Comparison of the Effects of DOTA and NOTA Chelators on 64Cu-Cudotadipep and 64Cu-Cunotadipep for Prostate Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of 64Cu-Cudotadipep and 64Cu-Cunotadipep
2.2. Stability Analysis
2.3. Cell Culture
2.4. Competitive Binding Assay
2.5. Cell Uptake
2.6. Saturation Binding Assay
2.7. Animal Experiment
2.8. Biodistribution Studies
2.9. MicroPET/CT Imaging
2.10. Radiation-Absorbed Dose Calculations
2.11. Statistical Analysis
3. Results
3.1. Assessment of PSMA-Binding Affinity
3.2. Serum Stability
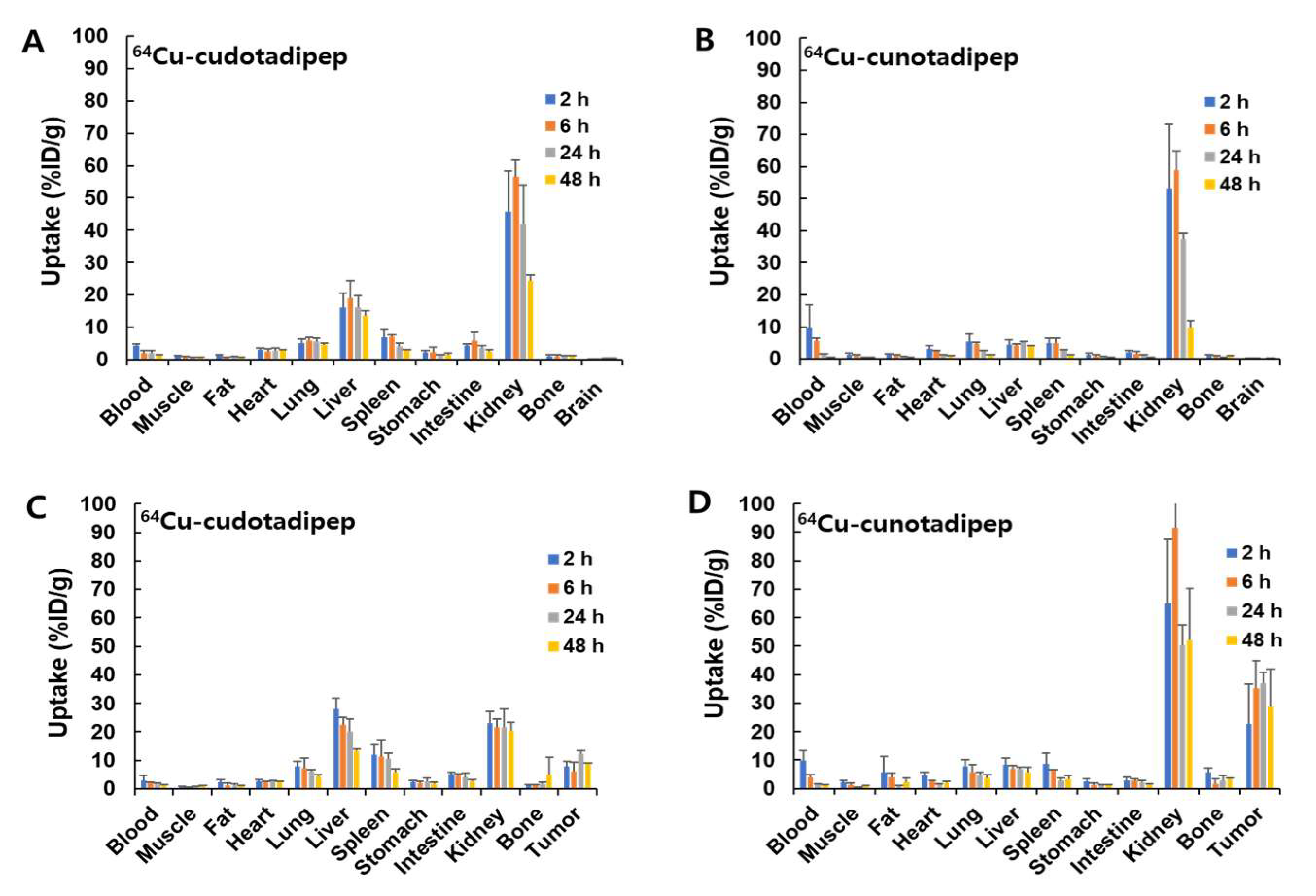
3.3. Biodistribution Analysis in Normal Mice
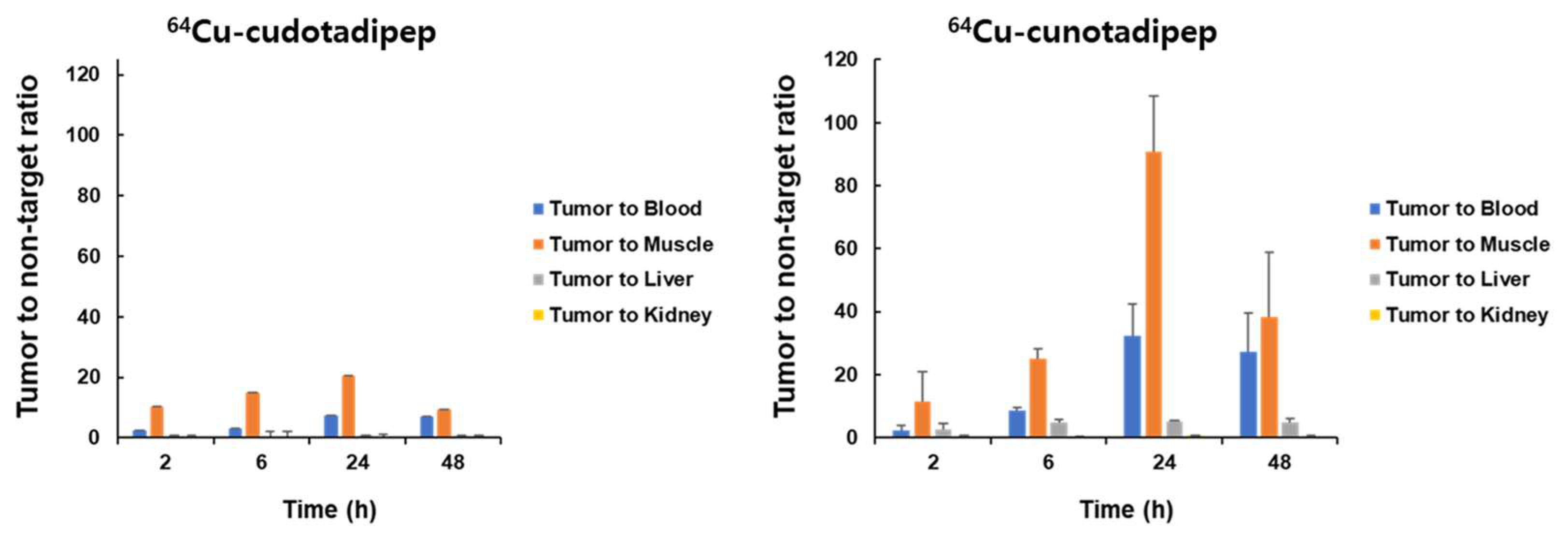
3.4. Biodistribution Analysis in Tumor Model
3.5. MicroPET/CT Imaging
3.6. Radiation Dosimetry of 64Cu-Cudotadipep and 64Cu-Cunotadipep
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, J.; Li, Y.; Yang, D.; Yang, C.; Mao, L. Current State of Biomarkers for the Diagnosis and Assessment of Treatment Efficacy of Prostate Cancer. Discov. Med. 2019, 27, 235–243. [Google Scholar] [PubMed]
- Hong, S.; Won, Y.J.; Lee, J.J.; Jung, K.W.; Kong, H.J.; Im, J.S.; Seo, H.G. Community of Population-Based Regional Cancer Registries Cancer Statistics in Korea: Incidence, Mortality, Survival, and Prevalence in 2018. Cancer Res. Treat. 2021, 53, 301–315. [Google Scholar] [CrossRef] [PubMed]
- Litwin, M.S.; Tan, H.J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.U.; Kim, J.S.; Kim, Y.S.; Cho, J.; Choi, S.H.; Nam, T.K.; Jeong, S.M.; Kim, Y.; Choi, Y.; Lee, D.E.; et al. Optimal Definition of Biochemical Recurrence in Patients Who Receive Salvage Radiotherapy Following Radical Prostatectomy for Prostate Cancer. Cancer Res. Treat. 2022, 54, 1191–1199. [Google Scholar] [CrossRef]
- Turpin, A.; Girard, E.; Baillet, C.; Pasquier, D.; Olivier, J.; Villers, A.; Puech, P.; Penel, N. Imaging for Metastasis in Prostate Cancer: A Review of the Literature. Front. Oncol. 2020, 10, 55. [Google Scholar] [CrossRef]
- Oh, S.W.; Cheon, G.J. Prostate-Specific Membrane Antigen PET Imaging in Prostate Cancer: Opportunities and Challenges. Korean J. Radiol. 2018, 19, 819–831. [Google Scholar] [CrossRef]
