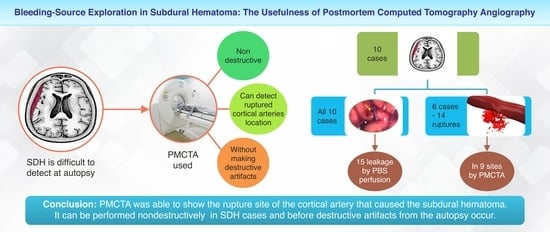
Bleeding-Source Exploration in Subdural Hematoma: Observational Study on the Usefulness of Postmortem Computed Tomography Angiography
Abstract
:1. Introduction
2. Materials and Methods
2.1. Case Selection
2.2. PMCT and PMCTA
2.3. Autopsy to Confirm Cortical Arterial Rupture
2.4. Verification of Artificial Rupture Resulting from PMCTA and Autopsy Technique
3. Results
3.1. Cortical Arterial Rupture and Extravascular Leakage on PMCTA
3.2. Artificial Rupture Attributed to PMCTA and Autopsy Technique
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Robinson, C.; Barber, J.; Amoroso, J.; Morgan, B.; Rutty, G. Pump injector system applied to targeted post-mortem coronary artery angiography. Int. J. Legal Med. 2013, 127, 661–666. [Google Scholar] [CrossRef]
- Ampanozi, G.; Halbheer, D.; Ebert, L.C.; Thali, M.J.; Held, U. Postmortem imaging findings and cause of death determination compared with autopsy: A systematic review of diagnostic test accuracy and meta-analysis. Int. J. Legal Med. 2020, 134, 321–337. [Google Scholar] [CrossRef]
- Saunders, S.L.; Morgan, B.; Raj, V.; Robinson, C.E.; Rutty, G.N. Targeted post-mortem computed tomography cardiac angiography: Proof of concept. Int. J. Leg. Med. 2011, 125, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Apitzsch, J.C.; Westphal, S.; Penzkofer, T.; Kuhl, C.K.; Knüchel, R.; Mahnken, A.H. The use of contrast-enhanced post mortem CT in the detection of cardiovascular deaths. PLoS ONE 2014, 9, e93101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saunders, S.L.; Morgan, B.; Raj, V.; Rutty, G.N. Post-mortem computed tomography: Past, present, and future. Forensic Sci. Med. Pathol. 2011, 7, 271–277. [Google Scholar] [CrossRef]
- Morgan, B.; Biggs, M.J.; Barber, J.; Raj, V.; Amoroso, J.; Hollingbury, F.E.; Robinson, C.; Rutty, G.N. Accuracy of targeted post-mortem computed tomography coronary angiography compared to assessment of serial histological sections. Int. J. Leg. Med. 2013, 127, 809–817. [Google Scholar] [CrossRef] [PubMed]
- van Rijn, R.R.; Leth, P.M. Targeted coronary post-mortem CT angiography, straight to the heart. Lancet. 2017, 390, 100–101. [Google Scholar] [CrossRef] [Green Version]
- Stassi, C.; Mondello, C.; Baldino, G.; Cardia, L.; Gualniera, P.; Calapai, F.; Sapienza, D.; Asmundo, A.; Ventura spagnolo, E. State of the art on the role of postmortem computed tomography angiography and magnetic resonance imaging in the diagnosis of cardiac causes of death: A narrative review. Tomography 2022, 8, 961–973. [Google Scholar] [CrossRef]
- Michaud, K.; Grabherr, S.; Doenz, F.; Mangin, P. Evaluation of postmortem MDCT and MDCT-angiography for the investigation of sudden cardiac death related to atherosclerotic coronary artery disease. Int. J. Cardiovasc. Imaging 2012, 28, 1807–1822. [Google Scholar] [CrossRef] [Green Version]
- Chainchel Singh, M.K.; Abdul Rashid, S.N.; Abdul Hamid, S.; Mahmood, M.S.; Feng, S.S.; Mohd Nawawi, H.; Omar, E. Correlation and assessment of coronary artery luminal stenosis: Post-mortem computed tomography angiogram versus histopathology. Forensic Sci. Int. 2020, 308, 110171. [Google Scholar] [CrossRef] [PubMed]
