Screening and Management of Coronary Artery Disease in Kidney Transplant Candidates
Abstract
:1. Introduction
2. CAD Presentation and Its Pathophysiology
2.1. Classic CV Risk Factors
2.2. Anemia
2.3. Volume Overload, Fluid Retention, and LVH
2.4. Secondary Hyperparathyroidism
2.5. Endothelial Dysfunction, Oxidative Stress, and Inflammation
2.6. Platelet Abnormalities
2.7. Uremic Toxins
2.8. Hemodialysis-Related Factors
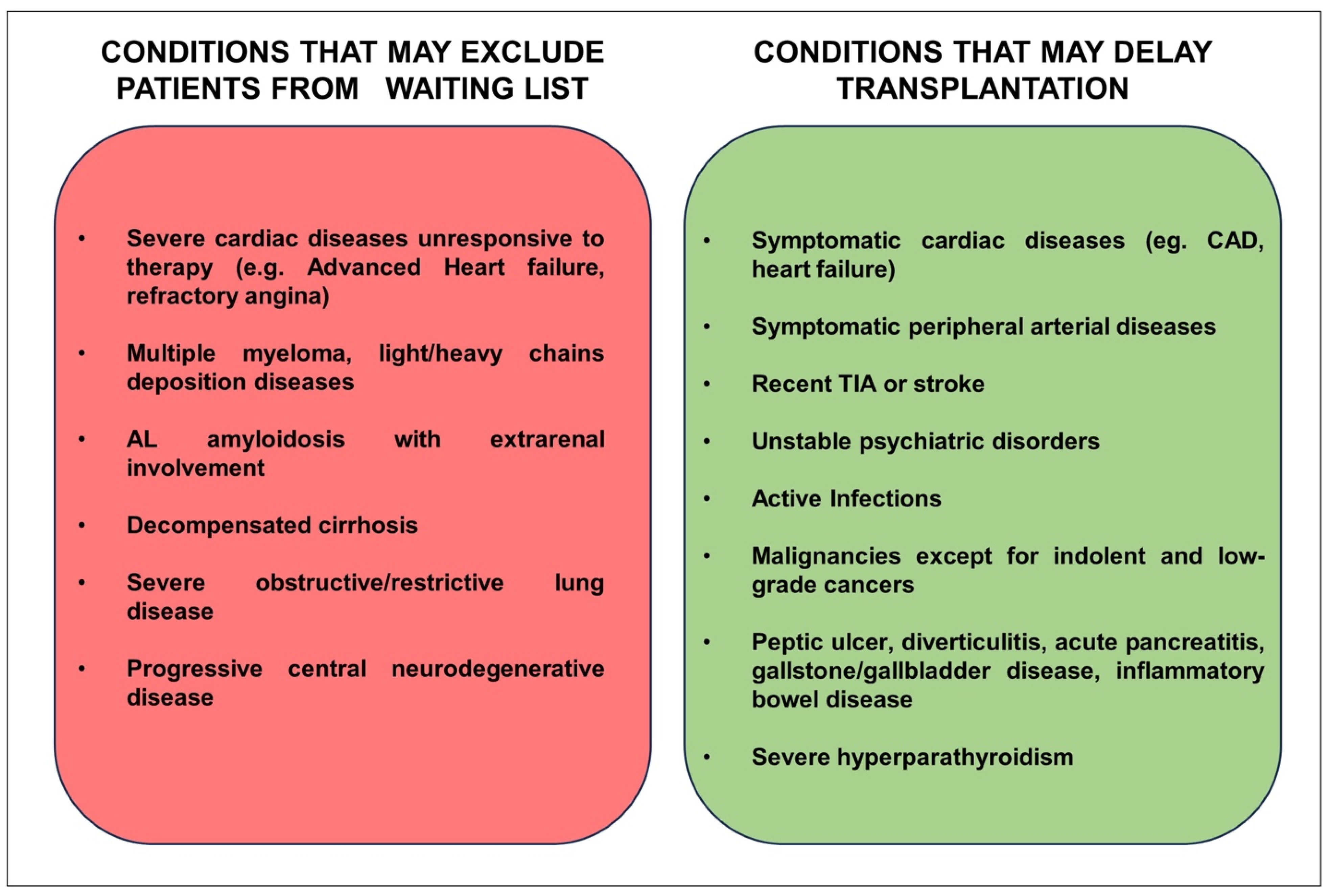
3. Cardiac Screening before Kidney Transplantation
| Society | High-Risk Criteria | Management |
|---|---|---|
| AHA/ACC (2012) [12] |
|
|
| ERBP (2013) [13] |
|
|
| AHA (2022) [69] |
| No prior CAD:
|
| ESC (2022) [70] |
|
|
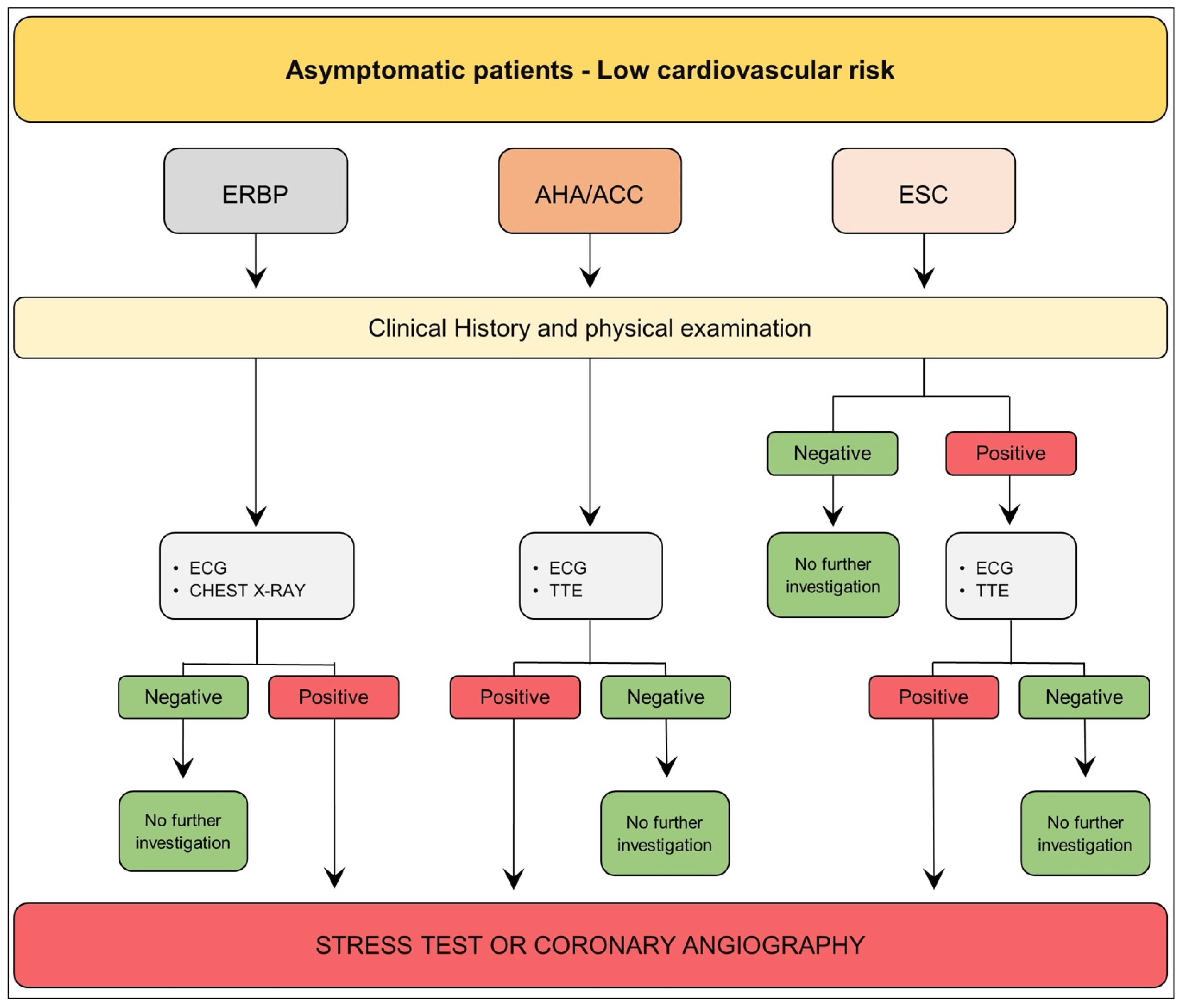
3.1. Asymptomatic Patient—Low Cardiovascular Risk
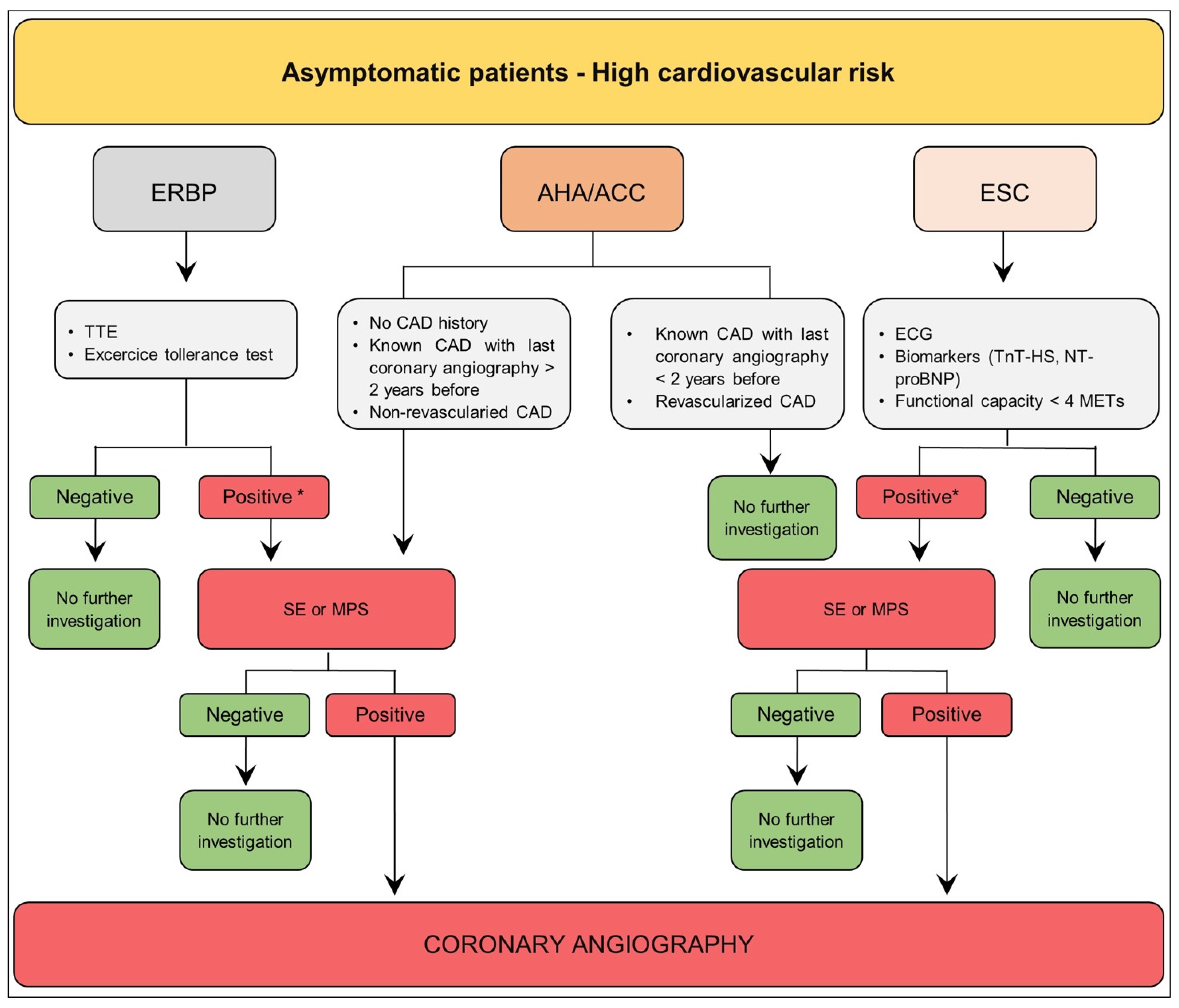
3.2. Asymptomatic Patient—High Cardiovascular Risk
4. Revascularization vs. Best Medical Therapy (BMT) before Transplantation: A Question Still Unanswered
4.1. Optimized Medical Therapy
4.1.1. Lipid-Lowering Drugs
4.1.2. Upcoming Role of Gliflozines
4.2. Revascularization
Dual Antiplatelet Therapy (DAPT) Duration after PCI
5. Future Perspectives
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, C.S.; Matsushita, K.; Woodward, M.; Bilo, H.J.G.; Chalmers, J.; Heerspink, H.J.L.; Lee, B.J.; Perkins, R.M.; Rossing, P.; Sairenchi, T.; et al. Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 2012, 380, 1662–1673. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Amann, K.; Bangalore, S.; Cavalcante, J.L.; Charytan, D.M.; Craig, J.C.; Gill, J.S.; Hlatky, M.A.; Jardine, A.G.; Landmesser, U.; et al. Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 1823–1838. [Google Scholar] [CrossRef] [PubMed]
- Batabyal, P.; Chapman, J.R.; Wong, G.; Craig, J.C.; Tong, A. Clinical practice guidelines on wait-listing for kidney transplantation: Consistent and equitable? Transplantation 2012, 94, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Brück, K.; Stel, V.S.; Gambaro, G.; Hallan, S.; Völzke, H.; Ärnlöv, J.; Kastarinen, M.; Guessous, I.; Vinhas, J.; Stengel, B.; et al. CKD Prevalence Varies across the European General Population. J. Am. Soc. Nephrol. 2016, 27, 2135–2147. [Google Scholar] [CrossRef] [PubMed]
- Tuttle, K.R.; Bakris, G.L.; Bilous, R.W.; Chiang, J.L.; de Boer, I.H.; Goldstein-Fuchs, J.; Hirsch, I.B.; Kalantar-Zadeh, K.; Narva, A.S.; Navaneethan, S.D.; et al. Diabetic kidney disease: A report from an ADA Consensus Conference. Diabetes Care 2014, 37, 2864–2883. [Google Scholar] [CrossRef] [PubMed]
- Ogurtsova, K.; da Rocha Fernandes, J.D.; Huang, Y.; Linnenkamp, U.; Guariguata, L.; Cho, N.H.; Cavan, D.; Shaw, J.E.; Makaroff, L.E. IDF Diabetes Atlas: Global estimates for the prevalence of diabetes for 2015 and 2040. Diabetes Res. Clin. Pract. 2017, 128, 40–50. [Google Scholar] [CrossRef]
- Rankin, A.J.; Mark, P.B. Cardiac screening prior to renal transplantation-good intentions, rather than good evidence, dictate practice. Kidney Int. 2021, 99, 306–308. [Google Scholar] [CrossRef]
- Bangalore, S.; Maron, D.J.; O’Brien, S.M.; Fleg, J.L.; Kretov, E.I.; Briguori, C.; Kaul, U.; Reynolds, H.R.; Mazurek, T.; Sidhu, M.S.; et al. Management of Coronary Disease in Patients with Advanced Kidney Disease. N. Engl. J. Med. 2020, 382, 1608–1618. [Google Scholar] [CrossRef]
- Chadban, S.J.; Ahn, C.; Axelrod, D.A.; Foster, B.J.; Kasiske, B.L.; Kher, V.; Kumar, D.; Oberbauer, R.; Pascual, J.; Pilmore, H.L.; et al. KDIGO Clinical Practice Guideline on the Evaluation and Management of Candidates for Kidney Transplantation. Transplantation 2020, 104, S11–S103. [Google Scholar] [CrossRef]