- Tateishi, U. Prostate-Specific Membrane Antigen (PSMA)-Ligand Positron Emission Tomography and Radioligand Therapy (RLT) of Prostate Cancer. Jpn. J. Clin. Oncol. 2020, 50, 349–356. [Google Scholar] [CrossRef]
- Lee, B.S.; Chu, S.Y.; Jung, W.J.; Jeong, H.J.; Lee, K.; Kim, M.H.; Kim, M.H.; Chi, D.Y.; Ahn, H.; Lee, Y.J.; et al. 18F-Labeled 1,2,3-Triazole-linked Glu-urea-Lys-based PSMA Ligands Have Good Pharmacokinetic Properties for Positron Emission Tomography Imaging of Prostate Cancer. Prostate 2020, 80, 1383–1393. [Google Scholar] [CrossRef]
- Lee, I.; Lim, I.; Byun, B.H.; Kim, B.I.; Choi, C.W.; Woo, S.K.; Lee, K.C.; Kang, J.H.; Kil, H.S.; Park, C.; et al. A Microdose Clinical Trial to Evaluate [18F]Florastamin as a Positron Emission Tomography Imaging Agent in Patients with Prostate Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 95–102. [Google Scholar] [CrossRef]
- Rowe, S.P.; Buck, A.; Bundschuh, R.A.; Lapa, C.; Serfling, S.E.; Derlin, T.; Higuchi, T.; Gorin, M.A.; Pomper, M.G.; Werner, R.A. [18F]DCFPyL PET/CT for Imaging of Prostate Cancer. [18F]DCFPyL PET/CT zur Prostatakarzinom-Bildgebung. Nuklearmedizin 2022, 61, 240–246. [Google Scholar]
- Rowe, S.P.; Gorin, M.A.; Pomper, M.G. Imaging of Prostate-Specific Membrane Antigen Using [18F]DCFPyL. PET Clin. 2017, 12, 289–296. [Google Scholar] [CrossRef]
- Kuten, J.; Fahoum, I.; Savin, Z.; Shamni, O.; Gitstein, G.; Hershkovitz, D.; Mabjeesh, N.J.; Yossepowitch, O.; Mishani, E.; Even-Sapir, E. Head-to-Head Comparison of 68Ga-PSMA-11 with 18F-PSMA-1007 PET/CT in Staging Prostate Cancer Using Histopathology and Immunohistochemical Analysis as a Reference Standard. J. Nucl. Med. 2020, 61, 527–532. [Google Scholar] [CrossRef] [Green Version]
- Sprute, K.; Kramer, V.; Koerber, S.A.; Meneses, M.; Fernandez, R.; Soza-Ried, C.; Eiber, M.; Weber, W.A.; Rauscher, I.; Rahbar, K.; et al. Diagnostic Accuracy of 18F-PSMA-1007 PET/CT Imaging for Lymph Node Staging of Prostate Carcinoma in Primary and Biochemical Recurrence. J. Nucl. Med. 2021, 62, 208–213. [Google Scholar] [CrossRef]