- Turillazzi, E.; Frati, P.; Pascale, N.; Pomara, C.; Grilli, G.; Viola, R.V.; Fineschi, V. Multi-phase post-mortem CT-angiography: A pathologic correlation study on cardiovascular sudden death. J. Geriatr. Cardiol. 2016, 13, 855–865. [Google Scholar] [CrossRef]
- Polacco, M.; Sedati, P.; Arena, V.; Pascali, V.L.; Beomonte Zobel, B.; Oliva, A.; Rossi, R. Visualization of myocardial Infarction by post-mortem single-organ coronary computed tomography: A feasibility study. Int. J. Leg. Med. 2015, 129, 517–524. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Shao, Y.; Zou, D.; Huang, P.; Li, Z.; Wang, M.; Chen, Y. Diagnosis of coronary artery disease using targeted post-mortem computed tomography coronary angiography: A case report. Forensic Sci. Res. 2017, 2, 107–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.; Park, H.; Cha, J.G.; Lee, S.; Yang, K. Myocardial contrast defect associated with thrombotic coronary occlusion: Pre-autopsy diagnosis of a cardiac death with post-mortem CT angiography. Korean J. Radiol. 2015, 16, 1024–1028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmiere, C.; Lobrinus, J.A.; Mangin, P.; Grabherr, S. Detection of coronary thrombosis after multi-phase postmortem CT-angiography. Leg. Med. 2013, 15, 12–18. [Google Scholar] [CrossRef]
- Takei, H.; Sano, R.; Takahashi, Y.; Takahashi, K.; Kominato, Y.; Tokue, H.; Shimada, T.; Awata, S.; Hirasawa, S.; Ohta, N. Usefulness of coronary postmortem computed tomography angiography to detect lesions in the coronary artery and myocardium in cases of sudden death. Leg. Med. 2018, 30, 46–51. [Google Scholar] [CrossRef]
- Jessika, C.; Anna Laura, S.; Stefano, D.; Giuliano, G.B.; Marco, B.; Riccardo, B.; Riccardo, R.; Enrico, S. Diagnosing coronary thrombosis using multiphase post-mortem CT angiography (MPMCTA): A case study. Med. Sci. Law. 2021, 61, 77–81. [Google Scholar] [CrossRef]
- Filograna, L.; Hatch, G.; Ruder, T.; Ross, S.G.; Bolliger, S.A.; Thali, M.J. The role of post-mortem imaging in a case of sudden death due to ascending aorta aneurysm rupture. Forensic Sci. Int. 2013, 228, e76–e80. [Google Scholar] [CrossRef]
- Kluschke, F.; Ross, S.; Flach, P.M.; Schweitzer, W.; Ampanozi, G.; Gascho, D.; Vonlanthen, B.; Thali, M.J.; Ruder, T.D. To see or not to see—Ambiguous findings on post-mortem cross-sectional imaging in a case of ruptured abdominal aortic aneurysm. Leg. Med. 2013, 15, 256–259. [Google Scholar] [CrossRef]
- Filograna, L.; Flach, P.M.; Bolliger, S.A.; Thali, M.J. The role of post-mortem CT (PMCT) imaging in the diagnosis of pericardial tamponade due to hemopericardium: A case report. Leg. Med. 2014, 16, 150–153. [Google Scholar] [CrossRef]
- Joffre, J.; Preda, G.; Arrivé, L.; Maury, E. Aortic dissection during extracorporeal membrane oxygenation axillary cannulation confirmed by postmortem computed tomography angiography. Am. J. Respir. Crit. Care Med. 2017, 195, 953–954. [Google Scholar] [CrossRef] [PubMed]
- Kummer, A.; Lhermitte, B.; Ödman, M.; Grabherr, S.; Mangin, P.; Palmiere, C. Carotid artery rupture and cervicofacial actinomycosis. Leg. Med. 2012, 14, 324–327. [Google Scholar] [CrossRef]
- Michaud, K.; Grabherr, S.; Lesta, M.d.M.; Augsburger, M.; Doenz, F.; Mangin, P. Ruptured pseudo-aneurysm of a femoral artery in a drug abuser revealed by post-mortem CT angiography. Int. J. Legal Med. 2013, 127, 819–823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ampanozi, G.; Preiss, U.; Hatch, G.M.; Zech, W.D.; Ketterer, T.; Bolliger, S.; Thali, M.J.; Ruder, T.D. Fatal lower extremity varicose vein rupture. Leg. Med. 2011, 13, 87–90. [Google Scholar] [CrossRef]