- Kasiske, B.L.; Cangro, C.B.; Hariharan, S.; Hricik, D.E.; Kerman, R.H.; Roth, D.; Rush, D.N.; Vazquez, M.A.; Weir, M.R.; American Society of Transplantation. The evaluation of renal transplantation candidates: Clinical practice guidelines. Am. J. Transplant. 2001, 1 (Suppl. S2), 3–95. [Google Scholar]
- American College of Cardiology/American Heart Association; Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery); American Society of Echocardiography; American Society of Nuclear Cardiology; Heart Rhythm Society; Society of Cardiovascular Anesthesiologists; Society for Cardiovascular Angiography and Interventions; Society for Vascular Medicine and Biology; Society for Vascular Surgery; Fleisher, L.A.; et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery: Executive summary: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Anesth. Analg. 2008, 106, 685–712. [Google Scholar] [CrossRef]
- Lentine, K.L.; Costa, S.P.; Weir, M.R.; Robb, J.F.; Fleisher, L.A.; Kasiske, B.L.; Carithers, R.L.; Ragosta, M.; Bolton, K.; Auerbach, A.D.; et al. Cardiac Disease Evaluation and Management Among Kidney and Liver Transplantation Candidates: A Scientific Statement From the American Heart Association and the American College of Cardiology Foundation. Circulation 2012, 126, 617–663. [Google Scholar] [CrossRef] [PubMed]
- European Renal Best Practice Transplantation Guideline Development Group. ERBP Guideline on the Management and Evaluation of the Kidney Donor and Recipient. Nephrol. Dial. Transplant. 2013, 28 (Suppl. S2), ii1–ii71. [Google Scholar] [CrossRef]
- Nakano, T.; Ninomiya, T.; Sumiyoshi, S.; Fujii, H.; Doi, Y.; Hirakata, H.; Tsuruya, K.; Iida, M.; Kiyohara, Y.; Sueishi, K. Association of kidney function with coronary atherosclerosis and calcification in autopsy samples from Japanese elders: The Hisayama study. Am. J. Kidney Dis. 2010, 55, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Mangano, M.; Stucchi, A.; Ciceri, P.; Conte, F.; Galassi, A. Cardiovascular disease in dialysis patients. Nephrol. Dial. Transplant. 2018, 33, iii28–iii34. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.W.; Fahim, M.A.; Hayen, A.; Mitchell, R.L.; Baines, L.; Lord, S.; Craig, J.C.; Webster, A.C. Cardiac testing for coronary artery disease in potential kidney transplant recipients. Cochrane Database Syst. Rev. 2011, 2011, CD008691. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Galassi, A.; Pivari, F.; Ciceri, P.; Conte, F. The Cardiovascular Burden in End-Stage Renal Disease. Contrib. Nephrol. 2017, 191, 44–57. [Google Scholar] [CrossRef] [PubMed]
- Sosnov, J.; Lessard, D.; Goldberg, R.J.; Yarzebski, J.; Gore, J.M. Differential symptoms of acute myocardial infarction in patients with kidney disease: A community-wide perspective. Am. J. Kidney Dis. 2006, 47, 378–384. [Google Scholar] [CrossRef]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef]
- Go, A.S.; Chertow, G.M.; Fan, D.; McCulloch, C.E.; Hsu, C. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N. Engl. J. Med. 2004, 351, 1296–1305. [Google Scholar] [CrossRef]
- Ronco, C.; Bellasi, A.; Di Lullo, L. Cardiorenal Syndrome: An Overview. Adv. Chronic Kidney Dis. 2018, 25, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, B.; Miskulin, D.; Zager, P. Epidemiology of hypertension in CKD. Adv. Chronic Kidney Dis. 2015, 22, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Pugh, D.; Gallacher, P.J.; Dhaun, N. Management of Hypertension in Chronic Kidney Disease. Drugs 2019, 79, 365–379. [Google Scholar] [CrossRef]