- Shin, D.; Yoon, C.E.; Kwon, H.J.; Moon, H.W.; Park, Y.H.; Cho, H.J.; Ha, U.S.; Hong, S.H.; Park, S.Y.; Ha, S.; et al. Irreversible Electroporation (IRE) for Prostate Cancer Using PSMA PET-CT. Prostate Int. 2022, 11, 40–45. [Google Scholar] [CrossRef]
- Gomes Marin, J.F.; Nunes, R.F.; Coutinho, A.M.; Zaniboni, E.C.; Costa, L.B.; Barbosa, F.G.; Queiroz, M.A.; Cerri, G.G.; Buchpiguel, C.A. Theranostics in Nuclear Medicine: Emerging and Re-emerging Integrated Imaging and Therapies in the Era of Precision Oncology. Radiographics 2020, 40, 1715–1740. [Google Scholar] [CrossRef]
- Mou, L.; Martini, P.; Pupillo, G.; Cieszykowska, I.; Cutler, C.S.; Mikołajczak, R. 67Cu Production Capabilities: A Mini Review. Molecules 2022, 27, 1501. [Google Scholar] [CrossRef]
- Huynh, T.T.; van Dam, E.M.; Sreekumar, S.; Mpoy, C.; Blyth, B.J.; Muntz, F.; Harris, M.J.; Rogers, B.E. Copper-67-Labeled Bombesin Peptide for Targeted Radionuclide Therapy of Prostate Cancer. Pharmaceuticals 2022, 15, 728. [Google Scholar] [CrossRef]
- Sevcenco, S.; Klingler, H.C.; Eredics, K.; Friedl, A.; Schneeweiss, J.; Knoll, P.; Kunit, T.; Lusuardi, L.; Mirzaei, S. Application of Cu-64 NODAGA-PSMA PET in Prostate Cancer. Adv. Ther. 2018, 35, 779–784. [Google Scholar] [CrossRef]
- Zhang, Y.; Hong, H.; Engle, J.W.; Bean, J.; Yang, Y.; Leigh, B.R.; Barnhart, T.E.; Cai, W. Positron Emission Tomography Imaging of CD105 Expression with a 64Cu-Labeled Monoclonal Antibody: NOTA Is Superior to DOTA. PloS One 2011, 6, e28005. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.S.; Kim, M.H.; Chu, S.Y.; Jung, W.J.; Jeong, H.J.; Lee, K.; Kim, H.S.; Kim, M.H.; Kil, H.S.; Han, S.J.; et al. Improving Theranostic Gallium-68/Lutetium-177–Labeled PSMA Inhibitors with an Albumin Binder for Prostate Cancer. Mol. Cancer Ther. 2021, 20, 2410–2419. [Google Scholar] [CrossRef]