- O’Donnell, C.; Hislop-Jambrich, J.; Woodford, N.; Baker, M. Demonstration of liver metastases on postmortem whole body CT angiography following inadvertent systemic venous infusion of the contrast medium. Int. J. Legal Med. 2012, 126, 311–314. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento, F.B.; dos Santos, G.A.; Melo, N.A.; Damasceno, E.B.; Mauad, T. Detection of the source of hemorrhage using postmortem computerized tomographic angiography in a case of a giant juvenile nasopharyngeal angiofibroma after surgical treatment. Forensic Sci. Med. Pathol. 2015, 11, 427–431. [Google Scholar] [CrossRef] [PubMed]
- Mokrane, F.Z.; Savall, F.; Rérolle, C.; Blanc, A.; Saint Martin, P.; Rousseau, H.; Rougé, D.; Telmon, N.; Dedouit, F. The usefulness of post-mortem CT angiography in injuries caused by falling from considerable heights: Three fatal cases. Diagn. Interv. Imaging 2014, 95, 1085–1090. [Google Scholar] [CrossRef] [Green Version]
- Inokuchi, G.; Makino, Y.; Motomura, A.; Chiba, F.; Torimitsu, S.; Hoshioka, Y.; Iwase, H. Fatal right coronary artery rupture following blunt chest trauma: Detection by postmortem selective coronary angiography. Int. J. Legal Med. 2016, 130, 759–763. [Google Scholar] [CrossRef]
- Hussami, M.; Grabherr, S.; Meuli, R.A.; Schmidt, S. Severe pelvic injury: Vascular lesions detected by ante- and post-mortem contrast medium-enhanced CT and associations with pelvic fractures. Int. J. Legal Med. 2017, 131, 731–738. [Google Scholar] [CrossRef] [Green Version]
- Shokry, D.A.; Hussein, M.N.; Hassan, F.M.; Heinemann, A.; Vogel, H.; Pueschel, K. Diagnostic value of multiphase postmortem computed tomography angiography in selected cases of blunt traumatic deaths. Leg. Med. 2018, 34, 1–6. [Google Scholar] [CrossRef]
- Savall, F.; Dedouit, F.; Mokrane, F.Z.; Rougé, D.; Saint-Martin, P.; Telmon, N. An unusual homicidal stab wound of the cervical spinal cord: A single case examined by post-mortem computed tomography angiography (PMCTA). Forensic Sci. Int. 2015, 254, e18–e21. [Google Scholar] [CrossRef]
- Zhou, S.; Wan, L.; Shao, Y.; Ying, C.; Wang, Y.; Zou, D.; Xia, W.; Chen, Y. Detection of aortic rupture using post-mortem computed tomography and post-mortem computed tomography angiography by cardiac puncture. Int. J. Legal Med. 2016, 130, 469–474. [Google Scholar] [CrossRef] [Green Version]
- Ruder, T.D.; Ross, S.; Preiss, U.; Thali, M.J. Minimally invasive post-mortem CT-angiography in a case involving a gunshot wound. Leg. Med. 2010, 12, 154–156. [Google Scholar] [CrossRef]
- Stamou, S.; Gascho, D.; Eggert, S.; Berger, F.; Thali, M.J.; Flach, P.M. A fatal case of a ruptured cerebral aneurysm detected by postmortem computed tomography angiography using a new contrast-medium solution. Am. J. Forensic Med. Pathol. 2016, 37, 227–230. [Google Scholar] [CrossRef] [Green Version]
- Kuninaka, H.; Takahashi, Y.; Sano, R.; Takahashi, K.; Kubo, R.; Kominato, Y.; Takei, H.; Kobayashi, S.; Shimada, T.; Tokue, H.; et al. Use of postmortem computed tomography angiography to detect vascular injuries accompanying skull base fracture. Leg. Med. 2016, 23, 55–58. [Google Scholar] [CrossRef]