- Garla, V.; Yanes-Cardozo, L.; Lien, L.F. Current therapeutic approaches in the management of hyperglycemia in chronic renal disease. Rev. Endocr. Metab. Disord. 2017, 18, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Atkins, R.C.; Zimmet, P. Diabetic kidney disease: Act now or pay later. Kidney Int. 2010, 77, 375–377. [Google Scholar] [CrossRef]
- Meyrier, A. Nephrosclerosis: A term in quest of a disease. Nephron 2015, 129, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D’Agostino, R.B.; Levy, D.; Belanger, A.M.; Silbershatz, H.; Kannel, W.B. Prediction of coronary heart disease using risk factor categories. Circulation 1998, 97, 1837–1847. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Shemanski, L.R.; Burke, G.L.; Hansen, K.J.; Appel, R.G. Tobacco, hypertension, and vascular disease: Risk factors for renal functional decline in an older population. Kidney Int. 2000, 57, 2072–2079. [Google Scholar] [CrossRef]
- Nagasawa, Y.; Yamamoto, R.; Rakugi, H.; Isaka, Y. Cigarette smoking and chronic kidney diseases. Hypertens. Res. 2012, 35, 261–265. [Google Scholar] [CrossRef]
- Yamagata, K.; Ishida, K.; Sairenchi, T.; Takahashi, H.; Ohba, S.; Shiigai, T.; Narita, M.; Koyama, A. Risk factors for chronic kidney disease in a community-based population: A 10-year follow-up study. Kidney Int. 2007, 71, 159–166. [Google Scholar] [CrossRef]
- Leonberg-Yoo, A.K.; Rudnick, M.R. Tobacco Use: A Chronic Kidney Disease Accelerant. Am. J. Nephrol. 2017, 46, 257–259. [Google Scholar] [CrossRef]
- Roehm, B.; Simoni, J.; Pruszynski, J.; Wesson, D.E. Cigarette Smoking Attenuates Kidney Protection by Angiotensin-Converting Enzyme Inhibition in Nondiabetic Chronic Kidney Disease. Am. J. Nephrol. 2017, 46, 260–267. [Google Scholar] [CrossRef]
- Zalba, G.; Fortuño, A.; Díez, J. Oxidative stress and atherosclerosis in early chronic kidney disease. Nephrol. Dial. Transplant. 2006, 21, 2686–2690. [Google Scholar] [CrossRef] [PubMed]
- Brand, S.; Amann, K.; Schupp, N. Angiotensin II-induced hypertension dose-dependently leads to oxidative stress and DNA damage in mouse kidneys and hearts. J. Hypertens. 2013, 31, 333–344. [Google Scholar] [CrossRef]
- Roehm, B.; Weiner, D.E. Blood pressure targets and kidney and cardiovascular disease: Same data but discordant guidelines. Curr. Opin. Nephrol. Hypertens. 2019, 28, 245–250. [Google Scholar] [CrossRef]
- ADVANCE Collaborative Group; Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Woodward, M.; Marre, M.; Cooper, M.; Glasziou, P.; et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [PubMed]
- Eckardt, K.U. Anaemia in end-stage renal disease: Pathophysiological considerations. Nephrol. Dial. Transplant. 2001, 16 (Suppl. S7), 2–8. [Google Scholar] [CrossRef] [PubMed]
- Portolés, J.; Martín, L.; Broseta, J.J.; Cases, A. Anemia in Chronic Kidney Disease: From Pathophysiology and Current Treatments, to Future Agents. Front. Med. 2021, 8, 642296. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.L. Fluid Volume Overload and Congestion in Heart Failure: Time to Reconsider Pathophysiology and How Volume Is Assessed. Circ. Heart Fail. 2016, 9, e002922. [Google Scholar] [CrossRef] [PubMed]
- Alhaj, E.; Alhaj, N.; Rahman, I.; Niazi, T.O.; Berkowitz, R.; Klapholz, M. Uremic cardiomyopathy: An underdiagnosed disease. Congest. Heart Fail. 2013, 19, E40–E45. [Google Scholar] [CrossRef]