- Cheng, H.C. The Power Issue: Determination of Kd or Ki from IC50. A Closer Look at the Cheng-Prusoff Equation, the Schild Plot and Related Power Equations. J. Pharmacol. Toxicol. Methods 2021, 46, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Dahlbom, M.; Lindner, T.; Vauclin, S.; Mona, C.; Slavik, R.; Czernin, J.; Haberkorn, U.; Calais, J. Radiation Dosimetry and Biodistribution of 68Ga-FAPI-46 PET Imaging in Cancer Patients. J. Nucl. Med. 2020, 61, 1171–1177. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Hadaschik, B.; Cardinale, J.; Radtke, J.; Vinsensia, M.; Lehnert, W.; Kesch, C.; Tolstov, Y.; Singer, S.; Grabe, N.; et al. F-18 Labelled PSMA-1007: Biodistribution, Radiation Dosimetry and Histopathological Validation of Tumor Lesions in Prostate Cancer Patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 678–688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grubmüller, B.; Baum, R.P.; Capasso, E.; Singh, A.; Ahmadi, Y.; Knoll, P.; Floth, A.; Righi, S.; Zandieh, S.; Meleddu, C.; et al. 64Cu-PSMA-617 PET/CT Imaging of Prostate Adenocarcinoma: First in-Human Studies. Cancer Biother. Radiopharm. 2016, 31, 277–286. [Google Scholar] [CrossRef]
- Baun, C.; Mitran, B.; Rinne, S.S.; Dam, J.H.; Olsen, B.B.; Tolmachev, V.; Orlova, A.; Thisgaard, H. Preclinical Evaluation of the Copper-64 Labeled GRPR-Antagonist RM26 in Comparison with the Cobalt-55 Labeled Counterpart for PET-Imaging of Prostate. Molecules 2020, 25, 5993. [Google Scholar] [CrossRef]
- Banerjee, S.R.; Pullambhatla, M.; Foss, C.A.; Nimmagadda, S.; Ferdani, R.; Anderson, C.J.; Mease, R.C.; Pomper, M.G. 64Cu-Labeled Inhibitors of Prostate-Specific Membrane Antigen for PET Imaging of Prostate Cancer. J. Med. Chem. 2014, 57, 2657–2669. [Google Scholar] [CrossRef]
- Cantiello, F.; Gangemi, V.; Cascini, G.L.; Calabria, F.; Moschini, M.; Ferro, M.; Musi, G.; Butticè, S.; Salonia, A.; Briganti, A.; et al. Diagnostic Accuracy of 64Copper Prostate-Specific Membrane Antigen Positron Emission Tomography/Computed Tomography for Primary Lymph Node Staging of Intermediate-To High-Risk Prostate Cancer: Our Preliminary Experience. Urology 2017, 106, 139–145. [Google Scholar] [CrossRef]