- Inokuchi, G.; Makino, Y.; Yajima, D.; Motomura, A.; Chiba, F.; Torimitsu, S.; Hoshioka, Y.; Iwase, H. A case of acute subdural hematoma due to ruptured aneurysm detected by postmortem angiography. Int. J. Legal Med. 2016, 130, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Chiba, F.; Inokuchi, G.; Makino, Y.; Torimitsu, S.; Motomura, A.; Yamaguchi, R.; Hashimoto, M.; Hoshioka, Y.; Nasgasawa, S.; Sakuma, A.; et al. Postmortem angiography revealing traumatic rupture of the intracranial internal carotid artery. Int. J. Legal Med. 2018, 132, 589–592. [Google Scholar] [CrossRef]
- Funayama, K.; Harada, K.; Koyama, A.; Katsuragi-Go, R.; Nishikawa-Harada, N.; Higuchi, R.; Aoyama, T.; Watanabe, H.; Takahashi, N.; Takatsuka, H. The usefulness of postmortem computed tomography angiography for subdural hematoma caused by rupture of the cortical artery: A report of two autopsy cases and a literature review. Leg. Med. 2021, 53, 101941. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Song, Y.; Li, Z.; Wang, M.; Song, F.; Zhang, J.; Zou, D.; Liu, N.; Shi, Y.; Zhang, Z. Detection of traumatic internal carotid artery pseudoaneurysm by postmortem imaging: A case report. Med 2022, 101, e28544. [Google Scholar] [CrossRef]
- Balser, D.; Farooq, S.; Mehmood, T.; Reyes, M.; Samadani, U. Actual and projected incidence rates for chronic subdural hematomas in United States Veterans Administration and civilian populations. J. Neurosurg. 2015, 123, 1209–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alshora, W.; Alfageeh, M.; Alshahrani, S.; Alqahtani, S.; Dajam, A.; Matar, M. Diagnosis and management of subdural hematoma: A review of recent literature. Int. J. Community Med. Public Health 2018, 5, 3709–3714. [Google Scholar] [CrossRef]
- Werner, C.; Engelhard, K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007, 99, 4–9. [Google Scholar] [CrossRef] [Green Version]
- Aromatario, M.; Torsello, A.; D’Errico, S.; Bertozzi, G.; Sessa, F.; Cipolloni, L.; Baldari, B. Traumatic epidural and subdural hematoma: Epidemiology, outcome, and dating. Medicina 2021, 57, 125. [Google Scholar] [CrossRef] [PubMed]
- Knight, B. Forensic Pathology, 2nd ed.; Oxford University Press: New York, NY, USA, 1996; ISBN 978-03-4058-897-0. [Google Scholar]
- Whitwell, H. Intracranial haematomas: Extradural and subdural. In Forensic Neuropathology, 2nd ed.; Whitwell, H., Milroy, C., du Plessis, D., Eds.; CRC Press: Boca Raton, FL, USA, 2021; ISBN 978-14-9870-616-2. [Google Scholar]
- Maxeiner, H.; Wolff, M. Pure subdural hematomas: A postmortem analysis of their form and bleeding points. Neurosurgery 2002, 50, 503–508, discussion 508–509. [Google Scholar] [CrossRef]
- Munro, D. The diagnosis and treatment of subdural hematoma. N. Engl. J. Med. 1934, 210, 1145–1160. [Google Scholar] [CrossRef]
- Talalla, A.; McKissock, W. Acute “spontaneous” subdural hemorrhage. An unusual form of cerebrovascular accident. Neurology 1971, 21, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Avis, S.P. Nontraumatic acute subdural hematoma. A case report and review of the literature. Am. J. Forensic Med. Pathol. 1993, 14, 130–134. [Google Scholar] [CrossRef]