- Foley, R.N.; Parfrey, P.S.; Harnett, J.D.; Kent, G.M.; Murray, D.C.; Barre, P.E. The impact of anemia on cardiomyopathy, morbidity, and and mortality in end-stage renal disease. Am. J. Kidney Dis. 1996, 28, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.A.; Asinger, R.W.; Berger, A.K.; Charytan, D.M.; Díez, J.; Hart, R.G.; Eckardt, K.-U.; Kasiske, B.L.; McCullough, P.A.; Passman, R.S.; et al. Cardiovascular disease in chronic kidney disease. A clinical update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 572–586. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, R.; Sullivan, L.M.; Fox, C.S.; Wang, T.J.; D’Agostino, R.B.; Gaziano, J.M.; Vasan, R.S. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch. Intern. Med. 2007, 167, 879–885. [Google Scholar] [CrossRef] [PubMed]
- Daenen, K.; Andries, A.; Mekahli, D.; Van Schepdael, A.; Jouret, F.; Bammens, B. Oxidative stress in chronic kidney disease. Pediatr. Nephrol. 2019, 34, 975–991. [Google Scholar] [CrossRef] [PubMed]
- Tbahriti, H.F.; Kaddous, A.; Bouchenak, M.; Mekki, K. Effect of different stages of chronic kidney disease and renal replacement therapies on oxidant-antioxidant balance in uremic patients. Biochem. Res. Int. 2013, 2013, 358985. [Google Scholar] [CrossRef] [PubMed]
- Popolo, A.; Autore, G.; Pinto, A.; Marzocco, S. Oxidative stress in patients with cardiovascular disease and chronic renal failure. Free Radic. Res. 2013, 47, 346–356. [Google Scholar] [CrossRef]
- Dounousi, E.; Duni, A.; Naka, K.K.; Vartholomatos, G.; Zoccali, C. The Innate Immune System and Cardiovascular Disease in ESKD: Monocytes and Natural Killer Cells. Curr. Vasc. Pharmacol. 2021, 19, 63–76. [Google Scholar] [CrossRef] [PubMed]
- Chmielewski, M.; Bryl, E.; Marzec, L.; Aleksandrowicz, E.; Witkowski, J.M.; Rutkowski, B. Expression of scavenger receptor CD36 in chronic renal failure patients. Artif. Organs 2005, 29, 608–614. [Google Scholar] [CrossRef]
- Kato, S.; Chmielewski, M.; Honda, H.; Pecoits-Filho, R.; Matsuo, S.; Yuzawa, Y.; Tranaeus, A.; Stenvinkel, P.; Lindholm, B. Aspects of immune dysfunction in end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2008, 3, 1526–1533. [Google Scholar] [CrossRef]
- Moghimpour Bijani, F.; Vallejo, J.G.; Rezaei, N. Toll-like receptor signaling pathways in cardiovascular diseases: Challenges and opportunities. Int. Rev. Immunol. 2012, 31, 379–395. [Google Scholar] [CrossRef]
- Kumar, V.; Rosenzweig, R.; Asalla, S.; Nehra, S.; Prabhu, S.D.; Bansal, S.S. TNFR1 Contributes to Activation-Induced Cell Death of Pathological CD4+ T Lymphocytes During Ischemic Heart Failure. JACC Basic Transl. Sci. 2022, 7, 1038–1049. [Google Scholar] [CrossRef] [PubMed]
- Rurik, J.G.; Aghajanian, H.; Epstein, J.A. Immune Cells and Immunotherapy for Cardiac Injury and Repair. Circ. Res. 2021, 128, 1766–1779. [Google Scholar] [CrossRef] [PubMed]
- Opherk, D.; Mall, G.; Zebe, H.; Schwarz, F.; Weihe, E.; Manthey, J.; Kübler, W. Reduction of coronary reserve: A mechanism for angina pectoris in patients with arterial hypertension and normal coronary arteries. Circulation 1984, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chade, A.R.; Brosh, D.; Higano, S.T.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Mild renal insufficiency is associated with reduced coronary flow in patients with non-obstructive coronary artery disease. Kidney Int. 2006, 69, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Fujii, H.; Takiuchi, S.; Kawano, Y.; Fukagawa, M. Putative role of asymmetric dimethylarginine in microvascular disease of kidney and heart in hypertensive patients. Am. J. Hypertens. 2008, 21, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Wilcox, C.S. Asymmetric dimethylarginine and reactive oxygen species: Unwelcome twin visitors to the cardiovascular and kidney disease tables. Hypertension 2012, 59, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Kaw, D.; Malhotra, D. Platelet dysfunction and end-stage renal disease. Semin. Dial. 2006, 19, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Duranton, F.; Cohen, G.; De Smet, R.; Rodriguez, M.; Jankowski, J.; Vanholder, R.; Argiles, A.; European Uremic Toxin Work Group. Normal and pathologic concentrations of uremic toxins. J. Am. Soc. Nephrol. 2012, 23, 1258–1270. [Google Scholar] [CrossRef]
