Unveiling the Hidden Power of Uromodulin: A Promising Potential Biomarker for Kidney Diseases
Abstract
:1. Introduction
2. Methodology
3. Uromodulin: General Characteristics
3.1. Synthesis of Uromodulin
3.2. Composition of Uromodulin
3.3. Production and Secretion of Uromodulin
3.4. Functions
3.5. Analytical Aspects
3.6. General Population
4. Uromodulin and Acute Kidney Disease
| Study Design | Study Population | Major Findings | Ref. |
|---|---|---|---|
| Animal studies | |||
| THP knockout mice vs. wild-type mice | THP−/− mice had worse damage and impaired renal macrophage M2 transformation compared to wild-type mice. | [46] | |
| THP knockout mice vs. wild-type mice | THP−/− mice showed more kidney inflammation and tubular necrosis. THP may stabilize the outer medulla by reducing inflammation, possibly affecting TLR4. | [81] | |
| THP knockout mice vs. wild-type mice | In AKI, S3 proximal segments suffer the most damage. TAL can affect S3 segment vulnerability by regulating THP-dependent MIP-2 expression. | [90] | |
| THP knockout mice vs. wild-type mice | The absence of THP significantly raised levels of circulating IFN-γ, IL1α, TNF-α, IL6, CXCL1, and IL13. | [123] | |
| Human studies | |||
| Cohort study | 218 adults undergoing on-pump cardiac surgery | A preoperative decreased urinary uromodulin-to-creatinine ratio is linked to increased AKI odds and elevated peak serum creatinine after cardiac surgery. | [104] |
| Experimental study | 10 normal human urine samples | THP seems to play a direct role in deactivating complement by serving as a cofactor for the breakdown of C3b. | [124] |
| Cohort study | 20 ICU patients with AKI after surgery | Elevation of fucose levels on THP plays a significant role through the complement lectin pathway in AKI. | [125] |
| Cohort study | 113 VLBW infants (weight ≤ 1200 g or < 31 weeks’ gestation) | Infants with AKI had lower minimum levels of urinary uromodulin on the first 4 postnatal days than those without AKI. | [126] |
| Cohort study | 2351 CKD participants with 184 patients with an AKI event | Lower urinary uromodulin levels predicted future AKI, independently of eGFR and albuminuria. | [127] |
| Cohort study | 101 children undergoing CPB | Children with the lowest pre-surgery urinary uromodulin levels faced a significantly higher risk of AKI following CPB. | [128] |
| Cross-sectional study | 30 cardiac surgery patients divided into two groups of 15 each: group I without kidney dysfunction and CCr > 60 mL/min; and group II with CCr < 60 mL/min | Group II showed lower urinary excretion of uromodulin. | [133] |
| Cross-sectional study | 21 ICU patients:. group 1 (n = 14) with no signs of kidney dysfunction vs. group 2 (n = 7) with the beginning of AKI | Patients who developed AKI exhibited reduced urinary excretions of uromodulin. | [134] |
| Cross-sectional study | 14 liver transplant patients suffered kidney insufficiency, and 20 showed no AKI after liver transplantation | A higher pretransplant THP synthesis/urinary secretion may protect the kidneys during and after liver transplantation. | [135] |
| Case-control study | 9 infants with AKI and 24 infants without AKI | Low urinary uromodulin levels may serve as a predictive indicator of AKI. | [136] |
| Cohort study | 656 participants hospitalized with AKI | A rise in urinary uromodulin from baseline to 12 months was linked to a 40% lower risk of developing CKD. | [137] |
| Cohort study | 98 patients with cirrhosis with subsequent hospital-acquired AKI | Reduced urinary uromodulin levels upon admission were linked to higher chances of later developing AKI in cirrhotic patients during hospitalization. | [138] |
| Case series | 5 cases of patients with acute tubular injury and/or acute interstitial nephritis | Elevated serum uromodulin levels were found in patients with acute tubular injury and/or acute interstitial nephritis. | [139] |
| Cross-sectional study | 66 adult patients with severe acute pancreatitis | While serum uromodulin correlated with kidney function in the early phase of severe acute pancreatitis, it did not reliably predict acute pancreatitis severity or AKI development. | [140] |
5. Uromodulin and Chronic Kidney Disease
5.1. IgA Nephropathy
5.2. Diabetic Nephropathy
5.3. Lupus Nephritis
5.4. ANCA-Associated Glomerulonephritis
5.5. Kidney Stones
5.6. Obstructive Nephropathy
5.7. Vesicoureteral Reflux
5.8. Uromodulin-Associated Kidney Diseases
5.9. Autosomal Dominant Polycystic Kidney Disease
5.10. Kidney Transplantation
5.11. Fanconi Syndrome
5.12. Fabry Disease
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rovida, C.L. Conclusione Degli Studi Intorno All’origine Istologica Dei Cilindri Dell’urina. Riv. Clin. Bologna 1873, 2a, 303–306. [Google Scholar]
- Tamm, I.; Horsfall, F.L. Characterization and Separation of an Inhibitor of Viral Hemagglutination Present in Urine. Proc. Soc. Exp. Biol. Med. 1950, 74, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Tamm, I.; Horsfall, F.L. A Mucoprotein Derived from Human Urine Which Reacts with Influenza, Mumps, and Newcastle Disease Viruses. J. Exp. Med. 1952, 95, 71–97. [Google Scholar] [CrossRef] [PubMed]
- Scherberich, J.E.; Gruber, R.; Nockher, W.A.; Christensen, E.I.; Schmitt, H.; Herbst, V.; Block, M.; Kaden, J.; Schlumberger, W. Serum Uromodulin—A Marker of Kidney Function and Renal Parenchymal Integrity. Nephrol. Dial. Transplant. 2018, 33, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Sikri, K.L.; Foster, C.L.; MacHugh, N.; Marshall, R.D. Localization of Tamm-Horsfall Glycoprotein in the Human Kidney Using Immuno-Fluorescence and Immuno-Electron Microscopical Techniques. J. Anat. 1981, 132, 597–605. [Google Scholar] [PubMed]
- Sikri, K.L.; Foster, C.L.; Alexander, D.P.; Marshall, R.D. Localization of Tamm-Horsfall Glycoprotein in the Fetal and Neonatal Hamster Kidney as Demonstrated by Immunofluorescence and Immunoelectron Microscopical Techniques. Biol. Neonate 1981, 39, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, R.H.; Van Ess, J.D.; May, A.G.; Schenk, E.A.; Freeman, R.B. Tamm-Horsfall Glycoproteinuria and Renal Allograft Rejection. Transplantation 1973, 16, 83–87. [Google Scholar] [CrossRef]
- Ollier-Hartmann, M.P.; Pouget-Abadie, C.; Bouillie, J.; Hartmann, L. Variations of Urinary Tamm-Horsfall Protein in Humans during the First Thirty Years of Life. Nephron 1984, 38, 163–166. [Google Scholar] [CrossRef]
- Mckenzie, J.K.; Patel, R.; Mcqueen, E.G. The excretion rate of tamm-horsfall urinary mucoprotein in normals and in patients with renal disease. Australas. Ann. Med. 1964, 13, 32–39. [Google Scholar] [CrossRef]
- Mazzuchi, N.; Pecarovich, R.; Ross, N.; Rodríguez, I.; Sanguinetti, C.M. Tamm-Horsfall Urinary Glycoprotein Quantitation by Radial Immunodiffusion: Normal Patterns. J. Lab. Clin. Med. 1974, 84, 771–776. [Google Scholar]
- Duława, J.; Kokot, F.; Kokot, M.; Pander, H. Urinary Excretion of Tamm-Horsfall Protein in Normotensive and Hypertensive Elderly Patients. J. Hum. Hypertens. 1998, 12, 635–637. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Kaye, D. Reduced Uromucoid Excretion in the Elderly. J. Infect. Dis. 1985, 152, 653. [Google Scholar] [CrossRef] [PubMed]
- Muchmore, A.V.; Decker, J.M. Uromodulin: A Unique 85-Kilodalton Immunosuppressive Glycoprotein Isolated from Urine of Pregnant Women. Science 1985, 229, 479–481. [Google Scholar] [CrossRef] [PubMed]
- Pennica, D.; Kohr, W.J.; Kuang, W.J.; Glaister, D.; Aggarwal, B.B.; Chen, E.Y.; Goeddel, D.V. Identification of Human Uromodulin as the Tamm-Horsfall Urinary Glycoprotein. Science 1987, 236, 83–88. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; McCracken, R.; Liu, Y.; Heitmeier, M.R.; Bourgeois, S.; Ryerse, J.; Wu, X.-R. Tamm-Horsfall Protein Translocates to the Basolateral Domain of Thick Ascending Limbs, Interstitium, and Circulation during Recovery from Acute Kidney Injury. Am. J. Physiol.-Ren. Physiol. 2013, 304, F1066–F1075. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; Wu, X.-R. Uromodulin in Kidney Injury: An Instigator, Bystander, or Protector? Am. J. Kidney Dis. 2012, 59, 452–461. [Google Scholar] [CrossRef] [PubMed]
- LaFavers, K.A.; Gaddy, A.R.; Micanovic, R.; Lingeman, J.; Williams, J.C.; Coe, F.L.; El-Achkar, T.M.; Worcester, E. Water Loading and Uromodulin Secretion in Healthy Individuals and Idiopathic Calcium Stone Formers. Clin. J. Am. Soc. Nephrol. 2023, 18, 1059–1067. [Google Scholar] [CrossRef]
- Pook, M.A.; Jeremiah, S.; Scheinman, S.J.; Povey, S.; Thakker, R.V. Localization of the Tamm-Horsfall Glycoprotein (Uromodulin) Gene to Chromosome 16p12.3–16p13.11. Ann. Hum. Genet. 1993, 57, 285–290. [Google Scholar] [CrossRef]
- Devuyst, O.; Pattaro, C. The UMOD Locus: Insights into the Pathogenesis and Prognosis of Kidney Disease. J. Am. Soc. Nephrol. 2018, 29, 713–726. [Google Scholar] [CrossRef]
- Hart, T.C.; Gorry, M.C.; Hart, P.S.; Woodard, A.S.; Shihabi, Z.; Sandhu, J.; Shirts, B.; Xu, L.; Zhu, H.; Barmada, M.M.; et al. Mutations of the UMOD Gene Are Responsible for Medullary Cystic Kidney Disease 2 and Familial Juvenile Hyperuricaemic Nephropathy. J. Med. Genet. 2002, 39, 882–892. [Google Scholar] [CrossRef]
- Scolari, F.; Caridi, G.; Rampoldi, L.; Tardanico, R.; Izzi, C.; Pirulli, D.; Amoroso, A.; Casari, G.; Ghiggeri, G.M. Uromodulin Storage Diseases: Clinical Aspects and Mechanisms. Am. J. Kidney Dis. 2004, 44, 987–999. [Google Scholar] [CrossRef] [PubMed]
- Padmanabhan, S.; Melander, O.; Johnson, T.; Di Blasio, A.M.; Lee, W.K.; Gentilini, D.; Hastie, C.E.; Menni, C.; Monti, M.C.; Delles, C.; et al. Genome-Wide Association Study of Blood Pressure Extremes Identifies Variant near UMOD Associated with Hypertension. PLoS Genet. 2010, 6, e1001177. [Google Scholar] [CrossRef] [PubMed]
- Köttgen, A.; Pattaro, C.; Böger, C.A.; Fuchsberger, C.; Olden, M.; Glazer, N.L.; Parsa, A.; Gao, X.; Yang, Q.; Smith, A.V.; et al. New Loci Associated with Kidney Function and Chronic Kidney Disease. Nat. Genet. 2010, 42, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Köttgen, A.; Glazer, N.L.; Dehghan, A.; Hwang, S.-J.; Katz, R.; Li, M.; Yang, Q.; Gudnason, V.; Launer, L.J.; Harris, T.B.; et al. Multiple Loci Associated with Indices of Renal Function and Chronic Kidney Disease. Nat. Genet. 2009, 41, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Gudbjartsson, D.F.; Holm, H.; Indridason, O.S.; Thorleifsson, G.; Edvardsson, V.; Sulem, P.; De Vegt, F.; d’Ancona, F.C.H.; Den Heijer, M.; Franzson, L.; et al. Association of Variants at UMOD with Chronic Kidney Disease and Kidney Stones—Role of Age and Comorbid Diseases. PLoS Genet. 2010, 6, e1001039. [Google Scholar] [CrossRef]