- Lütje, S.; Heskamp, S.; Cornelissen, A.S.; Poeppel, T.D.; van den Broek, S.A.; Rosenbaum-Krumme, S.; Bockisch, A.; Gotthardt, M.; Rijpkema, M.; Boerman, O.C. PSMA Ligands for Radionuclide Imaging and Therapy of Prostate Cancer: Clinical Status. Theranostics 2015, 5, 1388–1401. [Google Scholar] [CrossRef] [Green Version]
- Nedrow, J.R.; Latoche, J.D.; Day, K.E.; Modi, J.; Ganguly, T.; Zeng, D.; Kurland, B.F.; Berkman, C.E.; Anderson, C.J. Targeting PSMA with a Cu-64 Labeled Phosphoramidate Inhibitor for PET/CT Imaging of Variant PSMA-Expressing Xenografts in Mouse Models of Prostate Cancer. Mol. Imaging Biol. 2016, 18, 402–410. [Google Scholar] [CrossRef]
- Han, X.D.; Liu, C.; Liu, F.; Xie, Q.H.; Liu, T.L.; Guo, X.Y.; Xu, X.X.; Yang, X.; Zhu, H.; Yang, Z. 64Cu-PSMA-617: A Novel PSMA-Targeted Radio-tracer for PET Imaging in Gastric Adenocarcinoma Xenografted Mice Model. Oncotarget 2017, 8, 74159–74169. [Google Scholar] [CrossRef] [Green Version]
- Brandt, M.; Cardinale, J.; Aulsebrook, M.L.; Gasser, G.; Mindt, T.L. An Overview of PET Radiochemistry, Part 2: Radiometals. J. Nucl. Med. 2018, 59, 1500–1506. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.H.; Lim, I.; Woo, S.K.; Kim, K.I.; Lee, K.C.; Song, K.; Choi, C.W.; Lim, S.M. The Feasibility of 64Cu-PSMA I&T PET for Prostate Cancer. Cancer Biother. Radiopharm. 2022, 37, 417–423. [Google Scholar]
- Sprague, J.E.; Peng, Y.; Sun, X.; Weisman, G.R.; Wong, E.H.; Achilefu, S.; Anderson, C.J. Preparation and Biological Evaluation of Copper-64–Labeled Tyr3-Octreotate Using a Cross-Bridged Macrocyclic Chelator. Clin. Cancer Res. 2004, 10, 8674–8682. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Kim, D.W. Copper Chelation Chemistry with Various Chelators for Radiopharmaceuticals. J. Radiopharm. Mol. Probes 2019, 5, 129–134. [Google Scholar]
- Prasanphanich, A.F.; Nanda, P.K.; Rold, T.L.; Ma, L.; Lewis, M.R.; Garrison, J.C.; Hoffman, T.J.; Sieckman, G.L.; Figueroa, S.D.; Smith, C.J. [64Cu-NOTA-8-Aoc-BBN(7-14)NH2] Targeting Vector for Positron-Emission Tomography Imaging of Gastrin-Releasing Peptide Receptor-Expressing Tissues. Proc. Natl. Acad. Sci. USA 2007, 104, 2462–12467. [Google Scholar] [CrossRef]
