, 95% CI 0.96–0.99, p = 0.0053) |
| Author | Year of Publishing | No. of Patients | Study Type | Relevant Findings |
|---|---|---|---|---|
| Baussart, B. et al. [23] | 2021 | 114 | Retrospective Single Center Microadenomas | Preoperative PRL level is the only significant predictor of remission (p = 0.014) CSI was a predictor of lower remission (p = 0.009). |
| Vermeulen E. et al. [24] | 2020 | 69 | Retrospective Single Center | The 4 most powerful predictors are sex, tumor volume, the moment of prolactin normalization and the presence of a cystic, hemorrhagic, or necrotic component |
| Cander S. et al. [25] | 2014 | 113 | Retrospective Single Center | The rate of invasive pituitary adenoma is significantly higher in male patients |
| Han YL. et al. [26] | 2018 | 52 | Retrospective Single Center | Tumor size p = 0.007; OR = 5.748, 95% CI 1.621–20.379; and preoperative PRL levels p = 0.006; OR = 3.886, 95% CI 1.464–10.212 associated with unsatisfactory postoperative outcomes |
| Lv L. et al. [27] | 2019 | 42 | Retrospective Single Center Giant Prolactinomas | Male patients had larger tumors (14.57 vs. 7.74 cm3, p = 0.0179) |
| Author | Year of Publishing | No. of Patients | Study Type | Relevant Findings |
|---|---|---|---|---|
| LV L et al. [28] | 2019 | 94 | Retrospective | Female gender was associated with recurrence (p = 0.0003) Tumor volume and gender predict long-term remission (p < 0.0001) CSI, suprasellar/sellar extension, tumor volume independent predictors for long-term biological remission |
| Monsalves et al. [29] | 2014 | 153 | Retrospective | Preoperative growth rate associated with: -age (p = 0.0001), -FGFR-4 (p = 0.047) -p27 negativity (p = 0.007). Residual tumor volume associated with older age (p = 0.038), gender and suprasellar CS extension. |
| Nikitin PV et al. [30] | 2018 | 50 | Retrospective | Ki-67 cytocolorimetric index was correlated with tumor relapse (p = 0.0027 × 10–6, z = 5.3) |
| Pappy et al. [31] | 2019 | 501 | Retrospective | Best prediction model (M1): -tumor diameter > 2.9 cm -CSI -ki-67 > 3% |
| Chen Y et al. [32] | 2021 | 172 | Retrospective | Knosp classification grade 4, partial resection and ≥3% Ki-67 positive rate are independent risk factors of tumor recurrence or progression (p < 0.05) |
| Author | Year of Publishing | No. of Patients | Study Type | Relevant Findings |
|---|---|---|---|---|
| Qiao N et al. [33] | 2021 | 883 | Retrospective and Prospective | Knosp grade was the most important variable for the partial model. Postoperative day 1 GH level, total resection, and Knosp grade were the three most important variables for the full model. |
| Huber M. et al. [34] | 2022 | 86 | Prospective | Machine learning methods predict the outcome of surgery in prolactinomas. Super learner exhibits very good prediction for the primary outcome (mean AUC = 0.9, 95% CI 0.92–1.00). |
| Niu J. et al. [35] | 2019 | 194 | Retrospective | Nomogram using radiomics methods based on contrast-enhanced T1- and T2-weighted magnetic resonance predicted results better than the clinic-pathological model (AUC = 0.871, 95% CI 0.857–0.881 in test set, p = 0.021). |
| Zhang Y, Tu S et al. [36] | 2021 | 38 | Retrospective | The PPV of CS invasion of three-grade classifications is based on 3D MMI. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dumitriu-Stan, R.-I.; Burcea, I.-F.; Salmen, T.; Poiana, C. Prognostic Models in Growth-Hormone- and Prolactin-Secreting Pituitary Neuroendocrine Tumors: A Systematic Review. Diagnostics 2023, 13, 2118. https://doi.org/10.3390/diagnostics13122118
Dumitriu-Stan R-I, Burcea I-F, Salmen T, Poiana C. Prognostic Models in Growth-Hormone- and Prolactin-Secreting Pituitary Neuroendocrine Tumors: A Systematic Review. Diagnostics. 2023; 13(12):2118. https://doi.org/10.3390/diagnostics13122118
Chicago/Turabian StyleDumitriu-Stan, Roxana-Ioana, Iulia-Florentina Burcea, Teodor Salmen, and Catalina Poiana. 2023. "Prognostic Models in Growth-Hormone- and Prolactin-Secreting Pituitary Neuroendocrine Tumors: A Systematic Review" Diagnostics 13, no. 12: 2118. https://doi.org/10.3390/diagnostics13122118