- Cavallone, D.; Malagolini, N.; Serafini-Cessi, F. Mechanism of Release of Urinary Tamm-Horsfall Glycoprotein from the Kidney GPI-Anchored Counterpart. Biochem. Biophys. Res. Commun. 2001, 280, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ikonen, E. Functional Rafts in Cell Membranes. Nature 1997, 387, 569–572. [Google Scholar] [CrossRef] [PubMed]
- Hession, C.; Decker, J.M.; Sherblom, A.P.; Kumar, S.; Yue, C.C.; Mattaliano, R.J.; Tizard, R.; Kawashima, E.; Schmeissner, U.; Heletky, S. Uromodulin (Tamm-Horsfall Glycoprotein): A Renal Ligand for Lymphokines. Science 1987, 237, 1479–1484. [Google Scholar] [CrossRef]
- Prasadan, K.; Bates, J.; Badgett, A.; Dell, M.; Sukhatme, V.; Yu, H.; Kumar, S. Nucleotide Sequence and Peptide Motifs of Mouse Uromodulin (Tamm-Horsfall Protein)—The Most Abundant Protein in Mammalian Urine. Biochim. Biophys. Acta 1995, 1260, 328–332. [Google Scholar] [CrossRef]
- Rindler, M.J.; Naik, S.S.; Li, N.; Hoops, T.C.; Peraldi, M.N. Uromodulin (Tamm-Horsfall Glycoprotein/Uromucoid) Is a Phosphatidylinositol-Linked Membrane Protein. J. Biol. Chem. 1990, 265, 20784–20789. [Google Scholar] [CrossRef]
- Fletcher, A.P.; Neuberger, A.; Ratcliffe, W.A. Tamm-Horsfall Urinary Glycoprotein. The Chemical Composition. Biochem. J. 1970, 120, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Wenk, R.E.; Bhagavan, B.S.; Rudert, J. Tamm-Horsfall Uromucoprotein and the Pathogenesis of Casts, Reflux Nephropathy, and Nephritides. Pathobiol. Annu. 1981, 11, 229–257. [Google Scholar] [PubMed]
- van Rooijen, J.J.; Voskamp, A.F.; Kamerling, J.P.; Vliegenthart, J.F. Glycosylation Sites and Site-Specific Glycosylation in Human Tamm-Horsfall Glycoprotein. Glycobiology 1999, 9, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.Q.; Kirk, K.A.; Connelly, K.G.; Sanders, P.W. Bence Jones Proteins Bind to a Common Peptide Segment of Tamm-Horsfall Glycoprotein to Promote Heterotypic Aggregation. J. Clin. Investig. 1993, 92, 2975–2983. [Google Scholar] [CrossRef]
- Serafini-Cessi, F.; Malagolini, N.; Hoops, T.C.; Rindler, M.J. Biosynthesis and Oligosaccharide Processing of Human Tamm-Horsfall Glycoprotein Permanently Expressed in HeLa Cells. Biochem. Biophys. Res. Commun. 1993, 194, 784–790. [Google Scholar] [CrossRef]
- Pesce, A.J.; Clyne, D.H.; Pollak, V.E.; Kant, S.K.; Foulkes, E.C.; Selenke, W.M. Renal Tubular Interactions of Proteins. Clin. Biochem. 1980, 13, 209–215. [Google Scholar] [CrossRef]
- Donald, A.S.; Yates, A.D.; Soh, C.P.; Morgan, W.T.; Watkins, W.M. A Blood Group Sda-Active Pentasaccharide Isolated from Tamm-Horsfall Urinary Glycoprotein. Biochem. Biophys. Res. Commun. 1983, 115, 625–631. [Google Scholar] [CrossRef]
- Serafini-Cessi, F.; Malagolini, N.; Cavallone, D. Tamm-Horsfall Glycoprotein: Biology and Clinical Relevance. Am. J. Kidney Dis. 2003, 42, 658–676. [Google Scholar] [CrossRef]
- Easton, R.L.; Patankar, M.S.; Clark, G.F.; Morris, H.R.; Dell, A. Pregnancy-Associated Changes in the Glycosylation of Tamm-Horsfall Glycoprotein. Expression of Sialyl Lewis(x) Sequences on Core 2 Type O-Glycans Derived from Uromodulin. J. Biol. Chem. 2000, 275, 21928–21938. [Google Scholar] [CrossRef]
- Bokhove, M.; Nishimura, K.; Brunati, M.; Han, L.; de Sanctis, D.; Rampoldi, L.; Jovine, L. A Structured Interdomain Linker Directs Self-Polymerization of Human Uromodulin. Proc. Natl. Acad. Sci. USA 2016, 113, 1552–1557. [Google Scholar] [CrossRef]
- Jovine, L.; Qi, H.; Williams, Z.; Litscher, E.; Wassarman, P.M. The ZP Domain Is a Conserved Module for Polymerization of Extracellular Proteins. Nat. Cell Biol. 2002, 4, 457–461. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.D.; Bork, P. Epidermal Growth Factor-like Modules. Curr. Opin. Struc. Biol. 1993, 3, 385–392. [Google Scholar] [CrossRef]
- Schaeffer, C.; Santambrogio, S.; Perucca, S.; Casari, G.; Rampoldi, L. Analysis of Uromodulin Polymerization Provides New Insights into the Mechanisms Regulating ZP Domain-Mediated Protein Assembly. Mol. Biol. Cell 2009, 20, 589–599. [Google Scholar] [CrossRef] [PubMed]
- Santambrogio, S.; Cattaneo, A.; Bernascone, I.; Schwend, T.; Jovine, L.; Bachi, A.; Rampoldi, L. Urinary Uromodulin Carries an Intact ZP Domain Generated by a Conserved C-Terminal Proteolytic Cleavage. Biochem. Biophys. Res. Commun. 2008, 370, 410–413. [Google Scholar] [CrossRef] [PubMed]
- Brunati, M.; Perucca, S.; Han, L.; Cattaneo, A.; Consolato, F.; Andolfo, A.; Schaeffer, C.; Olinger, E.; Peng, J.; Santambrogio, S.; et al. The Serine Protease Hepsin Mediates Urinary Secretion and Polymerisation of Zona Pellucida Domain Protein Uromodulin. eLife 2015, 4, e08887. [Google Scholar] [CrossRef] [PubMed]
- Micanovic, R.; Khan, S.; Janosevic, D.; Lee, M.E.; Hato, T.; Srour, E.F.; Winfree, S.; Ghosh, J.; Tong, Y.; Rice, S.E.; et al. Tamm-Horsfall Protein Regulates Mononuclear Phagocytes in the Kidney. J. Am. Soc. Nephrol. 2018, 29, 841–856. [Google Scholar] [CrossRef] [PubMed]
- Maxfield, M. Molecular Forms of Human Urinary Mucoprotein Present under Physiological Conditions. Biochim. Biophys. Acta 1961, 49, 548–558. [Google Scholar] [CrossRef] [PubMed]
- Porter, K.R.; Tamm, I. Direct Visualization of a Mucoprotein Component of Urine. J. Biol. Chem. 1955, 212, 135–140. [Google Scholar] [CrossRef]
- Wiggins, R.C. Uromucoid (Tamm-Horsfall Glycoprotein) Forms Different Polymeric Arrangements on a Filter Surface under Different Physicochemical Conditions. Clin. Chim. Acta 1987, 162, 329–340. [Google Scholar] [CrossRef]
- Bachmann, S.; Dawnay, A.B.; Bouby, N.; Bankir, L. Tamm-Horsfall Protein Excretion during Chronic Alterations in Urinary Concentration and Protein Intake in the Rat. Ren. Physiol. Biochem. 1991, 14, 236–245. [Google Scholar] [CrossRef]
- Goodall, A.A.; Marshall, R.D. Effects of Freezing on the Estimated Amounts of Tamm-Horsfall Glycoprotein in Urine, as Determined by Radioimmunoassay. Biochem. J. 1980, 189, 533–539. [Google Scholar] [CrossRef] [PubMed]
- Thornley, C.; Dawnay, A.; Cattell, W.R. Human Tamm-Horsfall Glycoprotein: Urinary and Plasma Levels in Normal Subjects and Patients with Renal Disease Determined by a Fully Validated Radioimmunoassay. Clin. Sci. 1985, 68, 529–535. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Block, M.; Herbst, V.; Nockher, W.A.; Schlumberger, W.; Satanovskij, R.; Angermann, S.; Hasenau, A.-L.; Stecher, L.; Heemann, U.; et al. Plasma Uromodulin Correlates With Kidney Function and Identifies Early Stages in Chronic Kidney Disease Patients. Medicine 2016, 95, e3011. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.M.; Neuberger, A. The Turnover Rate of Rabbit Urinary Tamm-Horsfall Glycoprotein. Biochem. J. 1973, 136, 659–668. [Google Scholar] [CrossRef]
- Olden, M.; Corre, T.; Hayward, C.; Toniolo, D.; Ulivi, S.; Gasparini, P.; Pistis, G.; Hwang, S.-J.; Bergmann, S.; Campbell, H.; et al. Common Variants in UMOD Associate with Urinary Uromodulin Levels: A Meta-Analysis. J. Am. Soc. Nephrol. 2014, 25, 1869–1882. [Google Scholar] [CrossRef]
- Torffvit, O.; Jørgensen, P.E.; Kamper, A.-L.; Holstein-Rathlou, N.-H.; Leyssac, P.P.; Poulsen, S.S.; Strandgaard, S. Urinary Excretion of Tamm-Horsfall Protein and Epidermal Growth Factor in Chronic Nephropathy. Nephron 1998, 79, 167–172. [Google Scholar] [CrossRef]
- Ecelbarger, C.A.; Terris, J.; Hoyer, J.R.; Nielsen, S.; Wade, J.B.; Knepper, M.A. Localization and Regulation of the Rat Renal Na(+)-K(+)-2Cl- Cotransporter, BSC-1. Am. J. Physiol. 1996, 271, F619–F628. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, R.; Kahl, T.; Mutig, K.; Bachmann, S. Selectively Reduced Expression of Thick Ascending Limb Tamm-Horsfall Protein in Hypothyroid Kidneys. Histochem. Cell Biol. 2004, 121, 319–327. [Google Scholar] [CrossRef]
- Devuyst, O.; Olinger, E.; Rampoldi, L. Uromodulin: From Physiology to Rare and Complex Kidney Disorders. Nat. Rev. Nephrol. 2017, 13, 525–544. [Google Scholar] [CrossRef]
- Mutig, K.; Kahl, T.; Saritas, T.; Godes, M.; Persson, P.; Bates, J.; Raffi, H.; Rampoldi, L.; Uchida, S.; Hille, C.; et al. Activation of the Bumetanide-Sensitive Na+,K+,2Cl- Cotransporter (NKCC2) Is Facilitated by Tamm-Horsfall Protein in a Chloride-Sensitive Manner. J. Biol. Chem. 2011, 286, 30200–30210. [Google Scholar] [CrossRef]
- Renigunta, A.; Renigunta, V.; Saritas, T.; Decher, N.; Mutig, K.; Waldegger, S. Tamm-Horsfall Glycoprotein Interacts with Renal Outer Medullary Potassium Channel ROMK2 and Regulates Its Function. J. Biol. Chem. 2011, 286, 2224–2235. [Google Scholar] [CrossRef] [PubMed]
- Tokonami, N.; Takata, T.; Beyeler, J.; Ehrbar, I.; Yoshifuji, A.; Christensen, E.I.; Loffing, J.; Devuyst, O.; Olinger, E.G. Uromodulin Is Expressed in the Distal Convoluted Tubule, Where It Is Critical for Regulation of the Sodium Chloride Cotransporter NCC. Kidney Int. 2018, 94, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Wolf, M.T.F.; Wu, X.-R.; Huang, C.-L. Uromodulin Upregulates TRPV5 by Impairing Caveolin-Mediated Endocytosis. Kidney Int. 2013, 84, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Nie, M.; Bal, M.S.; Liu, J.; Yang, Z.; Rivera, C.; Wu, X.-R.; Hoenderop, J.G.J.; Bindels, R.J.M.; Marciano, D.K.; Wolf, M.T.F. Uromodulin Regulates Renal Magnesium Homeostasis through the Ion Channel Transient Receptor Potential Melastatin 6 (TRPM6). J. Biol. Chem. 2018, 293, 16488–16502. [Google Scholar] [CrossRef] [PubMed]
- Scolari, F.; Izzi, C.; Ghiggeri, G.M. Uromodulin: From Monogenic to Multifactorial Diseases. Nephrol. Dial. Transplant. 2015, 30, 1250–1256. [Google Scholar] [CrossRef] [PubMed]
- Wangsiripaisan, A.; Gengaro, P.E.; Edelstein, C.L.; Schrier, R.W. Role of Polymeric Tamm-Horsfall Protein in Cast Formation: Oligosaccharide and Tubular Fluid Ions. Kidney Int. 2001, 59, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Duncan, J.L. Differential Effect of Tamm-Horsfall Protein on Adherence of Escherichia Coli to Transitional Epithelial Cells. J. Infect. Dis. 1988, 158, 1379–1382. [Google Scholar] [CrossRef] [PubMed]
- Weichhart, T.; Zlabinger, G.J.; Säemann, M.D. The Multiple Functions of Tamm-Horsfall Protein in Human Health and Disease: A Mystery Clears Up. Wien. Klin. Wochenschr. 2005, 117, 316–322. [Google Scholar] [CrossRef]
- Garimella, P.S.; Bartz, T.M.; Ix, J.H.; Chonchol, M.; Shlipak, M.G.; Devarajan, P.; Bennett, M.R.; Sarnak, M.J. Urinary Uromodulin and Risk of Urinary Tract Infections: The Cardiovascular Health Study. Am. J. Kidney Dis. 2017, 69, 744–751. [Google Scholar] [CrossRef]