- Boswell, C.A.; Sun, X.; Niu, W.; Weisman, G.R.; Wong, E.H.; Rheingold, A.L.; Anderson, C.J. Comparative In Vivo Stability of Copper-64-Labeled Cross-Bridged and Conventional Tetraazamacrocyclic Complexes. J. Med. Chem. 2004, 47, 1465–1474. [Google Scholar] [CrossRef]
- Ahrens, B.J.; Li, L.; Ciminera, A.K.; Chea, J.; Poku, E.; Bading, J.R.; Weist, M.R.; Miller, M.M.; Colcher, D.M.; Shively, J.E. Diagnostic PET Imaging of Mammary Microcalcifications Using 64Cu-DOTA-Alendronate in a Rat Model of Breast Cancer. J. Nucl. Med. 2017, 58, 1373–1379. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.; Lim, I.; Byun, B.H.; Kim, B.I.; Choi, C.W.; Woo, S.K.; Kim, K.I.; Lee, K.C.; Kang, J.H.; Seong, M.K.; et al. A Preliminary Clinical Trial to Evaluate 64Cu-NOTA-Trastuzumab as a Positron Emission Tomography Imaging Agent in Patients with Breast Cancer. EJNMMI Res. 2021, 11, 8. [Google Scholar] [CrossRef]
- Woo, S.K.; Jang, S.J.; Seo, M.J.; Park, J.H.; Kim, B.S.; Kim, E.J.; Lee, Y.J.; Lee, T.S.; An, G.I.; Song, I.H.; et al. Development of 64Cu-NOTA-Trastuzumab for HER2 Targeting: A Radiopharmaceutical with Improved Pharmacokinetics for Human Studies. J. Nucl. Med. 2019, 60, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, K.; Larson, S.M.; Weber, W.A. Theranostic Concepts: More than Just a Fashion Trend—Introduction and Overview. J. Nucl. Med. 2017, 58, 1S–2S. [Google Scholar] [CrossRef] [Green Version]
- Rahbar, K.; Afshar-Oromieh, A.; Jadvar, H.; Ahmadzadehfar, H. PSMA Theranostics: Current Status and Future Directions. Mol. Imaging 2018, 17, 1536012118776068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, G.; Rong, D.; Li, Z.; Sun, G.; Wu, F.; Li, X.; Cao, H.; Cheng, Y.; Tang, W.; Sun, Y. Role of Small Molecule Targeted Compounds in Cancer: Progress, Opportunities, and Challenges. Front. Cell Dev. Biol. 2021, 9, 694363. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, C.; Guan, X.; Ma, H.; Cong, H.; Zhang, W.; Miao, Z. Small molecule-drug conjugates: A novel strategy for cancer-targeted treatment. Eur. J. Med. Chem. 2019, 163, 883–895. [Google Scholar] [CrossRef] [PubMed]
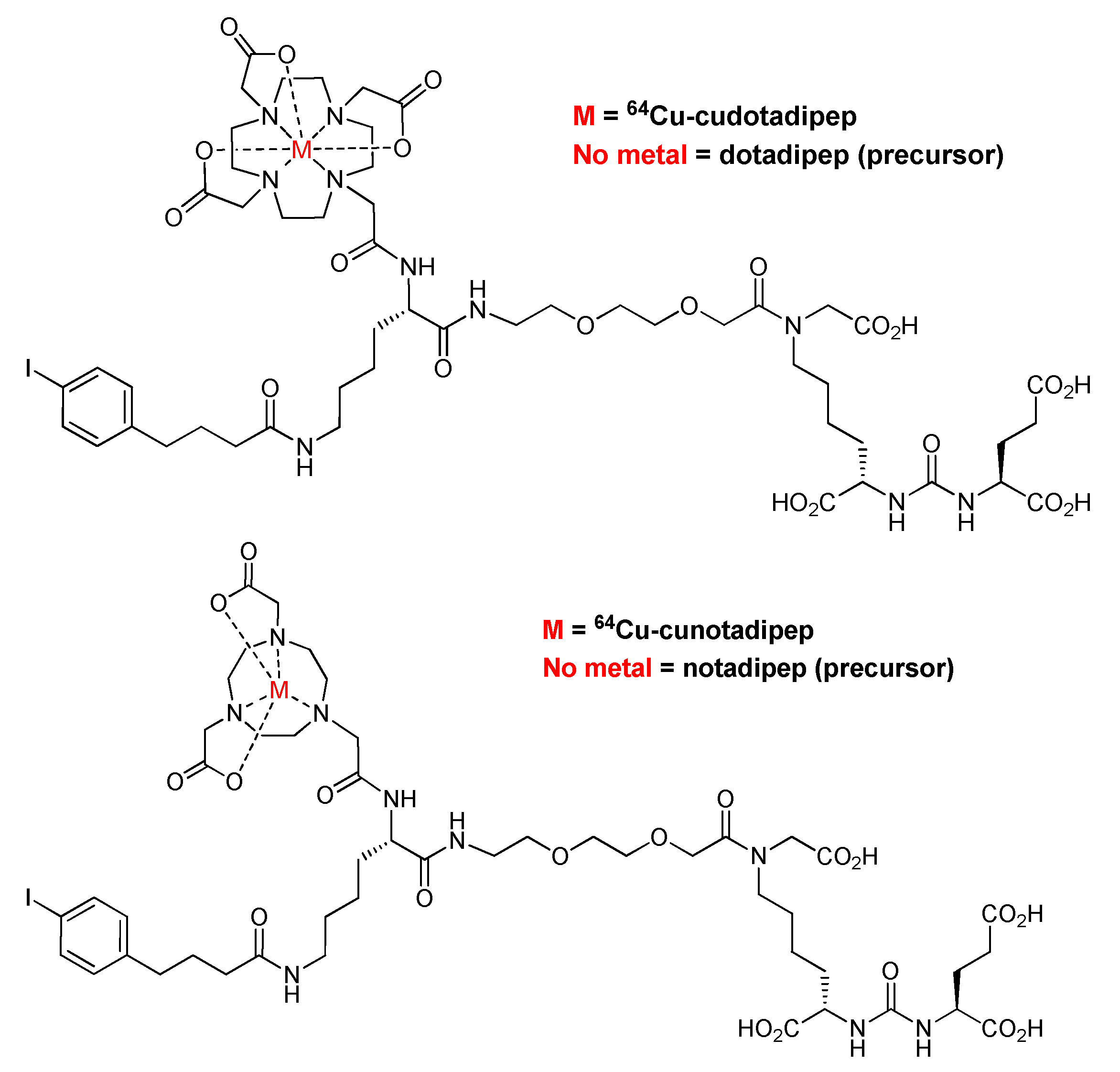