- Tokoro, K.; Nakajima, F.; Yamataki, A. Acute spontaneous subdural hematoma of arterial origin. Surg. Neurol. 1988, 29, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Scott, M. Spontaneous nontraumatic subdural hematomas. J. Am. Med. Assoc. 1949, 141, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Funayama, K.; Tainaka, K.; Koyama, A.; Katsuragi-Go, R.; Nishikawa-Harada, N.; Higuchi, R.; Aoyama, T.; Watanabe, H.; Takahashi, N.; Takatsuka, H. Detection and morphological analysis of micro-ruptured cortical arteries in subdural hematoma: Three-dimensional visualization using the tissue-clearing clear, unobstructed, brain/body imaging cocktails and computational analysis method. Diagnostics 2022, 12, 2875. [Google Scholar] [CrossRef]
- Vance, B.M. Ruptures of surface blood vessels on cerebral hemispheres as a cause of subdural hemorrhage. AMA Arch. Surg. 1950, 61, 992–1006. [Google Scholar] [CrossRef] [PubMed]
- Drake, C.G. Subdural haematoma from arterial rupture. J. Neurosurg. 1961, 18, 597–601. [Google Scholar] [CrossRef]
- Nizzoli, V.; Brambilla, P.; Tonnarelli, G.P. Acute subdural hematoma. Spontaneous forms of arterial origin. Eur. Neurol. 1981, 20, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Stephenson, G.; Gibson, R.M. Acute spontaneous subdural haematoma of arterial origin. Br. J. Neurosurg. 1989, 3, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Awaji, K.; Inokuchi, R.; Ikeda, R.; Haisa, T. Nontraumatic pure acute subdural hematoma caused by a ruptured cortical middle cerebral artery aneurysm: Case report and literature review. NMC Case Rep. J. 2016, 3, 63–66. [Google Scholar] [CrossRef] [Green Version]
- Uneda, A.; Hirashita, K.; Yabuno, S.; Kanda, T.; Suzuki, K.; Matsumoto, A.; Yunoki, M.; Yoshino, K. Repair of damaged cortical artery by direct micro-suture in surgical treatment of acute subdural hematoma: Technical note. Acta Neurochir. 2018, 160, 1931–1937. [Google Scholar] [CrossRef]
- Abecassis, Z.A.; Nistal, D.A.; Abecassis, I.J.; Sen, R.D.; Levitt, M.R. Ghost aneurysms in acute subdural hematomas: A report of two cases. World Neurosurg. 2020, 139, e159–e165. [Google Scholar] [CrossRef] [PubMed]
- Yanagawa, T.; Yamashita, K.; Harada, Y.; Hatayama, T.; Kono, T. Location of hemorrhage with nontraumatic acute subdural hematoma due to ruptured microaneurysm. Surg. Neurol. Int. 2021, 12, 401. [Google Scholar] [CrossRef]
- Byun, H.S.; Patel, P.P. Spontaneous subdural hematoma of arterial origin: Report of two cases. Neurosurgery 1979, 5, 611–613. [Google Scholar] [CrossRef] [PubMed]
- Verhey, L.H.; Wang, W.; Adel, J.G. True cortical saccular aneurysm presenting as an acute subdural hematoma. World Neurosurg. 2018, 113, 58–61. [Google Scholar] [CrossRef]
- Shi, X.Y.; Zhang, J.X.; Tang, Z.X.; Sun, H.; Shen, Z. Severe spontaneous acute arterial subdural hematoma as an initial symptom of chronic myeloid leukemia. Br. J. Neurosurg. 2021, 19, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Noufal, M.; Liang, C.W.; Yanez, P.; Calayag, M. Spontaneous chronic subdural hematoma due to cerebral cortical artery rupture: First case report and review of pertinent literature. Neuroradiol. J. 2021, 34, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health, Labour and Welfare. Available online: https://www.mhlw.go.jp/file/06-Seisakujouhou-10600000-Daijinkanboukouseikagakuka/0000080278.pdf (accessed on 15 October 2018).