- van der Starre, W.E.; van Nieuwkoop, C.; Thomson, U.; Zijderveld-Voshart, M.S.M.; Koopman, J.P.R.; van der Reijden, T.J.K.; van Dissel, J.T.; van de Vosse, E. Urinary Proteins, Vitamin D and Genetic Polymorphisms as Risk Factors for Febrile Urinary Tract Infection and Relation with Bacteremia: A Case Control Study. PLoS ONE 2015, 10, e0121302. [Google Scholar] [CrossRef]
- Bates, J.M.; Raffi, H.M.; Prasadan, K.; Mascarenhas, R.; Laszik, Z.; Maeda, N.; Hultgren, S.J.; Kumar, S. Tamm-Horsfall Protein Knockout Mice Are More Prone to Urinary Tract Infection: Rapid Communication. Kidney Int. 2004, 65, 791–797. [Google Scholar] [CrossRef] [PubMed]
- Raffi, H.S.; Bates, J.M.; Laszik, Z.; Kumar, S. Tamm-Horsfall Protein Acts as a General Host-Defense Factor against Bacterial Cystitis. Am. J. Nephrol. 2005, 25, 570–578. [Google Scholar] [CrossRef] [PubMed]
- Raffi, H.S.; Bates, J.M.; Laszik, Z.; Kumar, S. Tamm-Horsfall Protein Protects against Urinary Tract Infection by Proteus Mirabilis. J. Urol. 2009, 181, 2332–2338. [Google Scholar] [CrossRef] [PubMed]
- Pak, J.; Pu, Y.; Zhang, Z.T.; Hasty, D.L.; Wu, X.R. Tamm-Horsfall Protein Binds to Type 1 Fimbriated Escherichia Coli and Prevents E. Coli from Binding to Uroplakin Ia and Ib Receptors. J. Biol. Chem. 2001, 276, 9924–9930. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Zhu, X.-H.; Huang, H.-Y.; Shapiro, E.; Hasty, D.L.; Wu, X.-R. Ablation of the Tamm-Horsfall Protein Gene Increases Susceptibility of Mice to Bladder Colonization by Type 1-Fimbriated Escherichia coli. Am. J. Physiol. Renal Physiol. 2004, 286, F795–F802. [Google Scholar] [CrossRef] [PubMed]
- Ghirotto, S.; Tassi, F.; Barbujani, G.; Pattini, L.; Hayward, C.; Vollenweider, P.; Bochud, M.; Rampoldi, L.; Devuyst, O. The Uromodulin Gene Locus Shows Evidence of Pathogen Adaptation through Human Evolution. J. Am. Soc. Nephrol. 2016, 27, 2983–2996. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.L.; Stanisich, J.J.; Sauer, M.M.; Lin, C.-W.; Eras, J.; Zyla, D.S.; Trück, J.; Devuyst, O.; Aebi, M.; Pilhofer, M.; et al. Architecture and Function of Human Uromodulin Filaments in Urinary Tract Infections. Science 2020, 369, 1005–1010. [Google Scholar] [CrossRef] [PubMed]
- Coady, A.; Ramos, A.R.; Olson, J.; Nizet, V.; Patras, K.A. Tamm-Horsfall Protein Protects the Urinary Tract against Candida Albicans. Infect. Immun. 2018, 86, e00451-18. [Google Scholar] [CrossRef]
- Säemann, M.D.; Weichhart, T.; Zeyda, M.; Staffler, G.; Schunn, M.; Stuhlmeier, K.M.; Sobanov, Y.; Stulnig, T.M.; Akira, S.; von Gabain, A.; et al. Tamm-Horsfall Glycoprotein Links Innate Immune Cell Activation with Adaptive Immunity via a Toll-like Receptor-4-Dependent Mechanism. J. Clin. Investig. 2005, 115, 468–475. [Google Scholar] [CrossRef]
- Ruzhanskaya, A.V.; Kravtsov, E.G.; Dalin, M.V.; Gabrielyan, N.I. Study of Antibody Production to Tamm-Horsfall Protein in Renal Transplant Donors and Recipients. Bull. Exp. Biol. Med. 2006, 141, 620–623. [Google Scholar] [CrossRef]
- El-Achkar, T.M.; Wu, X.-R.; Rauchman, M.; McCracken, R.; Kiefer, S.; Dagher, P.C. Tamm-Horsfall Protein Protects the Kidney from Ischemic Injury by Decreasing Inflammation and Altering TLR4 Expression. Am. J. Physiol. Renal Physiol. 2008, 295, F534–F544. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, T.M.; Dagher, P.C. Tubular Cross Talk in Acute Kidney Injury: A Story of Sense and Sensibility. Am. J. Physiol. Renal Physiol. 2015, 308, F1317–F1323. [Google Scholar] [CrossRef] [PubMed]
- Micanovic, R.; Chitteti, B.R.; Dagher, P.C.; Srour, E.F.; Khan, S.; Hato, T.; Lyle, A.; Tong, Y.; Wu, X.-R.; El-Achkar, T.M. Tamm-Horsfall Protein Regulates Granulopoiesis and Systemic Neutrophil Homeostasis. J. Am. Soc. Nephrol. 2015, 26, 2172–2182. [Google Scholar] [CrossRef] [PubMed]
- Alesutan, I.; Luong, T.T.D.; Schelski, N.; Masyout, J.; Hille, S.; Schneider, M.P.; Graham, D.; Zickler, D.; Verheyen, N.; Estepa, M.; et al. Circulating Uromodulin Inhibits Vascular Calcification by Interfering with Pro-Inflammatory Cytokine Signalling. Cardiovasc. Res. 2021, 117, 930–941. [Google Scholar] [CrossRef] [PubMed]
- LaFavers, K.A.; Macedo, E.; Garimella, P.S.; Lima, C.; Khan, S.; Myslinski, J.; McClintick, J.; Witzmann, F.A.; Winfree, S.; Phillips, C.L.; et al. Circulating Uromodulin Inhibits Systemic Oxidative Stress by Inactivating the TRPM2 Channel. Sci. Transl. Med. 2019, 11, eaaw3639. [Google Scholar] [CrossRef]
- Rampoldi, L.; Scolari, F.; Amoroso, A.; Ghiggeri, G.; Devuyst, O. The Rediscovery of Uromodulin (Tamm–Horsfall Protein): From Tubulointerstitial Nephropathy to Chronic Kidney Disease. Kidney Int. 2011, 80, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Welker, P.; Geist, B.; Frühauf, J.-H.; Salanova, M.; Groneberg, D.A.; Krause, E.; Bachmann, S. Role of Lipid Rafts in Membrane Delivery of Renal Epithelial Na+-K+-ATPase, Thick Ascending Limb. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R1328–R1337. [Google Scholar] [CrossRef]
- Brown, D.A.; Crise, B.; Rose, J.K. Mechanism of Membrane Anchoring Affects Polarized Expression of Two Proteins in MDCK Cells. Science 1989, 245, 1499–1501. [Google Scholar] [CrossRef]
- Benting, J.H.; Rietveld, A.G.; Simons, K. N-Glycans Mediate the Apical Sorting of a GPI-Anchored, Raft-Associated Protein in Madin-Darby Canine Kidney Cells. J. Cell Biol. 1999, 146, 313–320. [Google Scholar] [CrossRef]
- El-Achkar, T.M.; McCracken, R.; Rauchman, M.; Heitmeier, M.R.; Al-Aly, Z.; Dagher, P.C.; Wu, X.-R. Tamm-Horsfall Protein-Deficient Thick Ascending Limbs Promote Injury to Neighboring S3 Segments in an MIP-2-Dependent Mechanism. Am. J. Physiol. Renal Physiol. 2011, 300, F999–F1007. [Google Scholar] [CrossRef]
- Leiherer, A.; Muendlein, A.; Saely, C.H.; Brandtner, E.M.; Geiger, K.; Fraunberger, P.; Drexel, H. The Value of Uromodulin as a New Serum Marker to Predict Decline in Renal Function. J. Hypertens. 2018, 36, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Block, M.; Herbst, V.; Schlumberger, W.; Nockher, A.; Angermann, S.; Schmaderer, C.; Heemann, U.; Renders, L.; Scherberich, J. Serum Uromodulin Predicts Graft Failure in Renal Transplant Recipients. Biomarkers 2017, 22, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.E.; Kleber, M.E.; Scharnagl, H.; Krämer, B.K.; März, W.; Scherberich, J.E. Serum Uromodulin and Mortality Risk in Patients Undergoing Coronary Angiography. J. Am. Soc. Nephrol. 2017, 28, 2201–2210. [Google Scholar] [CrossRef]
- Youhanna, S.; Weber, J.; Beaujean, V.; Glaudemans, B.; Sobek, J.; Devuyst, O. Determination of Uromodulin in Human Urine: Influence of Storage and Processing. Nephrol. Dial. Transplant. 2014, 29, 136–145. [Google Scholar] [CrossRef] [PubMed]
- Torffvit, O.; Agardh, C.-D.; Kjellsson, B.; Wieslander, J. Tubular Secretion of Tamm-Horsfall Protein in Type 1 (Insulin-Dependent) Diabetes Mellitus Using a Simplified Enzyme Linked Immunoassay. Clin. Chim. Acta 1992, 205, 31–41. [Google Scholar] [CrossRef]
- Shihabi, Z.K.; Hinsdale, M.E.; Bleyer, A.J. Analysis of Tamm-Horsfall Protein by High-Performance Liquid Chromatography with Native Fluorescence. J. Chromatogr. A 2004, 1027, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Lau, W.-H.; Leong, W.-S.; Ismail, Z.; Gam, L.-H. Qualification and Application of an ELISA for the Determination of Tamm Horsfall Protein (THP) in Human Urine and Its Use for Screening of Kidney Stone Disease. Int. J. Biol. Sci. 2008, 4, 215. [Google Scholar] [CrossRef]
- Van Duijl, T.T.; Ruhaak, L.R.; Hoogeveen, E.K.; De Mutsert, R.; Rosendaal, F.R.; Le Cessie, S.; De Fijter, J.W.; Cobbaert, C.M. Reference Intervals of Urinary Kidney Injury Biomarkers for Middle-Aged Men and Women Determined by Quantitative Protein Mass Spectrometry. Ann. Clin. Biochem. 2022, 59, 420–432. [Google Scholar] [CrossRef]
- Hammond, T.G.; Moes, S.; Youhanna, S.; Jennings, P.; Devuyst, O.; Odermatt, A.; Jenö, P. Development and Characterization of a Pseudo Multiple Reaction Monitoring Method for the Quantification of Human Uromodulin in Urine. Bioanalysis 2016, 8, 1279–1296. [Google Scholar] [CrossRef]
- Pruijm, M.; Ponte, B.; Ackermann, D.; Paccaud, F.; Guessous, I.; Ehret, G.; Pechère-Bertschi, A.; Vogt, B.; Mohaupt, M.G.; Martin, P.-Y.; et al. Associations of Urinary Uromodulin with Clinical Characteristics and Markers of Tubular Function in the General Population. Clin. J. Am. Soc. Nephrol. 2016, 11, 70–80. [Google Scholar] [CrossRef]
- Troyanov, S.; Delmas-Frenette, C.; Bollée, G.; Youhanna, S.; Bruat, V.; Awadalla, P.; Devuyst, O.; Madore, F. Clinical, Genetic, and Urinary Factors Associated with Uromodulin Excretion. Clin. J. Am. Soc. Nephrol. 2016, 11, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Pivin, E.; Ponte, B.; de Seigneux, S.; Ackermann, D.; Guessous, I.; Ehret, G.; Pechère-Bertschi, A.; Olinger, E.; Mohaupt, M.; Vogt, B.; et al. Uromodulin and Nephron Mass. Clin. J. Am. Soc. Nephrol. 2018, 13, 1556–1557. [Google Scholar] [CrossRef] [PubMed]
- Garimella, P.S.; Biggs, M.L.; Katz, R.; Ix, J.H.; Bennett, M.R.; Devarajan, P.; Kestenbaum, B.R.; Siscovick, D.S.; Jensen, M.K.; Shlipak, M.G.; et al. Urinary Uromodulin, Kidney Function, and Cardiovascular Disease in Elderly Adults. Kidney Int. 2015, 88, 1126–1134. [Google Scholar] [CrossRef] [PubMed]
- Garimella, P.S.; Jaber, B.L.; Tighiouart, H.; Liangos, O.; Bennett, M.R.; Devarajan, P.; El-Achkar, T.M.; Sarnak, M.J. Association of Preoperative Urinary Uromodulin with AKI after Cardiac Surgery. Clin. J. Am. Soc. Nephrol. 2017, 12, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Dawnay, A.B.; Cattell, W.R. Serum Tamm-Horsfall Glycoprotein Levels in Health and in Renal Disease. Clin. Nephrol. 1981, 15, 5–8. [Google Scholar] [PubMed]
- Usui, R.; Ogawa, T.; Takahashi, H.; Iwasaki, C.; Koike, M.; Morito, T.; Hatano, M.; Nitta, K. Serum Uromodulin Is a Novel Renal Function Marker in the Japanese Population. Clin. Exp. Nephrol. 2021, 25, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Smirnov, A.V.; Khasun, M.; Kayukov, I.G.; Galkina, O.V.; Sipovski, V.G.; Parastaeva, M.M.; Bogdanova, E.O. Serum Uromodulin as an Early Biomarker of Tubular Atrophy and Interstitial Fibrosis in Patients with Glomerulopathies. Ter. Arkhiv 2018, 90, 41–47. [Google Scholar] [CrossRef]
- Then, C.; Herder, C.; Then, H.; Thorand, B.; Huth, C.; Heier, M.; Meisinger, C.; Peters, A.; Koenig, W.; Rathmann, W.; et al. Serum Uromodulin Is Inversely Associated with Biomarkers of Subclinical Inflammation in the Population-Based KORA F4 Study. Clin. Kidney J. 2021, 14, 1618–1625. [Google Scholar] [CrossRef]
- Steubl, D.; Buzkova, P.; Ix, J.H.; Devarajan, P.; Bennett, M.R.; Chaves, P.H.M.; Shlipak, M.G.; Bansal, N.; Sarnak, M.J.; Garimella, P.S. Association of Serum and Urinary Uromodulin and Their Correlates in Older Adults—The Cardiovascular Health Study. Nephrology 2020, 25, 522–526. [Google Scholar] [CrossRef]