| 64Cu-Cudotadipep | 64Cu-Cunotadipep | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 6 h | 24 h | 48 h | 2 h | 6 h | 24 h | 48 h | |
| Blood | 4.27 ± 0.64 | 2.04 ± 0.71 | 2.01 ± 0.92 | 1.22 ± 0.36 | 9.51 ± 7.25 | 5.62 ± 0.95 | 1.39 ± 0.15 | 0.45 ± 0.15 |
| Muscle | 1.01 ± 0.15 | 0.74 ± 0.18 | 0.68 ± 0.18 | 0.62 ± 0.12 | 1.30 ± 0.42 | 1.05 ± 0.14 | 0.42 ± 0.05 | 0.45 ± 0.04 |
| Fat | 1.26 ± 0.28 | 0.72 ± 0.13 | 0.70 ± 0.33 | 0.51 ± 0.27 | 1.20 ± 0.35 | 0.99 ± 0.27 | 0.63 ± 0.26 | 0.40 ± 0.10 |
| Heart | 3.02 ± 0.52 | 2.67 ± 0.53 | 2.91 ± 0.66 | 2.72 ± 0.39 | 3.17 ± 1.12 | 2.25 ± 0.33 | 1.22 ± 0.06 | 0.95 ± 0.07 |
| Lung | 5.16 ± 1.26 | 5.77 ± 1.12 | 5.56 ± 1.13 | 4.66 ± 0.50 | 5.48 ± 2.24 | 4.76 ± 0.51 | 2.53 ± 0.20 | 1.32 ± 0.13 |
| Liver | 16.12 ± 4.51 | 19.13 ± 5.32 | 16.15 ± 3.65 | 13.48 ± 1.56 | 4.38 ± 1.51 | 4.11 ± 0.53 | 5.32 ± 0.12 | 3.86 ± 0.31 |
| Spleen | 6.80 ± 2.46 | 7.09 ± 0.68 | 4.14 ± 0.89 | 2.73 ± 0.28 | 5.07 ± 1.38 | 5.05 ± 1.56 | 2.56 ± 0.33 | 1.19 ± 0.11 |
| Stomach | 2.30 ± 0.60 | 2.26 ± 1.59 | 1.38 ± 0.15 | 1.45 ± 0.65 | 1.21 ± 0.49 | 0.92 ± 0.45 | 0.67 ± 0.22 | 0.44 ± 0.11 |
| Intestine | 4.22 ± 0.57 | 5.85 ± 2.69 | 3.64 ± 0.83 | 2.53 ± 0.54 | 2.21 ± 0.35 | 1.69 ± 0.55 | 1.16 ± 0.24 | 0.56 ± 0.08 |
| Kidney | 45.74 ± 12.68 | 56.43 ± 5.37 | 41.77 ± 12.09 | 24.37 ± 1.88 | 53.28 ± 19.92 | 58.85 ± 6.00 | 37.40 ± 1.84 | 9.55 ± 2.33 |
| Bone | 1.09 ± 0.32 | 1.10 ± 0.40 | 0.94 ± 0.41 | 0.96 ± 0.25 | 1.07 ± 0.24 | 0.89 ± 0.22 | 0.60 ± 0.03 | 0.83 ± 0.10 |
| Brain | 0.26 ± 0.07 | 0.25 ± 0.10 | 0.37 ± 0.08 | 0.44 ± 0.13 | 0.26 ± 0.12 | 0.19 ± 0.02 | 0.13 ± 0.01 | 0.18 ± 0.01 |
| 64Cu-Cudotadipep | 64Cu-Cunotadipep | |||||||
|---|---|---|---|---|---|---|---|---|
| 2 h | 6 h | 24 h | 48 h | 2 h | 6 h | 24 h | 48 h | |
| Blood | 2.79 ± 1.90 | 1.92 ± 0.53 | 1.75 ± 0.41 | 1.28 ± 0.24 | 9.81 ± 3.47 | 4.01 ± 0.94 | 1.23 ± 0.38 | 1.08 ± 0.27 |
| Muscle | 0.78 ± 0.13 | 0.40 ± 0.13 | 0.69 ± 0.33 | 0.98 ± 0.24 | 2.14 ± 0.59 | 1.44 ± 0.56 | 0.41 ± 0.06 | 0.80 ± 0.22 |
| Fat | 2.22 ± 1.07 | 1.12 ± 0.79 | 1.23 ± 0.52 | 0.88 ± 0.42 | 5.72 ± 5.53 | 4.14 ± 1.33 | 0.74 ± 0.20 | 2.36 ± 1.25 |