- Mckissock, W.; Richardson, A.; Bloom, W. Subdural haematoma: A review of 389 cases. Lancet 1960, 275, 1365–1369. [Google Scholar] [CrossRef]
- La Russa, R.; Catalano, C.; Di Sanzo, M.; Scopetti, M.; Gatto, V.; Santurro, A.; Viola, R.V.; Panebianco, V.; Frati, P.; Fineschi, V. Postmortem computed tomography angiography (PMCTA) and traditional autopsy in cases of sudden cardiac death due to coronary artery disease: A systematic review and meta-analysis. Radiol. Med. 2019, 124, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Chevallier, C.; Doenz, F.; Vaucher, P.; Palmiere, C.; Dominguez, A.; Binaghi, S.; Mangin, P.; Grabherr, S. Postmortem computed tomography angiography vs. conventional autopsy: Advantages and inconveniences of each method. Int. J. Legal Med. 2013, 127, 981–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Troncoso, J.C.; Pletnikova, O. Traumatic brain injuries and dural hemorrhages. In Essential Forensic Neuropathology, 1st ed.; Troncoso, J.C., Rubio, A., Fowler, D., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; p. 73. [Google Scholar]
- Matsuyama, T.; Shimomura, T.; Okumura, Y.; Sakaki, T. Acute subdural hematomas due to rupture of cortical arteries: A study of the points of rupture in 19 cases. Surg. Neurol. 1997, 47, 423–427. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, M.J.; Chaganti, J.; Dower, A.; Al-Khawaja, D. Spontaneous acute arterial subdural hematoma. World Neurosurg. 2018, 110, 403–406. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, C. When it goes wrong: Complications of post-mortem CT angiography (PMCTA) as performed at VIFM. J. Forensic Radiol. Imaging 2013, 2, 84–85. [Google Scholar] [CrossRef]
- Bruguier, C.; Mosimann, P.J.; Vaucher, P.; Uské, A.; Doenz, F.; Jackowski, C.; Mangin, P.; Grabherr, S. Multi-phase postmortem CT angiography: Recognizing technique-related artefacts and pitfalls. Int. J. Legal Med. 2013, 127, 639–652. [Google Scholar] [CrossRef] [Green Version]
- Berger, N.; Martinez, R.; Winklhofer, S.; Flach, P.M.; Ross, S.; Ampanozi, G.; Gascho, D.; Thali, M.J.; Ruder, T.D. Pitfalls in post-mortem CT-angiography—Intravascular contrast induces post-mortem pericardial effusion. Leg. Med. 2013, 15, 315–317. [Google Scholar] [CrossRef] [PubMed]
- Romero, J.M.; Kelly, H.R.; Delgado Almandoz, J.E.; Hernandez-Siman, J.; Passanese, J.C.; Lev, M.H.; González, R.G. Contrast extravasation on CT angiography predicts hematoma expansion and mortality in acute traumatic subdural hemorrhage. AJNR. Am. J. Neuroradiol. 2013, 34, 1528–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosa, M., Jr.; da Rocha, A.J.; Maia, A.C., Jr.; Saade, N.; Veiga, J.C.; Romero, J.M. Contusion contrast extravasation depicted on multidetector computed tomography angiography predicts growth and mortality in traumatic brain contusion. J. Neurotrauma 2016, 33, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Orito, K.; Hirohata, M.; Nakamura, Y.; Yamamoto, M.; Takeshige, N.; Aoki, T.; Hattori, G.; Sakata, K.; Takeuchi, Y.; Uzu, H.; et al. Predictive value of leakage signs for pure brain contusional hematoma expansion. J. Neurotrauma 2018, 35, 760–766. [Google Scholar] [CrossRef] [PubMed]