- Enko, D.; Meinitzer, A.; Scherberich, J.E.; März, W.; Herrmann, M.; Artinger, K.; Rosenkranz, A.R.; Zitta, S. Individual Uromodulin Serum Concentration Is Independent of Glomerular Filtration Rate in Healthy Kidney Donors. Clin. Chem. Lab. Med. (CCLM) 2021, 59, 563–570. [Google Scholar] [CrossRef]
- Yazdani, B.; Delgado, G.E.; Scharnagl, H.; Krämer, B.K.; Drexel, H.; März, W.; Scherberich, J.E.; Leiherer, A.; Kleber, M.E. Combined Use of Serum Uromodulin and eGFR to Estimate Mortality Risk. Front. Med. 2021, 8, 723546. [Google Scholar] [CrossRef] [PubMed]
- Pattaro, C.; Teumer, A.; Gorski, M.; Chu, A.Y.; Li, M.; Mijatovic, V.; Garnaas, M.; Tin, A.; Sorice, R.; Li, Y.; et al. Genetic Associations at 53 Loci Highlight Cell Types and Biological Pathways Relevant for Kidney Function. Nat. Commun. 2016, 7, 10023. [Google Scholar] [CrossRef] [PubMed]
- Trudu, M.; Janas, S.; Lanzani, C.; Debaix, H.; Schaeffer, C.; Ikehata, M.; Citterio, L.; Demaretz, S.; Trevisani, F.; Ristagno, G.; et al. Common Noncoding UMOD Gene Variants Induce Salt-Sensitive Hypertension and Kidney Damage by Increasing Uromodulin Expression. Nat. Med. 2013, 19, 1655–1660. [Google Scholar] [CrossRef] [PubMed]
- Ponte, B.; Sadler, M.C.; Olinger, E.; Vollenweider, P.; Bochud, M.; Padmanabhan, S.; Hayward, C.; Kutalik, Z.; Devuyst, O. Mendelian Randomization to Assess Causality between Uromodulin, Blood Pressure and Chronic Kidney Disease. Kidney Int. 2021, 100, 1282–1291. [Google Scholar] [CrossRef] [PubMed]
- Rossier, B.C.; Bochud, M.; Devuyst, O. The Hypertension Pandemic: An Evolutionary Perspective. Physiology 2017, 32, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Köttgen, A.; Hwang, S.-J.; Larson, M.G.; Van Eyk, J.E.; Fu, Q.; Benjamin, E.J.; Dehghan, A.; Glazer, N.L.; Kao, W.H.L.; Harris, T.B.; et al. Uromodulin Levels Associate with a Common UMOD Variant and Risk for Incident CKD. J. Am. Soc. Nephrol. 2010, 21, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Lifton, R.P. Genetic Dissection of Human Blood Pressure Variation: Common Pathways from Rare Phenotypes. Harvey Lect. 2004, 100, 71–101. [Google Scholar] [PubMed]
- Morris, A.P.; Le, T.H.; Wu, H.; Akbarov, A.; van der Most, P.J.; Hemani, G.; Smith, G.D.; Mahajan, A.; Gaulton, K.J.; Nadkarni, G.N.; et al. Trans-Ethnic Kidney Function Association Study Reveals Putative Causal Genes and Effects on Kidney-Specific Disease Aetiologies. Nat. Commun. 2019, 10, 29. [Google Scholar] [CrossRef]
- Wuttke, M.; Li, Y.; Li, M.; Sieber, K.B.; Feitosa, M.F.; Gorski, M.; Tin, A.; Wang, L.; Chu, A.Y.; Hoppmann, A.; et al. A Catalog of Genetic Loci Associated with Kidney Function from Analyses of a Million Individuals. Nat. Genet. 2019, 51, 957–972. [Google Scholar] [CrossRef]
- van Zuydam, N.R.; Ahlqvist, E.; Sandholm, N.; Deshmukh, H.; Rayner, N.W.; Abdalla, M.; Ladenvall, C.; Ziemek, D.; Fauman, E.; Robertson, N.R.; et al. A Genome-Wide Association Study of Diabetic Kidney Disease in Subjects With Type 2 Diabetes. Diabetes 2018, 67, 1414–1427. [Google Scholar] [CrossRef]
- Sveinbjornsson, G.; Mikaelsdottir, E.; Palsson, R.; Indridason, O.S.; Holm, H.; Jonasdottir, A.; Helgason, A.; Sigurdsson, S.; Jonasdottir, A.; Sigurdsson, A.; et al. Rare Mutations Associating with Serum Creatinine and Chronic Kidney Disease. Hum. Mol. Genet. 2014, 23, 6935–6943. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cheng, Y.; Consolato, F.; Schiano, G.; Chong, M.R.; Pietzner, M.; Nguyen, N.Q.H.; Scherer, N.; Biggs, M.L.; Kleber, M.E.; et al. Genome-Wide Studies Reveal Factors Associated with Circulating Uromodulin and Its Relationships to Complex Diseases. JCI Insight 2022, 7, e157035. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; El-Achkar, T.M.; Wu, X.-R. Tamm-Horsfall Protein Regulates Circulating and Renal Cytokines by Affecting Glomerular Filtration Rate and Acting as a Urinary Cytokine Trap. J. Biol. Chem. 2012, 287, 16365–16378. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.C.J. Importance of Carbohydrate in the Interaction of Tamm-Horsfall Protein with Complement 1q and Inhibition of Classical Complement Activation. Immunol. Cell Biol. 2006, 84, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Gong, K.; Xia, M.; Wang, Y.; Bai, L.; Ying, W.; Zhu, F.; Chen, Y. Importance of Glycosylation in the Interaction of Tamm-Horsfall Protein with Collectin-11 and Acute Kidney Injury. J. Cell. Mol. Med. 2020, 24, 3572–3581. [Google Scholar] [CrossRef] [PubMed]
- Askenazi, D.J.; Koralkar, R.; Patil, N.; Halloran, B.; Ambalavanan, N.; Griffin, R. Acute Kidney Injury Urine Biomarkers in Very Low-Birth-Weight Infants. Clin. J. Am. Soc. Nephrol. 2016, 11, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Bullen, A.L.; Katz, R.; Lee, A.K.; Anderson, C.A.M.; Cheung, A.K.; Garimella, P.S.; Jotwani, V.; Haley, W.E.; Ishani, A.; Lash, J.P.; et al. The SPRINT Trial Suggests That Markers of Tubule Cell Function in the Urine Associate with Risk of Subsequent Acute Kidney Injury While Injury Markers Elevate after the Injury. Kidney Int. 2019, 96, 470–479. [Google Scholar] [CrossRef]
- Bennett, M.R.; Pyles, O.; Ma, Q.; Devarajan, P. Preoperative Levels of Urinary Uromodulin Predict Acute Kidney Injury after Pediatric Cardiopulmonary Bypass Surgery. Pediatr. Nephrol. 2018, 33, 521–526. [Google Scholar] [CrossRef]
- Chertow, G.M.; Levy, E.M.; Hammermeister, K.E.; Grover, F.; Daley, J. Independent Association between Acute Renal Failure and Mortality Following Cardiac Surgery. Am. J. Med. 1998, 104, 343–348. [Google Scholar] [CrossRef]
- Conlon, P.J.; Stafford-Smith, M.; White, W.D.; Newman, M.F.; King, S.; Winn, M.P.; Landolfo, K. Acute Renal Failure Following Cardiac Surgery. Nephrol. Dial. Transplant. 1999, 14, 1158–1162. [Google Scholar] [CrossRef]
- Rosner, M.H.; Okusa, M.D. Acute Kidney Injury Associated with Cardiac Surgery. Clin. J. Am. Soc. Nephrol. 2006, 1, 19–32. [Google Scholar] [CrossRef] [PubMed]
- Parikh, C.R.; Coca, S.G.; Thiessen-Philbrook, H.; Shlipak, M.G.; Koyner, J.L.; Wang, Z.; Edelstein, C.L.; Devarajan, P.; Patel, U.D.; Zappitelli, M.; et al. Postoperative Biomarkers Predict Acute Kidney Injury and Poor Outcomes after Adult Cardiac Surgery. J. Am. Soc. Nephrol. 2011, 22, 1748–1757. [Google Scholar] [CrossRef] [PubMed]
- Dehne, M.G.; Boldt, J.; Heise, D.; Sablotzki, A.; Hempelmann, G. Tamm-Horsfall protein, alpha-1- and beta-2-microglobulin as kidney function markers in heart surgery. Anaesthesist 1995, 44, 545–551. [Google Scholar] [CrossRef] [PubMed]
- Dehne, M.G.; Sablotzki, A.; Mühling, J.; Papke, G.; Kuntzsch, U.; Hempelmann, G. Acute kidney failure. Non-invasive diagnosis of acute kidney failure in operative intensive care patients. Anaesthesist 1998, 47, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.C.; Zanaro, N.; González, L.; Trigo, P.; Imventarza, O.; Nesse, A. Tamm-Horsfall Protein Excretion to Predict the Onset of Renal Insufficiency. Clin. Biochem. 2002, 35, 65–68. [Google Scholar] [CrossRef]
- Askenazi, D.J.; Koralkar, R.; Hundley, H.E.; Montesanti, A.; Parwar, P.; Sonjara, S.; Ambalavanan, N. Urine Biomarkers Predict Acute Kidney Injury in Newborns. J. Pediatr. 2012, 161, 270–275.e1. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.; Xu, L.; Melchinger, I.; Thiessen-Philbrook, H.; Moledina, D.G.; Coca, S.G.; Hsu, C.-Y.; Go, A.S.; Liu, K.D.; Siew, E.D.; et al. Longitudinal Biomarkers and Kidney Disease Progression after Acute Kidney Injury. JCI Insight 2023, 8, e167731. [Google Scholar] [CrossRef]
- Patidar, K.R.; Garimella, P.S.; Macedo, E.; Slaven, J.E.; Ghabril, M.S.; Weber, R.E.; Anderson, M.; Orman, E.S.; Nephew, L.D.; Desai, A.P.; et al. Admission Plasma Uromodulin and the Risk of Acute Kidney Injury in Hospitalized Patients with Cirrhosis: A Pilot Study. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G447–G452. [Google Scholar] [CrossRef]
- Usui, R.; Ogawa, T.; Iwasaki, C.; Nitta, K.; Koike, M. Serum Uromodulin Is a Possible Auxiliary Diagnostic Tool for Acute Tubular Injury and Acute Interstitial Nephritis: A Case Series. Case Rep. Nephrol. Dial. 2022, 12, 185–192. [Google Scholar] [CrossRef]
- Kuśnierz-Cabala, B.; Gala-Błądzińska, A.; Mazur-Laskowska, M.; Dumnicka, P.; Sporek, M.; Matuszyk, A.; Gil, K.; Ceranowicz, P.; Walocha, J.; Kucharz, J.; et al. Serum Uromodulin Levels in Prediction of Acute Kidney Injury in the Early Phase of Acute Pancreatitis. Molecules 2017, 22, 988. [Google Scholar] [CrossRef]
- Fedak, D.; Kuźniewski, M.; Fugiel, A.; Wieczorek-Surdacka, E.; Przepiórkowska-Hoyer, B.; Jasik, P.; Miarka, P.; Dumnicka, P.; Kapusta, M.; Solnica, B.; et al. Serum Uromodulin Concentrations Correlate with Glomerular Filtration Rate in Patients with Chronic Kidney Disease. Pol. Arch. Med. Wewnętrznej 2016, 126, 995–1004. [Google Scholar] [CrossRef] [PubMed]
- Risch, L.; Lhotta, K.; Meier, D.; Medina-Escobar, P.; Nydegger, U.E.; Risch, M. The Serum Uromodulin Level Is Associated with Kidney Function. Clin. Chem. Lab. Med. 2014, 52, 1755–1761. [Google Scholar] [CrossRef] [PubMed]
- Jie, C.; Yi-Ying, Y.; Miao, C. Correlation of Serum Uromodulin Levels with Renal Fibrosis and Renal Function Progression in Patients with CKD. Pak. J. Pharm. Sci. 2021, 34, 2417–2422. [Google Scholar] [PubMed]
- Bächle, H.; Sekula, P.; Schlosser, P.; Steinbrenner, I.; Cheng, Y.; Kotsis, F.; Meiselbach, H.; Stockmann, H.; Schönherr, S.; Eckardt, K.-U.; et al. Uromodulin and Its Association with Urinary Metabolites: The German Chronic Kidney Disease Study. Nephrol. Dial. Transplant. 2023, 38, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Buzkova, P.; Garimella, P.S.; Ix, J.H.; Devarajan, P.; Bennett, M.R.; Chaves, P.H.M.; Shlipak, M.G.; Bansal, N.; Sarnak, M.J. Association of Serum Uromodulin With ESKD and Kidney Function Decline in the Elderly: The Cardiovascular Health Study. Am. J. Kidney Dis. 2019, 74, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.; Wang, J.; Gao, B.; Wu, L.; Wang, F.; Cui, Z.; He, K.; Zhang, L.; Chen, M.; Zhao, M.-H. Serum Uromodulin and Progression of Kidney Disease in Patients with Chronic Kidney Disease. J. Transl. Med. 2018, 16, 316. [Google Scholar] [CrossRef] [PubMed]
- Steubl, D.; Block, M.; Herbst, V.; Nockher, W.A.; Schlumberger, W.; Kemmner, S.; Bachmann, Q.; Angermann, S.; Wen, M.; Heemann, U.; et al. Urinary Uromodulin Independently Predicts End-Stage Renal Disease and Rapid Kidney Function Decline in a Cohort of Chronic Kidney Disease Patients. Medicine 2019, 98, e15808. [Google Scholar] [CrossRef]