| Heart | 2.68 ± 0.45 | 2.30 ± 0.30 | 2.56 ± 0.49 | 2.55 ± 0.12 | 4.56 ± 1.25 | 2.59 ± 0.38 | 1.64 ± 0.12 | 1.91 ± 0.57 |
| Lung | 8.02 ± 1.49 | 7.35 ± 3.45 | 6.00 ± 0.85 | 4.36 ± 0.62 | 7.72 ± 2.30 | 5.75 ± 2.72 | 4.58 ± 1.08 | 3.64 ± 1.14 |
| Liver | 28.09 ± 3.73 | 22.44 ± 2.56 | 20.08 ± 4.40 | 13.34 ± 0.55 | 8.33 ± 2.29 | 6.96 ± 1.00 | 6.99 ± 0.45 | 5.74 ± 1.83 |
| Spleen | 11.94 ± 3.50 | 11.43 ± 5.81 | 10.43 ± 1.98 | 5.74 ± 1.34 | 8.58 ± 3.99 | 6.63 ± 0.12 | 2.79 ± 0.87 | 3.56 ± 1.14 |
| Stomach | 2.35 ± 0.63 | 2.18 ± 0.47 | 2.66 ± 1.28 | 1.72 ± 0.70 | 2.50 ± 1.05 | 1.42 ± 0.53 | 1.27 ± 0.08 | 1.14 ± 0.31 |
| Intestine | 5.19 ± 0.68 | 4.55 ± 0.77 | 4.17 ± 1.45 | 2.56 ± 0.62 | 2.97 ± 1.15 | 2.74 ± 0.80 | 2.38 ± 0.42 | 1.54 ± 0.16 |
| Kidney | 23.11 ± 4.11 | 21.51 ± 3.13 | 21.57 ± 6.53 | 20.41 ± 3.03 | 65.02 ± 22.42 | 91.48 ± 12.98 | 50.29 ± 7.06 | 52.07 ± 18.32 |
| Bone | 1.34 ± 0.23 | 1.25 ± 0.20 | 1.88 ± 0.35 | 5.05 ± 6.09 | 5.71 ± 1.47 | 1.81 ± 1.50 | 2.81 ± 1.71 | 3.42 ± 0.40 |
| Tumor | 7.95 ± 1.58 | 6.03 ± 3.20 | 12.12 ± 1.17 | 8.62 ± 0.44 | 22.61 ± 14.10 | 35.14 ± 9.68 | 37.13 ± 3.65 | 28.84 ± 13.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, I.; Kim, M.H.; Lee, K.; Oh, K.; Lim, H.; Ahn, J.H.; Lee, Y.J.; Cheon, G.J.; Chi, D.Y.; Lim, S.M. Comparison of the Effects of DOTA and NOTA Chelators on 64Cu-Cudotadipep and 64Cu-Cunotadipep for Prostate Cancer. Diagnostics 2023, 13, 2649. https://doi.org/10.3390/diagnostics13162649
Lee I, Kim MH, Lee K, Oh K, Lim H, Ahn JH, Lee YJ, Cheon GJ, Chi DY, Lim SM. Comparison of the Effects of DOTA and NOTA Chelators on 64Cu-Cudotadipep and 64Cu-Cunotadipep for Prostate Cancer. Diagnostics. 2023; 13(16):2649. https://doi.org/10.3390/diagnostics13162649
Chicago/Turabian StyleLee, Inki, Min Hwan Kim, Kyongkyu Lee, Keumrok Oh, Hyunwoo Lim, Jae Hun Ahn, Yong Jin Lee, Gi Jeong Cheon, Dae Yoon Chi, and Sang Moo Lim. 2023. "Comparison of the Effects of DOTA and NOTA Chelators on 64Cu-Cudotadipep and 64Cu-Cunotadipep for Prostate Cancer" Diagnostics 13, no. 16: 2649. https://doi.org/10.3390/diagnostics13162649