- Tumur, Z.; Shimizu, H.; Enomoto, A.; Miyazaki, H.; Niwa, T. Indoxyl sulfate upregulates expression of ICAM-1 and MCP-1 by oxidative stress-induced NF-kappaB activation. Am. J. Nephrol. 2010, 31, 435–441. [Google Scholar] [CrossRef]
- Meijers, B.K.I.; Van Kerckhoven, S.; Verbeke, K.; Dehaen, W.; Vanrenterghem, Y.; Hoylaerts, M.F.; Evenepoel, P. The uremic retention solute p-cresyl sulfate and markers of endothelial damage. Am. J. Kidney Dis. 2009, 54, 891–901. [Google Scholar] [CrossRef]
- Boini, K.M.; Hussain, T.; Li, P.-L.; Koka, S. Trimethylamine-N-Oxide Instigates NLRP3 Inflammasome Activation and Endothelial Dysfunction. Cell Physiol. Biochem. 2017, 44, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Meyer, T.W.; Hostetter, T.H. Uremia. N. Engl. J. Med. 2007, 357, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Saran, R.; Robinson, B.; Abbott, K.C.; Agodoa, L.Y.C.; Albertus, P.; Ayanian, J.; Balkrishnan, R.; Bragg-Gresham, J.; Cao, J.; Chen, J.L.T.; et al. US Renal Data System 2016 Annual Data Report: Epidemiology of Kidney Disease in the United States. Am. J. Kidney Dis. 2017, 69, A7–A8. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R. How can we prevent intradialytic hypotension? Curr. Opin. Nephrol. Hypertens. 2012, 21, 593–599. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Regidor, D.L.; Kovesdy, C.P.; Van Wyck, D.; Bunnapradist, S.; Horwich, T.B.; Fonarow, G.C. Fluid retention is associated with cardiovascular mortality in patients undergoing long-term hemodialysis. Circulation 2009, 119, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Ekdahl, K.N.; Soveri, I.; Hilborn, J.; Fellström, B.; Nilsson, B. Cardiovascular disease in haemodialysis: Role of the intravascular innate immune system. Nat. Rev. Nephrol. 2017, 13, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.; Heiss, C.; Drexhage, C.; Kehmeier, E.S.; Balzer, J.; Mühlfeld, A.; Merx, M.W.; Lauer, T.; Kühl, H.; Floege, J.; et al. Hemodialysis-induced release of hemoglobin limits nitric oxide bioavailability and impairs vascular function. J. Am. Coll. Cardiol. 2010, 55, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Sharif, A. The Argument for Abolishing Cardiac Screening of Asymptomatic Kidney Transplant Candidates. Am. J. Kidney Dis. 2020, 75, 946–954. [Google Scholar] [CrossRef]
- Cheng, X.S.; VanWagner, L.B.; Costa, S.P.; Axelrod, D.A.; Bangalore, S.; Norman, S.P.A.; Herzog, C.; Lentine, K.L.; the American Heart Association Council on the Kidney in Cardiovascular Disease and Council on Cardiovascular Radiology and Intervention Emerging. Evidence on Coronary Heart Disease Screening in Kidney and Liver Transplantation Candidates: A Scientific Statement From the American Heart Association. Circulation 2022, 146, e299–e324. [Google Scholar] [CrossRef]
- Halvorsen, S.; Mehilli, J.; Cassese, S.; Hall, T.S.; Abdelhamid, M.; Barbato, E.; De Hert, S.; de Laval, I.; Geisler, T.; Hinterbuchner, L.; et al. 2022 ESC Guidelines on cardiovascular assessment and management of patients undergoing non-cardiac surgery. Eur. Heart J. 2022, 43, 3826–3924. [Google Scholar] [CrossRef]