- Devuyst, O.; Bochud, M. Uromodulin, Kidney Function, Cardiovascular Disease, and Mortality. Kidney Int. 2015, 88, 944–946. [Google Scholar] [CrossRef]
- Pattaro, C.; Köttgen, A.; Teumer, A.; Garnaas, M.; Böger, C.A.; Fuchsberger, C.; Olden, M.; Chen, M.-H.; Tin, A.; Taliun, D.; et al. Genome-Wide Association and Functional Follow-up Reveals New Loci for Kidney Function. PLoS Genet. 2012, 8, e1002584. [Google Scholar] [CrossRef]
- Pattaro, C.; De Grandi, A.; Vitart, V.; Hayward, C.; Franke, A.; Aulchenko, Y.S.; Johansson, A.; Wild, S.H.; Melville, S.A.; Isaacs, A.; et al. A Meta-Analysis of Genome-Wide Data from Five European Isolates Reveals an Association of COL22A1, SYT1, and GABRR2 with Serum Creatinine Level. BMC Med. Genet. 2010, 11, 41. [Google Scholar] [CrossRef]
- Liu, C.-T.; Garnaas, M.K.; Tin, A.; Kottgen, A.; Franceschini, N.; Peralta, C.A.; de Boer, I.H.; Lu, X.; Atkinson, E.; Ding, J.; et al. Genetic Association for Renal Traits among Participants of African Ancestry Reveals New Loci for Renal Function. PLoS Genet. 2011, 7, e1002264. [Google Scholar] [CrossRef] [PubMed]
- Okada, Y.; Sim, X.; Go, M.J.; Wu, J.-Y.; Gu, D.; Takeuchi, F.; Takahashi, A.; Maeda, S.; Tsunoda, T.; Chen, P.; et al. Meta-Analysis Identifies Multiple Loci Associated with Kidney Function-Related Traits in East Asian Populations. Nat. Genet. 2012, 44, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Böger, C.A.; Gorski, M.; Li, M.; Hoffmann, M.M.; Huang, C.; Yang, Q.; Teumer, A.; Krane, V.; O’Seaghdha, C.M.; Kutalik, Z.; et al. Association of eGFR-Related Loci Identified by GWAS with Incident CKD and ESRD. PLoS Genet. 2011, 7, e1002292. [Google Scholar] [CrossRef] [PubMed]
- Reznichenko, A.; Böger, C.A.; Snieder, H.; Van Den Born, J.; De Borst, M.H.; Damman, J.; Van Dijk, M.C.; Van Goor, H.; Hepkema, B.G.; Hillebrands, J.-L.; et al. UMOD as a Susceptibility Gene for End-Stage Renal Disease. BMC Med. Genet. 2012, 13, 78. [Google Scholar] [CrossRef] [PubMed]
- Gorski, M.; Tin, A.; Garnaas, M.; McMahon, G.M.; Chu, A.Y.; Tayo, B.O.; Pattaro, C.; Teumer, A.; Chasman, D.I.; Chalmers, J.; et al. Genome-Wide Association Study of Kidney Function Decline in Individuals of European Descent. Kidney Int. 2015, 87, 1017–1029. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.-A.; Engström, G.; Christensson, A.; Nilsson, P.M.; Melander, O.; Orho-Melander, M. Genetic Predisposition for Renal Dysfunction and Incidence of CKD in the Malmö Diet and Cancer Study. Kidney Int. Rep. 2019, 4, 1143–1151. [Google Scholar] [CrossRef] [PubMed]
- Wunnenburger, S.; Schultheiss, U.T.; Walz, G.; Hausknecht, B.; Ekici, A.B.; Kronenberg, F.; Eckardt, K.-U.; Köttgen, A.; Wuttke, M. Associations between Genetic Risk Variants for Kidney Diseases and Kidney Disease Etiology. Sci. Rep. 2017, 7, 13944. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, L.; He, K.; Gao, B.; Wang, F.; Zhao, M.; Zhang, L.; on behalf of the Chinese Cohort Study of Chronic Kidney Disease (C-STRIDE). UMOD Polymorphisms Associated with Kidney Function, Serum Uromodulin and Risk of Mortality among Patients with Chronic Kidney Disease, Results from the C-STRIDE Study. Genes 2021, 12, 1687. [Google Scholar] [CrossRef]
- Su, S.J.; Chang, K.L.; Lin, T.M.; Huang, Y.H.; Yeh, T.M. Uromodulin and Tamm-Horsfall Protein Induce Human Monocytes to Secrete TNF and Express Tissue Factor. J. Immunol. 1997, 158, 3449–3456. [Google Scholar] [CrossRef]
- Schaeffer, C.; Devuyst, O.; Rampoldi, L. Uromodulin: Roles in Health and Disease. Annu. Rev. Physiol. 2021, 83, 477–501. [Google Scholar] [CrossRef]
- Han, J.; Liu, Y.; Rao, F.; Nievergelt, C.M.; O’Connor, D.T.; Wang, X.; Liu, L.; Bu, D.; Liang, Y.; Wang, F.; et al. Common Genetic Variants of the Human Uromodulin Gene Regulate Transcription and Predict Plasma Uric Acid Levels. Kidney Int. 2013, 83, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, S.; Iyoda, M.; Suzuki, T.; Kanazawa, N.; Honda, H. Serum Uromodulin Levels Reflect Severity of Clinicopathological Findings in Early Stage IgA Nephropathy. Am. J. Nephrol. 2022, 53, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Chen, Y.; Liu, Y.; Shi, S.; Wang, S.; Li, X.; Zhang, H.; Wang, H. Urinary Uromodulin Excretion Predicts Progression of Chronic Kidney Disease Resulting from IgA Nephropathy. PLoS ONE 2013, 8, e71023. [Google Scholar] [CrossRef] [PubMed]
- Obara, T.; Mizoguchi, S.; Shimozuru, Y.; Sato, T.; Hotta, O. The Complex of Immunoglobulin A and Uromodulin as a Diagnostic Marker for Immunoglobulin A Nephropathy. Clin. Exp. Nephrol. 2012, 16, 713–721. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, N.; Wang, J.; Xie, Y.; Li, Y.; Liang, T.; Wang, J.; Yin, Z.; He, K.; Chen, X. Identification of a Uromodulin Fragment for Diagnosis of IgA Nephropathy: IgA Nephropathy Diagnosis with a Uromodulin Fragment. Rapid Commun. Mass. Spectrom. 2010, 24, 1971–1978. [Google Scholar] [CrossRef] [PubMed]
- Rudnicki, M.; Siwy, J.; Wendt, R.; Lipphardt, M.; Koziolek, M.J.; Maixnerova, D.; Peters, B.; Kerschbaum, J.; Leierer, J.; Neprasova, M.; et al. Urine Proteomics for Prediction of Disease Progression in Patients with IgA Nephropathy. Nephrol. Dial. Transplant. 2021, 37, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Neprasova, M.; Maixnerova, D.; Novak, J.; Reily, C.; Julian, B.A.; Boron, J.; Novotny, P.; Suchanek, M.; Tesar, V.; Kacer, P. Toward Noninvasive Diagnosis of IgA Nephropathy: A Pilot Urinary Metabolomic and Proteomic Study. Dis. Markers 2016, 2016, 3650909. [Google Scholar] [CrossRef] [PubMed]
- Schiel, R.; Block, M.; Steveling, A.; Stein, G.; Lücking, S.; Scherberich, J. Serum Uromodulin in Children and Adolescents with Type 1 Diabetes Mellitus and Controls: Its Potential Role in Kidney Health. Exp. Clin. Endocrinol. Diabetes 2023, 131, 142–152. [Google Scholar] [CrossRef]
- Wiromrat, P.; Bjornstad, P.; Roncal, C.; Pyle, L.; Johnson, R.J.; Cherney, D.Z.; Lipina, T.; Bishop, F.; Maahs, D.M.; Wadwa, R.P. Serum Uromodulin Is Associated with Urinary Albumin Excretion in Adolescents with Type 1 Diabetes. J. Diabetes Complicat. 2019, 33, 648–650. [Google Scholar] [CrossRef]
- Bjornstad, P.; Singh, S.K.; Snell-Bergeon, J.K.; Lovshin, J.A.; Lytvyn, Y.; Lovblom, L.E.; Rewers, M.J.; Boulet, G.; Lai, V.; Tse, J.; et al. The Relationships between Markers of Tubular Injury and Intrarenal Haemodynamic Function in Adults with and without Type 1 Diabetes: Results from the Canadian Study of Longevity in Type 1 Diabetes. Diabetes Obes. Metab. 2019, 21, 575–583. [Google Scholar] [CrossRef]
- Catalano, C.; Torffvit, O. Urinary Excretion of Tamm-Horsfall Protein in Normotensive, Normo-Albuminuric Type 1 Diabetic Patients. Nephron 1996, 72, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Pfleiderer, S.; Zimmerhackl, L.B.; Kinne, R.; Manz, F.; Schuler, G.; Brandis, M. Renal Proximal and Distal Tubular Function Is Attenuated in Diabetes Mellitus Type 1 as Determined by the Renal Excretion of α1-Microglobulin and Tamm-Horsfall Protein. Clin. Investig. 1993, 71, 972–977. [Google Scholar] [CrossRef] [PubMed]
- Zimmerhackl, L.B.; Pfleiderer, S.; Kinne, R.; Manz, F.; Schuler, G.; Brandis, M. Tamm-Horsfall-Protein Excretion as a Marker of Ascending Limb Transport Indicates Early Renal Tubular Damage in Diabetes Mellitus Type I. J. Diabet. Complicat. 1991, 5, 112–114. [Google Scholar] [CrossRef] [PubMed]
- Van, J.A.D.; Clotet-Freixas, S.; Zhou, J.; Batruch, I.; Sun, C.; Glogauer, M.; Rampoldi, L.; Elia, Y.; Mahmud, F.H.; Sochett, E.; et al. Peptidomic Analysis of Urine from Youths with Early Type 1 Diabetes Reveals Novel Bioactivity of Uromodulin Peptides In Vitro. Mol. Cell. Proteom. 2020, 19, 501–517. [Google Scholar] [CrossRef] [PubMed]
- Torffvit, O. Urinary Sulphated Glycosaminoglycans and Tamm-Horsfall Protein in Type 1 Diabetic Patients. Scand. J. Urol. Nephrol. 1999, 33, 328–332. [Google Scholar] [CrossRef] [PubMed]
- Torffvit, O.; Agardh, C.-D.; Thulin, T. A Study of Tamm-Horsfall Protein Excretion in Hypertensive Patients and Type 1 Diabetic Patients. Scand. J. Urol. Nephrol. 1999, 33, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Sejdiu, I.; Torffvit, O. Decreased Urinary Concentration of Tamm-Horsfall Protein Is Associated with Development of Renal Failure and Cardiovascular Death within 20 Years in Type 1 but Not in Type 2 Diabetic Patients. Scand. J. Urol. Nephrol. 2008, 42, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Bjornstad, P.; Wiromrat, P.; Johnson, R.J.; Sippl, R.; Cherney, D.Z.I.; Wong, R.; Rewers, M.J.; Snell-Bergeon, J.K. Serum Uromodulin Predicts Less Coronary Artery Calcification and Diabetic Kidney Disease Over 12 Years in Adults With Type 1 Diabetes: The CACTI Study. Diabetes Care 2019, 42, 297–302. [Google Scholar] [CrossRef]
- Abbott, K.C.; Bakris, G.L. Cardiology Patient Page. Kidney Failure and Cardiovascular Disease. Circulation 2003, 108, e114–e115. [Google Scholar] [CrossRef]
- Cruz, D.N.; Bagshaw, S.M. Heart-Kidney Interaction: Epidemiology of Cardiorenal Syndromes. Int. J. Nephrol. 2010, 2011, 351291. [Google Scholar] [CrossRef]
- Orchard, T.J.; Costacou, T.; Kretowski, A.; Nesto, R.W. Type 1 Diabetes and Coronary Artery Disease. Diabetes Care 2006, 29, 2528–2538. [Google Scholar] [CrossRef] [PubMed]
- Burke, A.P.; Kolodgie, F.D.; Zieske, A.; Fowler, D.R.; Weber, D.K.; Varghese, P.J.; Farb, A.; Virmani, R. Morphologic Findings of Coronary Atherosclerotic Plaques in Diabetics: A Postmortem Study. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 1266–1271. [Google Scholar] [CrossRef] [PubMed]
- Mautner, S.L.; Lin, F.; Roberts, W.C. Composition of Atherosclerotic Plaques in the Epicardial Coronary Arteries in Juvenile (Type I) Diabetes Mellitus. Am. J. Cardiol. 1992, 70, 1264–1268. [Google Scholar] [CrossRef] [PubMed]
- Moreno, P.R.; Murcia, A.M.; Palacios, I.F.; Leon, M.N.; Bernardi, V.H.; Fuster, V.; Fallon, J.T. Coronary Composition and Macrophage Infiltration in Atherectomy Specimens from Patients with Diabetes Mellitus. Circulation 2000, 102, 2180–2184. [Google Scholar] [CrossRef] [PubMed]
- Spagnoli, L.G.; Mauriello, A.; Palmieri, G.; Santeusanio, G.; Amante, A.; Taurino, M. Relationships between Risk Factors and Morphological Patterns of Human Carotid Atherosclerotic Plaques. A Multivariate Discriminant Analysis. Atherosclerosis 1994, 108, 39–60. [Google Scholar] [CrossRef] [PubMed]