- McDermott, M.; Fleming, J.F.; Vanderlinden, R.G.; Tucker, W.S. Spontaneous arterial subdural hematoma. Neurosurgery 1984, 14, 13–18. [Google Scholar] [CrossRef]
- O’Brien, P.K.; Norris, J.W.; Tator, C.H. Acute subdural hematomas of arterial origin. J. Neurosurg. 1974, 41, 435–439. [Google Scholar] [CrossRef]
- Akioka, N.; Fukuda, O.; Takaba, M.; Kameda, H.; Saito, T.; Endo, S. Clinical investigation of acute spontaneous subdural hematoma cases. J. Stroke Cerebrovasc. Dis. 2007, 16, 109–113. [Google Scholar] [CrossRef]
- Martins, W.A.; Teixeira, A.B.; Frigeri, T.M.; Paglioli, E. Spontaneous subdural hematoma associated to Duret hemorrhage. Interdiscip. Neurosurg. 2015, 2, 13–15. [Google Scholar] [CrossRef] [Green Version]




| No. | Age (Years), Sex | Form of SDH 1 | Arterial Rupture Site | Contrast Agent Leakage | Total Volume of the Contrast Agent |
|---|---|---|---|---|---|
| 1 | 95 Female | Subacute | Right parietal lobe | + | 20 mL |
| 2 | 84 Female | Acute | Right temporal lobe | + | 20 mL |
| 3 | 77 Male | Acute | Right temporal lobe | + | 20 mL |
| 4 | 96 Male | Acute | Right parietal lobe | + | 10 mL |
| 5 | 62 Male | Acute | Right frontal pole Right frontal lobe Right temporal lobe Right parietal lobe | + + + + | 20 mL |
| 6 | 62 Male | Acute | Left parietal lobe Right parietal lobe | + - | 40 mL (Left) 40 mL (Right) |
| 7 | 70 Male | Acute | Left occipital lobe | - | 65 mL |
| 8 | 82 Female | Acute | Left parietal lobe | - | 65 mL |
| 9 | 85 Female | Acute | Left temporal lobe | - | 55 mL |
| 10 | 83 Male | Acute | Left parietal lobe | - | 65 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Funayama, K.; Koyama, A.; Katsuragi-Go, R.; Aoyama, T.; Watanabe, H.; Takahashi, N.; Takatsuka, H. Bleeding-Source Exploration in Subdural Hematoma: Observational Study on the Usefulness of Postmortem Computed Tomography Angiography. Diagnostics 2023, 13, 2286. https://doi.org/10.3390/diagnostics13132286
Funayama K, Koyama A, Katsuragi-Go R, Aoyama T, Watanabe H, Takahashi N, Takatsuka H. Bleeding-Source Exploration in Subdural Hematoma: Observational Study on the Usefulness of Postmortem Computed Tomography Angiography. Diagnostics. 2023; 13(13):2286. https://doi.org/10.3390/diagnostics13132286
Chicago/Turabian StyleFunayama, Kazuhisa, Akihide Koyama, Rieka Katsuragi-Go, Takashi Aoyama, Hiraku Watanabe, Naoya Takahashi, and Hisakazu Takatsuka. 2023. "Bleeding-Source Exploration in Subdural Hematoma: Observational Study on the Usefulness of Postmortem Computed Tomography Angiography" Diagnostics 13, no. 13: 2286. https://doi.org/10.3390/diagnostics13132286