- Nimmo, A.; Forsyth, J.L.; Oniscu, G.C.; Robb, M.; Watson, C.; Fotheringham, J.; Roderick, P.J.; Ravanan, R.; Taylor, D.M. A propensity score–matched analysis indicates screening for asymptomatic coronary artery disease does not predict cardiac events in kidney transplant recipients. Kidney Int. 2021, 99, 431–442. [Google Scholar] [CrossRef] [PubMed]
- Narala, K.R.; Hassan, S.; LaLonde, T.A.; McCullough, P.A. Management of coronary atherosclerosis and acute coronary syndromes in patients with chronic kidney disease. Curr. Probl. Cardiol. 2013, 38, 165–206. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Qiao, Y.; Shin, J.-I.; Sang, Y.; Inker, L.A.; Secora, A.; Luo, S.; Coresh, J.; Alexander, G.C.; Jackson, J.W.; Chang, A.R.; et al. Discontinuation of Angiotensin Converting Enzyme Inhibitors and Angiotensin Receptor Blockers in Chronic Kidney Disease. Mayo Clin. Proc. 2019, 94, 2220–2229. [Google Scholar] [CrossRef] [PubMed]
- Badve, S.V.; Roberts, M.A.; Hawley, C.M.; Cass, A.; Garg, A.X.; Krum, H.; Tonkin, A.; Perkovic, V. Effects of beta-adrenergic antagonists in patients with chronic kidney disease: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2011, 58, 1152–1161. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Liu, Y.; Perkovic, V.; Li, X.; Ninomiya, T.; Hou, W.; Zhao, N.; Liu, L.; Lv, J.; Zhang, H.; et al. Renin-Angiotensin System Inhibitors and Kidney and Cardiovascular Outcomes in Patients With CKD: A Bayesian Network Meta-analysis of Randomized Clinical Trials. Am. J. Kidney Dis. 2016, 67, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Edner, M.; Benson, L.; Dahlström, U.; Lund, L.H. Association between renin-angiotensin system antagonist use and mortality in heart failure with severe renal insufficiency: A prospective propensity score-matched cohort study. Eur. Heart J. 2015, 36, 2318–2326. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Landray, M.J.; Reith, C.; Emberson, J.; Wheeler, D.C.; Tomson, C.; Wanner, C.; Krane, V.; Cass, A.; Craig, J.; et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): A randomised placebo-controlled trial. Lancet 2011, 377, 2181–2192. [Google Scholar] [CrossRef]
- Soohoo, M.; Moradi, H.; Obi, Y.; Rhee, C.M.; Gosmanova, E.O.; Molnar, M.Z.; Kashyap, M.L.; Gillen, D.L.; Kovesdy, C.P.; Kalantar-Zadeh, K.; et al. Statin Therapy Before Transition to End-Stage Renal Disease With Posttransition Outcomes. J. Am. Heart Assoc. 2019, 8, e011869. [Google Scholar] [CrossRef]
- McGuire, D.K.; Shih, W.J.; Cosentino, F.; Charbonnel, B.; Cherney, D.Z.I.; Dagogo-Jack, S.; Pratley, R.; Greenberg, M.; Wang, S.; Huyck, S.; et al. Association of SGLT2 Inhibitors With Cardiovascular and Kidney Outcomes in Patients With Type 2 Diabetes: A Meta-analysis. JAMA Cardiol. 2021, 6, 148–158. [Google Scholar] [CrossRef]
- Mahaffey, K.W.; Neal, B.; Perkovic, V.; de Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Fabbrini, E.; Sun, T.; Li, Q.; et al. Canagliflozin for Primary and Secondary Prevention of Cardiovascular Events: Results From the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation 2018, 137, 323–334. [Google Scholar] [CrossRef]
- Wiviott, S.D.; Raz, I.; Bonaca, M.P.; Mosenzon, O.; Kato, E.T.; Cahn, A.; Silverman, M.G.; Zelniker, T.A.; Kuder, J.F.; Murphy, S.A.; et al. Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2019, 380, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Hindi, J.; Farouk, S.S. Sodium-Glucose Cotransporter 2 Inhibitors and Kidney Transplantation: What Are We Waiting For? Kidney360 2021, 2, 1174–1178. [Google Scholar] [CrossRef] [PubMed]