- Scheurlen, K.M.; Billeter, A.T.; Kopf, S.; Herbst, V.; Block, M.; Nawroth, P.P.; Zeier, M.; Scherberich, J.E.; Müller-Stich, B.P. Serum Uromodulin and Roux-En-Y Gastric Bypass: Improvement of a Marker Reflecting Nephron Mass. Surg. Obes. Relat. Dis. 2019, 15, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.M.; Murakami, T.; Oakes, M.L.; Mitsuhashi, M.; Kelly, C.; Henry, R.R.; Sharma, K. Uromodulin mRNA from Urinary Extracellular Vesicles Correlate to Kidney Function Decline in Type 2 Diabetes Mellitus. Am. J. Nephrol. 2018, 47, 283–291. [Google Scholar] [CrossRef]
- Lou, N.; Ni, Y.; Jia, H.; Deng, J.; Jiang, L.; Zheng, F.; Sun, A. Urinary Microvesicle-Bound Uromodulin: A Potential Molecular Biomarker in Diabetic Kidney Disease. J. Diabetes Res. 2017, 2017, 3918681. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chen, C.-Y.; Huang, C.-H.; Wu, C.-L.; Wu, H.-M.; Chiu, P.-F.; Kor, C.-T.; Chen, T.-H.; Chang, G.-D.; Kuo, C.-C.; et al. Urinary Glycated Uromodulin in Diabetic Kidney Disease. Clin. Sci. 2017, 131, 1815–1829. [Google Scholar] [CrossRef]
- Möllsten, A.; Torffvit, O. Tamm–Horsfall Protein Gene Is Associated with Distal Tubular Dysfunction in Patients with Type 1 Diabetes. Scand. J. Urol. Nephrol. 2010, 44, 438–444. [Google Scholar] [CrossRef]
- Ahluwalia, T.S.; Lindholm, E.; Groop, L.; Melander, O. Uromodulin Gene Variant Is Associated with Type 2 Diabetic Nephropathy. J. Hypertens. 2011, 29, 1731–1734. [Google Scholar] [CrossRef] [PubMed]
- Prudente, S.; Di Paola, R.; Copetti, M.; Lucchesi, D.; Lamacchia, O.; Pezzilli, S.; Mercuri, L.; Alberico, F.; Giusti, L.; Garofolo, M.; et al. The Rs12917707 Polymorphism at the UMOD Locus and Glomerular Filtration Rate in Individuals with Type 2 Diabetes: Evidence of Heterogeneity across Two Different European Populations. Nephrol. Dial. Transplant. 2017, 32, 1718–1722. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.; Ma, J.; Keaton, J.M.; Dimitrov, L.; Mudgal, P.; Stromberg, M.; Bonomo, J.A.; Hicks, P.J.; Freedman, B.I.; Bowden, D.W.; et al. Association of Kidney Structure-Related Gene Variants with Type 2 Diabetes-Attributed End-Stage Kidney Disease in African Americans. Hum. Genet. 2016, 135, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Yadav, A.; Kumar, V.; Bhansali, A.; Jha, V. Uromodulin Rs4293393 T>C Variation Is Associated with Kidney Disease in Patients with Type 2 Diabetes. Indian J. Med. Res. 2017, 146, 15. [Google Scholar] [CrossRef]
- Hanly, J.G.; O’Keeffe, A.G.; Su, L.; Urowitz, M.B.; Romero-Diaz, J.; Gordon, C.; Bae, S.-C.; Bernatsky, S.; Clarke, A.E.; Wallace, D.J.; et al. The Frequency and Outcome of Lupus Nephritis: Results from an International Inception Cohort Study. Rheumatology 2016, 55, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Norby, G.E.; Mjøen, G.; Bjørneklett, R.; Vikse, B.E.; Holdaas, H.; Svarstad, E.; Aasarød, K. Outcome in Biopsy-Proven Lupus Nephritis: Evaluation of Biopsies from the Norwegian Kidney Biopsy Registry. Lupus 2017, 26, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Teh, C.L.; Phui, V.E.; Ling, G.R.; Ngu, L.-S.; Wan, S.A.; Tan, C.H.-H. Causes and Predictors of Mortality in Biopsy-Proven Lupus Nephritis: The Sarawak Experience. Clin. Kidney J. 2018, 11, 56–61. [Google Scholar] [CrossRef]
- Rovin, B.H.; Parikh, S.V. Lupus Nephritis: The Evolving Role of Novel Therapeutics. Am. J. Kidney Dis. 2014, 63, 677–690. [Google Scholar] [CrossRef]
- Kostopoulou, M.; Fanouriakis, A.; Cheema, K.; Boletis, J.; Bertsias, G.; Jayne, D.; Boumpas, D.T. Management of Lupus Nephritis: A Systematic Literature Review Informing the 2019 Update of the Joint EULAR and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) Recommendations. RMD Open 2020, 6, e001263. [Google Scholar] [CrossRef]
- David, B.-L.; Ivan, G.-N.J.; Emilio, P.-G.E.; Daniela, M.-S.J.; Betsabe, C.-H.; Luisa, V.-V.M.; Selene, F.-R.N.; Guadalupe, A.-C.E.A.; Miriam, S.-C.A.; Alfredo, C.; et al. Low Serum Uromodulin Levels and Their Association with Lupus Flares. PLoS ONE 2022, 17, e0276481. [Google Scholar] [CrossRef]
- Boenisch, M.; Hurst, R.; Huber, S.; Koehn, J.; Krapfenbauer, K. Improved Prognostic Diagnosis of Systemic Lupus Erythematosus in an Early Stage of Disease by a Combination of Different Predictive Biomarkers Identified by Proteome Analysis. EPMA J. 2014, 5, 5. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Liu, M.; Pei, F.; Yu, L.; Yang, X. Role of Uromodulin and Complement Activation in the Progression of Kidney Disease. Oncol. Lett. 2021, 22, 829. [Google Scholar] [CrossRef] [PubMed]
- Bedair, R.N.; Amin Ismail, M.M.; Gaber, E.W.; Kader Mahmoud, R.A.; Mowafy, M.N. Study of the Relationship between Urinary Level of Uromodulin, Renal Involvement and Disease Activity in Patients with Systemic Lupus Erythrematosus. Saudi J. Kidney Dis. Transpl. 2020, 31, 32–43. [Google Scholar] [CrossRef]
- Tsai, C.-Y.; Wu, T.-H.; Yu, C.-L.; Lu, J.-Y.; Tsai, Y.-Y. Increased Excretions of β2-Microglobulin, IL-6, and IL-8 and Decreased Excretion of Tamm-Horsfall Glycoprotein in Urine of Patients with Active Lupus Nephritis. Nephron 2000, 85, 207–214. [Google Scholar] [CrossRef]
- Tachibana, S.; Iyoda, M.; Suzuki, T.; Kanazawa, N.; Iseri, K.; Wada, Y.; Matsumoto, K.; Shibata, T. Serum Uromodulin Is Associated with the Severity of Clinicopathological Findings in ANCA-Associated Glomerulonephritis. PLoS ONE 2019, 14, e0224690. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.-H.; Hsieh, S.-C.; Li, K.-J.; Wu, C.-H.; Yu, C.-L.; Yang, A.-H.; Tsai, C.-Y. Altered Glycosylation of Tamm-Horsfall Glycoprotein Derived from Renal Allograft Recipients Leads to Changes in Its Biological Function. Transpl. Immunol. 2008, 18, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, D.C.; Hinsman, E.J.; Rhodes, J.A. Tamm-Horsfall Glycoprotein Binds IgG with High Affinity. Kidney Int. 1993, 44, 1014–1021. [Google Scholar] [CrossRef]
- Schmid, M.; Prajczer, S.; Gruber, L.N.; Bertocchi, C.; Gandini, R.; Pfaller, W.; Jennings, P.; Joannidis, M. Uromodulin Facilitates Neutrophil Migration across Renal Epithelial Monolayers. Cell. Physiol. Biochem. 2010, 26, 311–318. [Google Scholar] [CrossRef]
- Wai-Hoe, L.; Wing-Seng, L.; Ismail, Z.; Lay-Harn, G. Proteomics and Detection of Uromodulin in First-Time Renal Calculi Patients and Recurrent Renal Calculi Patients. Appl. Biochem. Biotechnol. 2009, 159, 221–232. [Google Scholar] [CrossRef]
- Romero, M.C.; Nocera, S.; Nesse, A.B. Decreased Tamm-Horsfall Protein in Lithiasic Patients. Clin. Biochem. 1997, 30, 63–67. [Google Scholar] [CrossRef]
- Glauser, A.; Hochreiter, W.; Jaeger, P.; Hess, B. Determinants of Urinary Excretion of Tamm–Horsfall Protein in Non-selected Kidney Stone Formers and Healthy Subjects. Nephrol. Dial. Transplant. 2000, 15, 1580–1587. [Google Scholar] [CrossRef] [PubMed]
- Argade, S.; Chen, T.; Shaw, T.; Berecz, Z.; Shi, W.; Choudhury, B.; Lowell Parsons, C.; Sur, R.L. An Evaluation of Tamm–Horsfall Protein Glycans in Kidney Stone Formers Using Novel Techniques. Urolithiasis 2015, 43, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, P.; Rimer, J.D.; Kolbach, A.M.; Ward, M.D.; Kleinman, J.G.; Wesson, J.A. Calcium Oxalate Monohydrate Aggregation Induced by Aggregation of Desialylated Tamm-Horsfall Protein. Urol. Res. 2011, 39, 269–282. [Google Scholar] [CrossRef] [PubMed]
- Grant, A.M.; Baker, L.R.; Neuberger, A. Urinary Tamm-Horsfall Glycoprotein in Certain Kidney Diseases and Its Content in Renal and Bladder Calculi. Clin. Sci. 1973, 44, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Shen, S.J. The Role of Tamm-Horsfall Mucoprotein in Calcium Oxalate Crystallization. N-Acetylcysteine-a New Therepy for Calcium Oxalate Urolithiasis. Br. J. Urol. 1994, 74, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Jaggi, M.; Nakagawa, Y.; Zipperle, L.; Hess, B. Tamm-Horsfall Protein in Recurrent Calcium Kidney Stone Formers with Positive Family History: Abnormalities in Urinary Excretion, Molecular Structure and Function. Urol. Res. 2007, 35, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Chaiyarit, S.; Thongboonkerd, V. Oxidized Forms of Uromodulin Promote Calcium Oxalate Crystallization and Growth, but Not Aggregation. Int. J. Biol. Macromol. 2022, 214, 542–553. [Google Scholar] [CrossRef] [PubMed]
- Hess, B. Tamm-Horsfall Glycoprotein—Inhibitor or Promoter of Calcium Oxalate Monohydrate Crystallization Processes? Urol. Res. 1992, 20, 83–86. [Google Scholar] [CrossRef]
- Noonin, C.; Peerapen, P.; Yoodee, S.; Kapincharanon, C.; Kanlaya, R.; Thongboonkerd, V. Systematic Analysis of Modulating Activities of Native Human Urinary Tamm-Horsfall Protein on Calcium Oxalate Crystallization, Growth, Aggregation, Crystal-Cell Adhesion and Invasion through Extracellular Matrix. Chem.-Biol. Interact. 2022, 357, 109879. [Google Scholar] [CrossRef]
- Liu, Y.; Mo, L.; Goldfarb, D.S.; Evan, A.P.; Liang, F.; Khan, S.R.; Lieske, J.C.; Wu, X.-R. Progressive Renal Papillary Calcification and Ureteral Stone Formation in Mice Deficient for Tamm-Horsfall Protein. Am. J. Physiol. Renal Physiol. 2010, 299, F469–F478. [Google Scholar] [CrossRef]
- Mo, L.; Huang, H.-Y.; Zhu, X.-H.; Shapiro, E.; Hasty, D.L.; Wu, X.-R. Tamm-Horsfall Protein Is a Critical Renal Defense Factor Protecting against Calcium Oxalate Crystal Formation. Kidney Int. 2004, 66, 1159–1166. [Google Scholar] [CrossRef] [PubMed]
- Mo, L.; Liaw, L.; Evan, A.P.; Sommer, A.J.; Lieske, J.C.; Wu, X.-R. Renal Calcinosis and Stone Formation in Mice Lacking Osteopontin, Tamm-Horsfall Protein, or Both. Am. J. Physiol. Renal Physiol. 2007, 293, F1935–F1943. [Google Scholar] [CrossRef] [PubMed]
- Fuselier, H.A.; Ward, D.M.; Lindberg, J.S.; Allen, J.M.; Husserl, F.E.; Marcucci, P.A.; Cole, F.E.; Turnipseed, J.; Alam, J.; Kok, D.J.; et al. Urinary Tamm-Horsfall Protein Increased after Potassium Citrate Therapy in Calcium Stone Formers. Urology 1995, 45, 942–946. [Google Scholar] [CrossRef] [PubMed]
- Patel, Y.P.; Patel, S.G.; Patel, S.B.; Parikh, A.; Soni, S.; Srivastava, R.; Raval, C.; Raval, M.A.; Nand Pandey, S.; Ganpule, A.P.; et al. SPP1 and UMOD Gene Variants Are Synergistically Associated with Risk of Renal Stone Disease. Gene 2023, 863, 147264. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.-H.; He, J.-Q.; Qin, W.-W.; Zhao, Y.-Y.; Tan, N.-H. Biomarkers of Obstructive Nephropathy Using a Metabolomics Approach in Rat. Chem. Biol. Interact. 2018, 296, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Mezzano, S.A.; Ruiz-Ortega, M.; Egido, J. Angiotensin II and Renal Fibrosis. Hypertension 2001, 38, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Rüster, C.; Wolf, G. Renin-Angiotensin-Aldosterone System and Progression of Renal Disease. J. Am. Soc. Nephrol. 2006, 17, 2985–2991. [Google Scholar] [CrossRef]
- Chevalier, R.L. Chronic Partial Ureteral Obstruction in the Neonatal Guinea Pig. II. Pressure Gradients Affecting Glomerular Filtration Rate. Pediatr. Res. 1984, 18, 1271–1277. [Google Scholar] [CrossRef]
- Mao, W.; Liu, S.; Wang, K.; Wang, M.; Shi, H.; Liu, Q.; Bao, M.; Peng, B.; Geng, J. Cystatin C in Evaluating Renal Function in Ureteral Calculi Hydronephrosis in Adults. Kidney Blood Press. Res. 2020, 45, 109–121. [Google Scholar] [CrossRef]
- Washino, S.; Hosohata, K.; Miyagawa, T. Roles Played by Biomarkers of Kidney Injury in Patients with Upper Urinary Tract Obstruction. Int. J. Mol. Sci. 2020, 21, 5490. [Google Scholar] [CrossRef]
- Dziukas, L.J.; Sterzel, R.B.; Hodson, C.J.; Hoyer, J.R. Renal Localization of Tamm-Horsfall Protein in Unilateral Obstructive Uropathy in Rats. Lab. Investig. 1982, 47, 185–193. [Google Scholar] [PubMed]
- Vukmirović Papuga, M.; Bukumirić, Z.; Ilinčić, B.; Mijović, R.; Šašić Ostojić, T.; Žeravica, R. Serum Uromodulin, a Potential Biomarker of Tubulointerstitial Damage, Correlates Well with Measured GFR and ERPF in Patients with Obstructive Nephropathy. Medicina 2022, 58, 1729. [Google Scholar] [CrossRef]
- Garin, E.H. Primary Vesicoureteral Reflux; What Have We Learnt from the Recently Published Randomized, Controlled Trials? Pediatr. Nephrol. 2019, 34, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Tullus, K. Vesicoureteric Reflux in Children. Lancet 2015, 385, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Ammenti, A.; Alberici, I.; Brugnara, M.; Chimenz, R.; Guarino, S.; La Manna, A.; La Scola, C.; Maringhini, S.; Marra, G.; Materassi, M.; et al. Updated Italian Recommendations for the Diagnosis, Treatment and Follow-up of the First Febrile Urinary Tract Infection in Young Children. Acta Paediatr. 2020, 109, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Breinbjerg, A.; Jørgensen, C.S.; Frøkiær, J.; Tullus, K.; Kamperis, K.; Rittig, S. Risk Factors for Kidney Scarring and Vesicoureteral Reflux in 421 Children after Their First Acute Pyelonephritis, and Appraisal of International Guidelines. Pediatr. Nephrol. 2021, 36, 2777–2787. [Google Scholar] [CrossRef] [PubMed]
- Hewitt, I.K.; Montini, G. Kidney Damage Associated with Vesico Ureteric Reflux. Curr. Opin. Pediatr. 2021, 33, 247–251. [Google Scholar] [CrossRef]
- Andriole, V.T. The Role of Tamm-Horsfall Protein in the Pathogenesis of Reflux Nephropathy and Chronic Pyelonephritis. Yale J. Biol. Med. 1985, 58, 91–100. [Google Scholar]
- Lynn, K.L.; Bailey, R.R.; Groufsky, A.; Hunt, J.S.; Bean, A.R.; McGiven, A.R. 23. Antibodies to Tamm-Horsfall Urinary Glycoprotein in Patients with Urinary Tract Infection, Reflux Nephropathy, Urinary Obstruction and Paraplegia. In Reflux Nephropathy Update: 1983; Hodson, C.J., Hepinstall, R.H., Winberg, J., Eds.; Contributions to Nephrology; S. Karger AG: Basel, Switzerland, 1984; Volume 39, pp. 296–304. ISBN 978-3-8055-3807-7. [Google Scholar]
- Akioka, Y.; Chikamoto, H.; Horita, S.; Yago, R.; Tanabe, K.; Yamaguchi, Y.; Hattori, M. Screening of Vesicoureteral Reflux in Pediatric Patients with Kidney Transplantation Showing Non-Specific Interstitial Fibrosis and Tubular Atrophy with Interstitial Tamm-Horsfall Protein Deposits in Protocol Allograft Biopsy. Clin. Transplant. 2009, 23, 2–5. [Google Scholar] [CrossRef]
- Uto, I.; Ishimatsu, T.; Hirayama, H.; Ueda, S.; Nishi, K.; Tsuruta, J.; Kambara, T. Urinary Tamm-Horsfall Protein Excretion in Patients with Primary Vesicoureteral Reflux. Eur. Urol. 1991, 19, 315–318. [Google Scholar] [CrossRef]
- Maringhini, S.; Cusumano, R.; Corrado, C.; Puccio, G.; Pavone, G.; D’Alessandro, M.M.; Sapia, M.C.; Devuyst, O.; Abbate, S. Uromodulin and Vesico-Ureteral Reflux: A Genetic Study. Biomedicines 2023, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Joseph, C.B.; Mariniello, M.; Yoshifuji, A.; Schiano, G.; Lake, J.; Marten, J.; Richmond, A.; Huffman, J.E.; Campbell, A.; Harris, S.E.; et al. Meta-GWAS Reveals Novel Genetic Variants Associated with Urinary Excretion of Uromodulin. J. Am. Soc. Nephrol. 2022, 33, 511–529. [Google Scholar] [CrossRef] [PubMed]
- Rampoldi, L. Allelism of MCKD, FJHN and GCKD Caused by Impairment of Uromodulin Export Dynamics. Human. Mol. Genet. 2003, 12, 3369–3384. [Google Scholar] [CrossRef] [PubMed]
- Lens, X.M.; Banet, J.F.; Outeda, P.; Barrio-Lucía, V. A Novel Pattern of Mutation in Uromodulin Disorders: Autosomal Dominant Medullary Cystic Kidney Disease Type 2, Familial Juvenile Hyperuricemic Nephropathy, and Autosomal Dominant Glomerulocystic Kidney Disease. Am. J. Kidney Dis. 2005, 46, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Massari, P.U.; Hsu, C.H.; Barnes, R.V.; Fox, I.H.; Gikas, P.W.; Weller, J.M. Familial Hyperuricemia and Renal Disease. Arch. Intern. Med. 1980, 140, 680–684. [Google Scholar] [CrossRef] [PubMed]
- Thompson, G.R.; Weiss, J.J.; Goldman, R.T.; Rigg, G.A. Familial Occurrence of Hyperuricemia, Gout, and Medullary Cystic Disease. Arch. Intern. Med. 1978, 138, 1614–1617. [Google Scholar] [CrossRef] [PubMed]
- Schaeffer, C.; Cattaneo, A.; Trudu, M.; Santambrogio, S.; Bernascone, I.; Giachino, D.; Caridi, G.; Campo, A.; Murtas, C.; Benoni, S.; et al. Urinary Secretion and Extracellular Aggregation of Mutant Uromodulin Isoforms. Kidney Int. 2012, 81, 769–778. [Google Scholar] [CrossRef]
- Dahan, K.; Fuchshuber, A.; Adamis, S.; Smaers, M.; Kroiss, S.; Loute, G.; Cosyns, J.-P.; Hildebrandt, F.; Verellen-Dumoulin, C.; Pirson, Y. Familial Juvenile Hyperuricemic Nephropathy and Autosomal Dominant Medullary Cystic Kidney Disease Type 2: Two Facets of the Same Disease? J. Am. Soc. Nephrol. 2001, 12, 2348–2357. [Google Scholar] [CrossRef]
- Hateboer, N.; Gumbs, C.; Teare, M.D.; Coles, G.A.; Griffiths, D.; Ravine, D.; Futreal, P.A.; Rahman, N. Confirmation of a Gene Locus for Medullary Cystic Kidney Disease (MCKD2) on Chromosome 16p12. Kidney Int. 2001, 60, 1233–1239. [Google Scholar] [CrossRef]
- Bernascone, I.; Vavassori, S.; Di Pentima, A.; Santambrogio, S.; Lamorte, G.; Amoroso, A.; Scolari, F.; Ghiggeri, G.M.; Casari, G.; Polishchuk, R.; et al. Defective Intracellular Trafficking of Uromodulin Mutant Isoforms. Traffic 2006, 7, 1567–1579. [Google Scholar] [CrossRef]
- Wolf, M.T.F.; Beck, B.B.; Zaucke, F.; Kunze, A.; Misselwitz, J.; Ruley, J.; Ronda, T.; Fischer, A.; Eifinger, F.; Licht, C.; et al. The Uromodulin C744G Mutation Causes MCKD2 and FJHN in Children and Adults and May Be Due to a Possible Founder Effect. Kidney Int. 2007, 71, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Vylet’al, P.; Kublová, M.; Kalbáčová, M.; Hodaňová, K.; Barešová, V.; Stibůrková, B.; Sikora, J.; Hůlková, H.; Živný, J.; Majewski, J.; et al. Alterations of Uromodulin Biology: A Common Denominator of the Genetically Heterogeneous FJHN/MCKD Syndrome. Kidney Int. 2006, 70, 1155–1169. [Google Scholar] [CrossRef] [PubMed]
- Rezende-Lima, W.; Parreira, K.S.; García-González, M.; Riveira, E.; Banet, J.F.; Lens, X.M. Homozygosity for Uromodulin Disorders: FJHN and MCKD-Type 2. Kidney Int. 2004, 66, 558–563. [Google Scholar] [CrossRef]
- Jennings, P.; Aydin, S.; Kotanko, P.; Lechner, J.; Lhotta, K.; Williams, S.; Thakker, R.V.; Pfaller, W. Membrane Targeting and Secretion of Mutant Uromodulin in Familial Juvenile Hyperuricemic Nephropathy. J. Am. Soc. Nephrol. 2007, 18, 264–273. [Google Scholar] [CrossRef]
- Lhotta, K.; Gehringer, A.; Jennings, P.; Kronenberg, F.; Brezinka, C.; Andersone, I.; Strazdins, V. Familial Juvenile Hyperuricemic Nephropathy: Report on a New Mutation and a Pregnancy. Clin. Nephrol. 2009, 71, 80–83. [Google Scholar] [CrossRef] [PubMed]
- Nasr, S.H.; Lucia, J.P.; Galgano, S.J.; Markowitz, G.S.; D’Agati, V.D. Uromodulin Storage Disease. Kidney Int. 2008, 73, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Adam, J.; Bollée, G.; Fougeray, S.; Noël, L.-H.; Antignac, C.; Knebelman, B.; Pallet, N. Endoplasmic Reticulum Stress in UMOD-Related Kidney Disease: A Human Pathologic Study. Am. J. Kidney Dis. 2012, 59, 117–121. [Google Scholar] [CrossRef]
- Kemter, E.; Rathkolb, B.; Rozman, J.; Hans, W.; Schrewe, A.; Landbrecht, C.; Klaften, M.; Ivandic, B.; Fuchs, H.; Gailus-Durner, V.; et al. Novel Missense Mutation of Uromodulin in Mice Causes Renal Dysfunction with Alterations in Urea Handling, Energy, and Bone Metabolism. Am. J. Physiol. Renal Physiol. 2009, 297, F1391–F1398. [Google Scholar] [CrossRef]
- Schröder, M.; Kaufman, R.J. ER Stress and the Unfolded Protein Response. Mutat. Res. 2005, 569, 29–63. [Google Scholar] [CrossRef]
- Choi, S.W.; Ryu, O.H.; Choi, S.J.; Song, I.S.; Bleyer, A.J.; Hart, T.C. Mutant Tamm-Horsfall Glycoprotein Accumulation in Endoplasmic Reticulum Induces Apoptosis Reversed by Colchicine and Sodium 4-Phenylbutyrate. J. Am. Soc. Nephrol. 2005, 16, 3006–3014. [Google Scholar] [CrossRef]
- Williams, S.E.; Reed, A.A.C.; Galvanovskis, J.; Antignac, C.; Goodship, T.; Karet, F.E.; Kotanko, P.; Lhotta, K.; Morinière, V.; Williams, P.; et al. Uromodulin Mutations Causing Familial Juvenile Hyperuricaemic Nephropathy Lead to Protein Maturation Defects and Retention in the Endoplasmic Reticulum. Hum. Mol. Genet. 2009, 18, 2963–2974. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Trachtman, H.; Sandhu, J.; Gorry, M.C.; Hart, T.C. Renal Manifestations of a Mutation in the Uromodulin (Tamm Horsfall Protein) Gene. Am. J. Kidney Dis. 2003, 42, e8.1–e8.7. [Google Scholar] [CrossRef] [PubMed]
- Devuyst, O.; Olinger, E.; Weber, S.; Eckardt, K.-U.; Kmoch, S.; Rampoldi, L.; Bleyer, A.J. Autosomal Dominant Tubulointerstitial Kidney Disease. Nat. Rev. Dis. Primers 2019, 5, 60. [Google Scholar] [CrossRef] [PubMed]
- Olinger, E.; Schaeffer, C.; Kidd, K.; Elhassan, E.A.E.; Cheng, Y.; Dufour, I.; Schiano, G.; Mabillard, H.; Pasqualetto, E.; Hofmann, P.; et al. An Intermediate-Effect Size Variant in UMOD Confers Risk for Chronic Kidney Disease. Proc. Natl. Acad. Sci. USA 2022, 119, e2114734119. [Google Scholar] [CrossRef] [PubMed]
- Dahan, K.; Devuyst, O.; Smaers, M.; Vertommen, D.; Loute, G.; Poux, J.-M.; Viron, B.; Jacquot, C.; Gagnadoux, M.-F.; Chauveau, D.; et al. A Cluster of Mutations in the UMOD Gene Causes Familial Juvenile Hyperuricemic Nephropathy with Abnormal Expression of Uromodulin. J. Am. Soc. Nephrol. 2003, 14, 2883–2893. [Google Scholar] [CrossRef] [PubMed]
- Olinger, E.; Hofmann, P.; Kidd, K.; Dufour, I.; Belge, H.; Schaeffer, C.; Kipp, A.; Bonny, O.; Deltas, C.; Demoulin, N.; et al. Clinical and Genetic Spectra of Autosomal Dominant Tubulointerstitial Kidney Disease Due to Mutations in UMOD and MUC1. Kidney Int. 2020, 98, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Bleyer, A.J.; Hart, T.C.; Shihabi, Z.; Robins, V.; Hoyer, J.R. Mutations in the Uromodulin Gene Decrease Urinary Excretion of Tamm-Horsfall Protein. Kidney Int. 2004, 66, 974–977. [Google Scholar] [CrossRef]