- Perkovic, V.; Jardine, M.J.; Neal, B.; Bompoint, S.; Heerspink, H.J.L.; Charytan, D.M.; Edwards, R.; Agarwal, R.; Bakris, G.; Bull, S.; et al. Canagliflozin and Renal Outcomes in Type 2 Diabetes and Nephropathy. N. Engl. J. Med. 2019, 380, 2295–2306. [Google Scholar] [CrossRef] [PubMed]
- Heerspink, H.J.L.; Stefánsson, B.V.; Correa-Rotter, R.; Chertow, G.M.; Greene, T.; Hou, F.-F.; Mann, J.F.E.; McMurray, J.J.V.; Lindberg, M.; Rossing, P.; et al. Dapagliflozin in Patients with Chronic Kidney Disease. N. Engl. J. Med. 2020, 383, 1436–1446. [Google Scholar] [CrossRef]
- Mende, C.W. Chronic Kidney Disease and SGLT2 Inhibitors: A Review of the Evolving Treatment Landscape. Adv. Ther. 2022, 39, 148–164. [Google Scholar] [CrossRef] [PubMed]
- Ujjawal, A.; Schreiber, B.; Verma, A. Sodium-glucose cotransporter-2 inhibitors (SGLT2i) in kidney transplant recipients: What is the evidence? Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221090000. [Google Scholar] [CrossRef]
- Bittl, J.A.; He, Y.; Jacobs, A.K.; Yancy, C.W.; Normand, S.-L.T.; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Bayesian methods affirm the use of percutaneous coronary intervention to improve survival in patients with unprotected left main coronary artery disease. Circulation 2013, 127, 2177–2185. [Google Scholar] [CrossRef]
- Dzavik, V.; Ghali, W.A.; Norris, C.; Mitchell, L.B.; Koshal, A.; Saunders, L.D.; Galbraith, P.D.; Hui, W.; Faris, P.; Knudtson, M.L.; et al. Long-term survival in 11,661 patients with multivessel coronary artery disease in the era of stenting: A report from the Alberta Provincial Project for Outcome Assessment in Coronary Heart Disease (APPROACH) Investigators. Am. Heart J. 2001, 142, 119–126. [Google Scholar] [CrossRef]
- Lee, P.H.; Ahn, J.-M.; Chang, M.; Baek, S.; Yoon, S.-H.; Kang, S.-J.; Lee, S.-W.; Kim, Y.-H.; Lee, C.W.; Park, S.-W.; et al. Left Main Coronary Artery Disease: Secular Trends in Patient Characteristics, Treatments, and Outcomes. J. Am. Coll. Cardiol. 2016, 68, 1233–1246. [Google Scholar] [CrossRef]
- Hannan, E.L.; Samadashvili, Z.; Cozzens, K.; Walford, G.; Jacobs, A.K.; Holmes, D.R.; Stamato, N.J.; Gold, J.P.; Sharma, S.; Venditti, F.J.; et al. Comparative outcomes for patients who do and do not undergo percutaneous coronary intervention for stable coronary artery disease in New York. Circulation 2012, 125, 1870–1879. [Google Scholar] [CrossRef] [PubMed]
- Neumann, F.-J.; Sousa-Uva, M.; Ahlsson, A.; Alfonso, F.; Banning, A.P.; Benedetto, U.; Byrne, R.A.; Collet, J.-P.; Falk, V.; Head, S.J.; et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2019, 40, 87–165. [Google Scholar] [CrossRef]
- Kumar, N.; Baker, C.S.R.; Chan, K.; Duncan, N.; Malik, I.; Frankel, A.; Ashby, D.R.; McLean, A.; Palmer, A.; Cairns, T.D.; et al. Cardiac survival after pre-emptive coronary angiography in transplant patients and those awaiting transplantation. Clin. J. Am. Soc. Nephrol. 2011, 6, 1912–1919. [Google Scholar] [CrossRef] [PubMed]
- Bangalore, S.; Guo, Y.; Samadashvili, Z.; Blecker, S.; Xu, J.; Hannan, E.L. Revascularization in Patients With Multivessel Coronary Artery Disease and Chronic Kidney Disease: Everolimus-Eluting Stents Versus Coronary Artery Bypass Graft Surgery. J. Am. Coll. Cardiol. 2015, 66, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Herzog, C.A.; Simegn, M.A.; Xu, Y.; Costa, S.P.; Mathew, R.O.; El-Hajjar, M.C.; Gulati, S.; Maldonado, R.A.; Daugas, E.; Madero, M.; et al. Kidney Transplant List Status and Outcomes in the ISCHEMIA-CKD Trial. J. Am. Coll. Cardiol. 2021, 78, 348–361. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.U.; Junarta, J.; Marhefka, G.D. Coronary Revascularization Versus Optimal Medical Therapy in Renal Transplant Candidates With Coronary Artery Disease: A Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e023548. [Google Scholar] [CrossRef]
- Doğan, A.; Özdemir, E.; Kahraman, S.; Açıl, T.; Saltan, Y.; Kurtoğlu, N. Impact of early (3 months) dual antiplatelet treatment interruption prior to renal transplantation in patients with second-generation DES on perioperative stent thrombosis and MACEs. Anatol. J. Cardiol. 2017, 18, 391–396. [Google Scholar] [CrossRef]
- Mehran, R.; Cao, D.; Angiolillo, D.J.; Bangalore, S.; Bhatt, D.L.; Ge, J.; Hermiller, J.; Makkar, R.R.; Neumann, F.-J.; Saito, S.; et al. 3- or 1-Month DAPT in Patients at High Bleeding Risk Undergoing Everolimus-Eluting Stent Implantation. JACC Cardiovasc. Interv. 2021, 14, 1870–1883. [Google Scholar] [CrossRef]
- Smits, P.C.; Frigoli, E.; Tijssen, J.; Jüni, P.; Vranckx, P.; Ozaki, Y.; Morice, M.-C.; Chevalier, B.; Onuma, Y.; Windecker, S.; et al. Abbreviated Antiplatelet Therapy in Patients at High Bleeding Risk With or Without Oral Anticoagulant Therapy After Coronary Stenting: An Open-Label, Randomized, Controlled Trial. Circulation 2021, 144, 1196–1211. [Google Scholar] [CrossRef]
- Pivato, C.A.; Reimers, B.; Testa, L.; Pacchioni, A.; Briguori, C.; Musto, C.; Esposito, G.; Piccolo, R.; Lucisano, L.; De Luca, L.; et al. One-Month Dual Antiplatelet Therapy After Bioresorbable Polymer Everolimus-Eluting Stents in High Bleeding Risk Patients. J. Am. Heart Assoc. 2022, 11, e023454. [Google Scholar] [CrossRef]
- Ying, T.; Gill, J.; Webster, A.; Kim, S.J.; Morton, R.; Klarenbach, S.W.; Kelly, P.; Ramsay, T.; Knoll, G.A.; Pilmore, H.; et al. Canadian-Australasian Randomised trial of screening kidney transplant candidates for coronary artery disease-A trial protocol for the CARSK study. Am. Heart J. 2019, 214, 175–183. [Google Scholar] [CrossRef]
- Kotta, P.A.; Elango, M.; Papalois, V. Preoperative Cardiovascular Assessment of the Renal Transplant Recipient: A Narrative Review. J. Clin. Med. 2021, 10, 2525. [Google Scholar] [CrossRef]
| Traditional Risk Factors | CKD-Related Risk Factors | Haemodialysis-Related Risk Factors |
|---|---|---|
| Age | Inflammation and oxidative stress (ROS) | A-V fistula |
| Sex | Anemia | Biomaterials contact |
| Family history | Uremic toxins | Hemodynamic stress |
| Hypertension | Hypervolemia | Hemoglobin decompartmentalization |
| Diabetes mellitus | Secondary hyperparathyroidism | |
| Dyslipidemia | Prothrombotic state and platelet dysfunction | |
| Tobacco use | ||
| Physical inactivity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vadalà, G.; Alaimo, C.; Buccheri, G.; Di Fazio, L.; Di Caccamo, L.; Sucato, V.; Cipriani, M.; Galassi, A.R. Screening and Management of Coronary Artery Disease in Kidney Transplant Candidates. Diagnostics 2023, 13, 2709. https://doi.org/10.3390/diagnostics13162709
Vadalà G, Alaimo C, Buccheri G, Di Fazio L, Di Caccamo L, Sucato V, Cipriani M, Galassi AR. Screening and Management of Coronary Artery Disease in Kidney Transplant Candidates. Diagnostics. 2023; 13(16):2709. https://doi.org/10.3390/diagnostics13162709
Chicago/Turabian StyleVadalà, Giuseppe, Chiara Alaimo, Giancarlo Buccheri, Luca Di Fazio, Leandro Di Caccamo, Vincenzo Sucato, Manlio Cipriani, and Alfredo Ruggero Galassi. 2023. "Screening and Management of Coronary Artery Disease in Kidney Transplant Candidates" Diagnostics 13, no. 16: 2709. https://doi.org/10.3390/diagnostics13162709