- Satanovskij, R.; Bader, A.; Block, M.; Herbst, V.; Schlumberger, W.; Haack, T.; Nockher, W.A.; Heemann, U.; Renders, L.; Schmaderer, C.; et al. A New Missense Mutation in UMOD Gene Leads to Severely Reduced Serum Uromodulin Concentrations—A Tool for the Diagnosis of Uromodulin-Associated Kidney Disease. Clin. Biochem. 2017, 50, 155–158. [Google Scholar] [CrossRef]
- Edwards, N.; Olinger, E.; Adam, J.; Kelly, M.; Schiano, G.; Ramsbottom, S.A.; Sandford, R.; Devuyst, O.; Sayer, J.A. A Novel Homozygous UMOD Mutation Reveals Gene Dosage Effects on Uromodulin Processing and Urinary Excretion. Nephrol. Dial. Transplant. 2017, 32, 1994–1999. [Google Scholar] [CrossRef]
- Labriola, L.; Olinger, E.; Belge, H.; Pirson, Y.; Dahan, K.; Devuyst, O. Paradoxical Response to Furosemide in Uromodulin-Associated Kidney Disease. Nephrol. Dial. Transplant. 2015, 30, 330–335. [Google Scholar] [CrossRef]
- Trudu, M.; Schaeffer, C.; Riba, M.; Ikehata, M.; Brambilla, P.; Messa, P.; Martinelli-Boneschi, F.; Rastaldi, M.P.; Rampoldi, L. Early Involvement of Cellular Stress and Inflammatory Signals in the Pathogenesis of Tubulointerstitial Kidney Disease Due to UMOD Mutations. Sci. Rep. 2017, 7, 7383. [Google Scholar] [CrossRef] [PubMed]
- Cansever, H.N.; Sari, F.; Cevikol, C.; Cetinkaya, R.; Süleymanlar, G.; Ersoy, F. Serum Uromodulin Levels, MR Imaging Findings, and Their Relationship with eGFR-Based CKD Staging in ADPKD Patients. Int. Urol. Nephrol. 2021, 53, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Petzold, K.; Poster, D.; Krauer, F.; Spanaus, K.; Andreisek, G.; Nguyen-Kim, T.D.L.; Pavik, I.; Ho, T.A.; Serra, A.L.; Rotar, L. Urinary Biomarkers at Early ADPKD Disease Stage. PLoS ONE 2015, 10, e0123555. [Google Scholar] [CrossRef] [PubMed]
- Bahl, D.; Haddad, Z.; Datoo, A.; Qazi, Y.A. Delayed Graft Function in Kidney Transplantation. Curr. Opin. Organ. Transplant. 2019, 24, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Perico, N.; Cattaneo, D.; Sayegh, M.H.; Remuzzi, G. Delayed Graft Function in Kidney Transplantation. Lancet 2004, 364, 1814–1827. [Google Scholar] [CrossRef] [PubMed]
- Yarlagadda, S.G.; Coca, S.G.; Garg, A.X.; Doshi, M.; Poggio, E.; Marcus, R.J.; Parikh, C.R. Marked Variation in the Definition and Diagnosis of Delayed Graft Function: A Systematic Review. Nephrol. Dial. Transplant. 2008, 23, 2995–3003. [Google Scholar] [CrossRef] [PubMed]
- Siedlecki, A.; Irish, W.; Brennan, D.C. Delayed Graft Function in the Kidney Transplant. Am. J. Transplant. 2011, 11, 2279–2296. [Google Scholar] [CrossRef]
- Schröppel, B.; Legendre, C. Delayed Kidney Graft Function: From Mechanism to Translation. Kidney Int. 2014, 86, 251–258. [Google Scholar] [CrossRef]
- Venkatachalam, M.A.; Weinberg, J.M.; Kriz, W.; Bidani, A.K. Failed Tubule Recovery, AKI-CKD Transition, and Kidney Disease Progression. J. Am. Soc. Nephrol. 2015, 26, 1765–1776. [Google Scholar] [CrossRef]
- Linkermann, A.; Stockwell, B.R.; Krautwald, S.; Anders, H.-J. Regulated Cell Death and Inflammation: An Auto-Amplification Loop Causes Organ Failure. Nat. Rev. Immunol. 2014, 14, 759–767. [Google Scholar] [CrossRef]
- Chawla, L.S.; Eggers, P.W.; Star, R.A.; Kimmel, P.L. Acute Kidney Injury and Chronic Kidney Disease as Interconnected Syndromes. N. Engl. J. Med. 2014, 371, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.R. Progress in Transplantation: Will It Be Achieved in Big Steps or by Marginal Gains? Am. J. Kidney Dis. 2017, 69, 287–295. [Google Scholar] [CrossRef] [PubMed]
- Lohkamp, L.-N.; Öllinger, R.; Chatzigeorgiou, A.; Illigens, B.M.-W.; Siepmann, T. Intraoperative Biomarkers in Renal Transplantation. Nephrology 2016, 21, 188–199. [Google Scholar] [CrossRef] [PubMed]
- Kemmner, S.; Holzmann-Littig, C.; Sandberger, H.; Bachmann, Q.; Haberfellner, F.; Torrez, C.; Schmaderer, C.; Heemann, U.; Renders, L.; Assfalg, V.; et al. Pretransplant Serum Uromodulin and Its Association with Delayed Graft Function Following Kidney Transplantation—A Prospective Cohort Study. J. Clin. Med. 2021, 10, 2586. [Google Scholar] [CrossRef] [PubMed]
- Borštnar, Š.; Večerić-Haler, Ž.; Boštjančič, E.; Pipan Tkalec, Ž.; Kovač, D.; Lindič, J.; Kojc, N. Uromodulin and microRNAs in Kidney Transplantation—Association with Kidney Graft Function. Int. J. Mol. Sci. 2020, 21, 5592. [Google Scholar] [CrossRef] [PubMed]
- Bostom, A.; Steubl, D.; Garimella, P.S.; Franceschini, N.; Roberts, M.B.; Pasch, A.; Ix, J.H.; Tuttle, K.R.; Ivanova, A.; Shireman, T.; et al. Serum Uromodulin: A Biomarker of Long-Term Kidney Allograft Failure. Am. J. Nephrol. 2018, 47, 275–282. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, P.J.; Alkawa, A.; Davies, H.M.; Ward, R.G.; Bakran, A.; Sells, R.A.; Johnson, P.M. Uromodulin Levels Are Decreased in Urine during Acute Tubular Necrosis but Not during Immune Rejection after Renal Transplantation. Clin. Sci. 1993, 84, 243–246. [Google Scholar] [CrossRef] [PubMed]
- Torffvit, O.; Kamper, A.-L.; Strandgaard, S. Tamm-Horsfall Protein in Urine After Uninephrectomy/Transplantation in Kidney Donors and Their Recipients. Scand. J. Urol. Nephrol. 1997, 31, 555–559. [Google Scholar] [CrossRef]
- Stokman, G.; Kers, J.; Yapici, Ü.; Hoelbeek, J.J.; Claessen, N.; de Boer, O.J.; Netea, M.G.; Hilbrands, L.; Bemelman, F.J.; Ten Berge, I.J.M.; et al. Predominant Tubular Interleukin-18 Expression in Polyomavirus-Associated Nephropathy. Transplantation 2016, 100, e88–e95. [Google Scholar] [CrossRef]
- Nankivell, B.J.; Borrows, R.J.; Fung, C.L.-S.; O’Connell, P.J.; Allen, R.D.M.; Chapman, J.R. The Natural History of Chronic Allograft Nephropathy. N. Engl. J. Med. 2003, 349, 2326–2333. [Google Scholar] [CrossRef]
- Wekerle, T.; Segev, D.; Lechler, R.; Oberbauer, R. Strategies for Long-Term Preservation of Kidney Graft Function. Lancet 2017, 389, 2152–2162. [Google Scholar] [CrossRef] [PubMed]
- Uslu, A.; Hür, E.; Şen, Ç.; Şen, S.; Akgün, A.; Taşlı, F.A.; Nart, A.; Yilmaz, M.; Töz, H. To What Extent Estimated or Measured GFR Could Predict Subclinical Graft Fibrosis: A Comparative Prospective Study with Protocol Biopsies. Transpl. Int. 2015, 28, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.; Svensson, M.; Tannæs, T.M.; Waldum-Grevbo, B.; Jenssen, T.; Eide, I.A. Associations of Serum Uromodulin and Urinary Epidermal Growth Factor with Measured Glomerular Filtration Rate and Interstitial Fibrosis in Kidney Transplantation. Am. J. Nephrol. 2022, 53, 108–117. [Google Scholar] [CrossRef]
- Lynn, K.L.; Shenkin, A.; Marshall, R.D. Factors Affecting Excretion of Human Urinary Tamm-Horsfall Glycoprotein. Clin. Sci. 1982, 62, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Schuurs, T.A.; Morariu, A.M.; Ottens, P.J.; ’t Hart, N.A.; Popma, S.H.; Leuvenink, H.G.D.; Ploeg, R.J. Time-Dependent Changes in Donor Brain Death Related Processes. Am. J. Transplant. 2006, 6, 2903–2911. [Google Scholar] [CrossRef] [PubMed]
- Devarajan, P. Update on Mechanisms of Ischemic Acute Kidney Injury. J. Am. Soc. Nephrol. 2006, 17, 1503–1520. [Google Scholar] [CrossRef] [PubMed]
- Mansour, S.G.; Puthumana, J.; Reese, P.P.; Hall, I.E.; Doshi, M.D.; Weng, F.L.; Schröppel, B.; Thiessen-Philbrook, H.; Bimali, M.; Parikh, C.R. Associations between Deceased-Donor Urine MCP-1 and Kidney Transplant Outcomes. Kidney Int. Rep. 2017, 2, 749–758. [Google Scholar] [CrossRef]
- Reese, P.P.; Hall, I.E.; Weng, F.L.; Schröppel, B.; Doshi, M.D.; Hasz, R.D.; Thiessen-Philbrook, H.; Ficek, J.; Rao, V.; Murray, P.; et al. Associations between Deceased-Donor Urine Injury Biomarkers and Kidney Transplant Outcomes. J. Am. Soc. Nephrol. 2016, 27, 1534–1543. [Google Scholar] [CrossRef]
- Basile, D.P.; Bonventre, J.V.; Mehta, R.; Nangaku, M.; Unwin, R.; Rosner, M.H.; Kellum, J.A.; Ronco, C. ADQI XIII Work Group Progression after AKI: Understanding Maladaptive Repair Processes to Predict and Identify Therapeutic Treatments. J. Am. Soc. Nephrol. 2016, 27, 687–697. [Google Scholar] [CrossRef]
- Puthumana, J.; Hall, I.E.; Reese, P.P.; Schröppel, B.; Weng, F.L.; Thiessen-Philbrook, H.; Doshi, M.D.; Rao, V.; Lee, C.G.; Elias, J.A.; et al. YKL-40 Associates with Renal Recovery in Deceased Donor Kidney Transplantation. J. Am. Soc. Nephrol. 2017, 28, 661–670. [Google Scholar] [CrossRef]
- Mansour, S.G.; Liu, C.; Jia, Y.; Reese, P.P.; Hall, I.E.; El-Achkar, T.M.; LaFavers, K.A.; Obeid, W.; Rosenberg, A.Z.; Daneshpajouhnejad, P.; et al. Uromodulin to Osteopontin Ratio in Deceased Donor Urine Is Associated With Kidney Graft Outcomes. Transplantation 2021, 105, 876–885. [Google Scholar] [CrossRef] [PubMed]
- Reznichenko, A.; Van Dijk, M.C.R.F.; Van Der Heide, J.H.; Bakker, S.J.L.; Seelen, M.; Navis, G. Uromodulin in Renal Transplant Recipients: Elevated Urinary Levels and Bimodal Association with Graft Failure. Am. J. Nephrol. 2011, 34, 445–451. [Google Scholar] [CrossRef] [PubMed]
- Vyletal, P.; Bleyer, A.J.; Kmoch, S. Uromodulin Biology and Pathophysiology—An Update. Kidney Blood Press. Res. 2010, 33, 456–475. [Google Scholar] [CrossRef] [PubMed]
- Drube, J.; Schiffer, E.; Mischak, H.; Kemper, M.J.; Neuhaus, T.; Pape, L.; Lichtinghagen, R.; Ehrich, J.H.H. Urinary Proteome Pattern in Children with Renal Fanconi Syndrome. Nephrol. Dial. Transplant. 2009, 24, 2161–2169. [Google Scholar] [CrossRef] [PubMed]
- Van’T Hoff, W.G. Molecular Developments in Renal Tubulopathies. Arch. Dis. Child. 2000, 83, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Masson, C.; Cissé, I.; Simon, V.; Insalaco, P.; Audran, M. Fabry Disease: A Review. Jt. Bone Spine 2004, 71, 381–383. [Google Scholar] [CrossRef] [PubMed]
- Vylet’al, P.; Hůlková, H.; Živná, M.; Berná, L.; Novák, P.; Elleder, M.; Kmoch, S. Abnormal Expression and Processing of Uromodulin in Fabry Disease Reflects Tubular Cell Storage Alteration and Is Reversible by Enzyme Replacement Therapy. J. Inherit. Metab. Dis. 2008, 31, 508–517. [Google Scholar] [CrossRef] [PubMed]
- Matafora, V.; Cuccurullo, M.; Beneduci, A.; Petrazzuolo, O.; Simeone, A.; Anastasio, P.; Mignani, R.; Feriozzi, S.; Pisani, A.; Comotti, C.; et al. Early Markers of Fabry Disease Revealed by Proteomics. Mol. BioSyst. 2015, 11, 1543–1551. [Google Scholar] [CrossRef]
- Doykov, I.D.; Heywood, W.E.; Nikolaenko, V.; Śpiewak, J.; Hällqvist, J.; Clayton, P.T.; Mills, P.; Warnock, D.G.; Nowak, A.; Mills, K. Rapid, Proteomic Urine Assay for Monitoring Progressive Organ Disease in Fabry Disease. J. Med. Genet. 2020, 57, 38–47. [Google Scholar] [CrossRef]
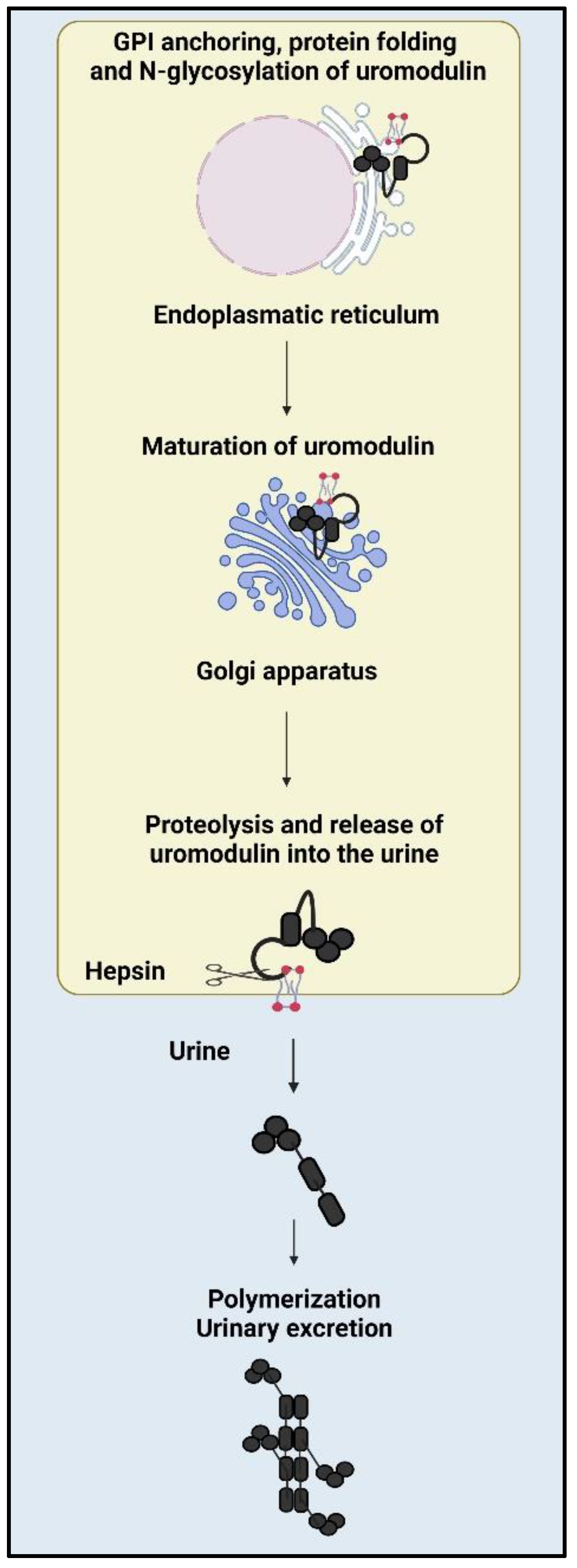
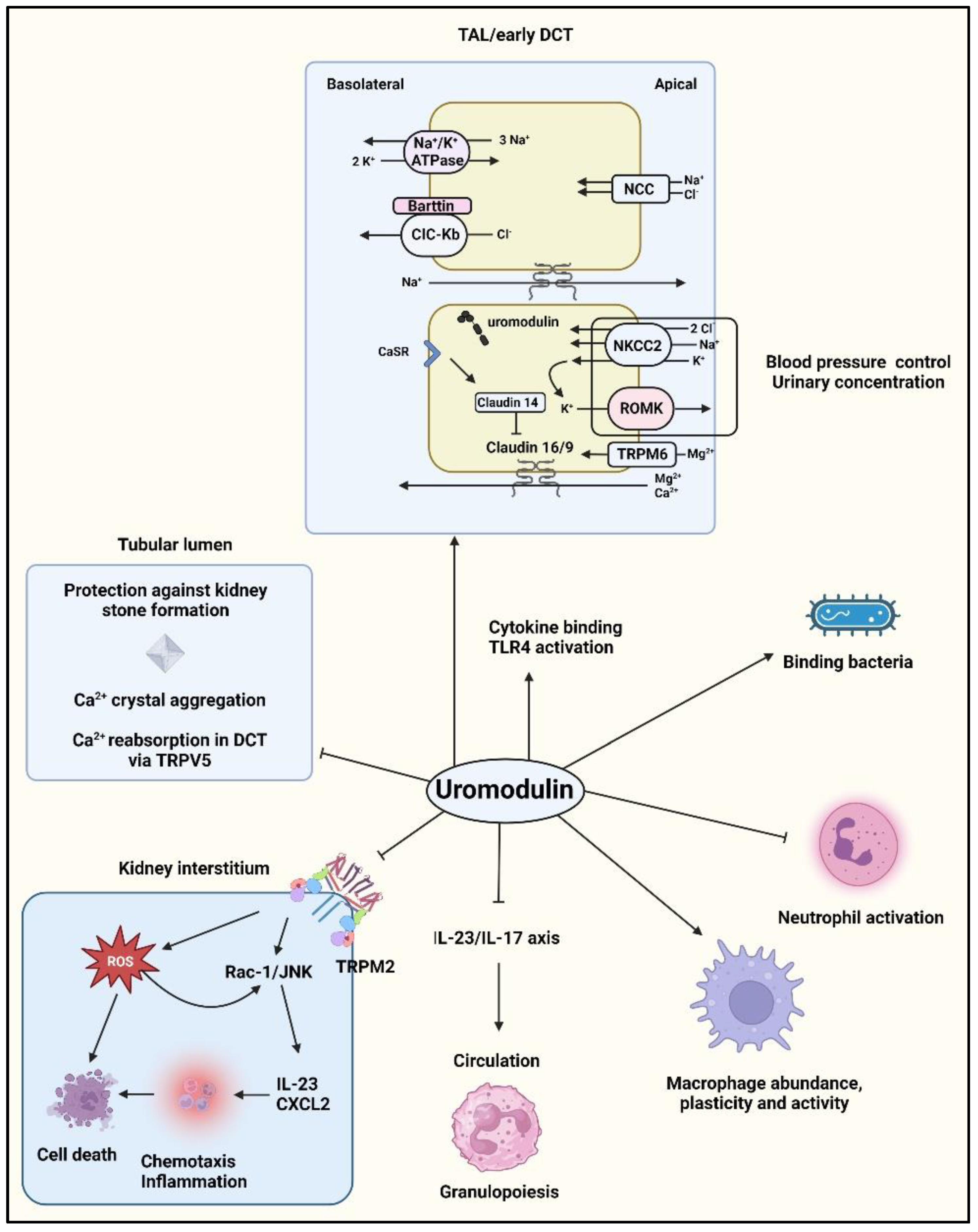
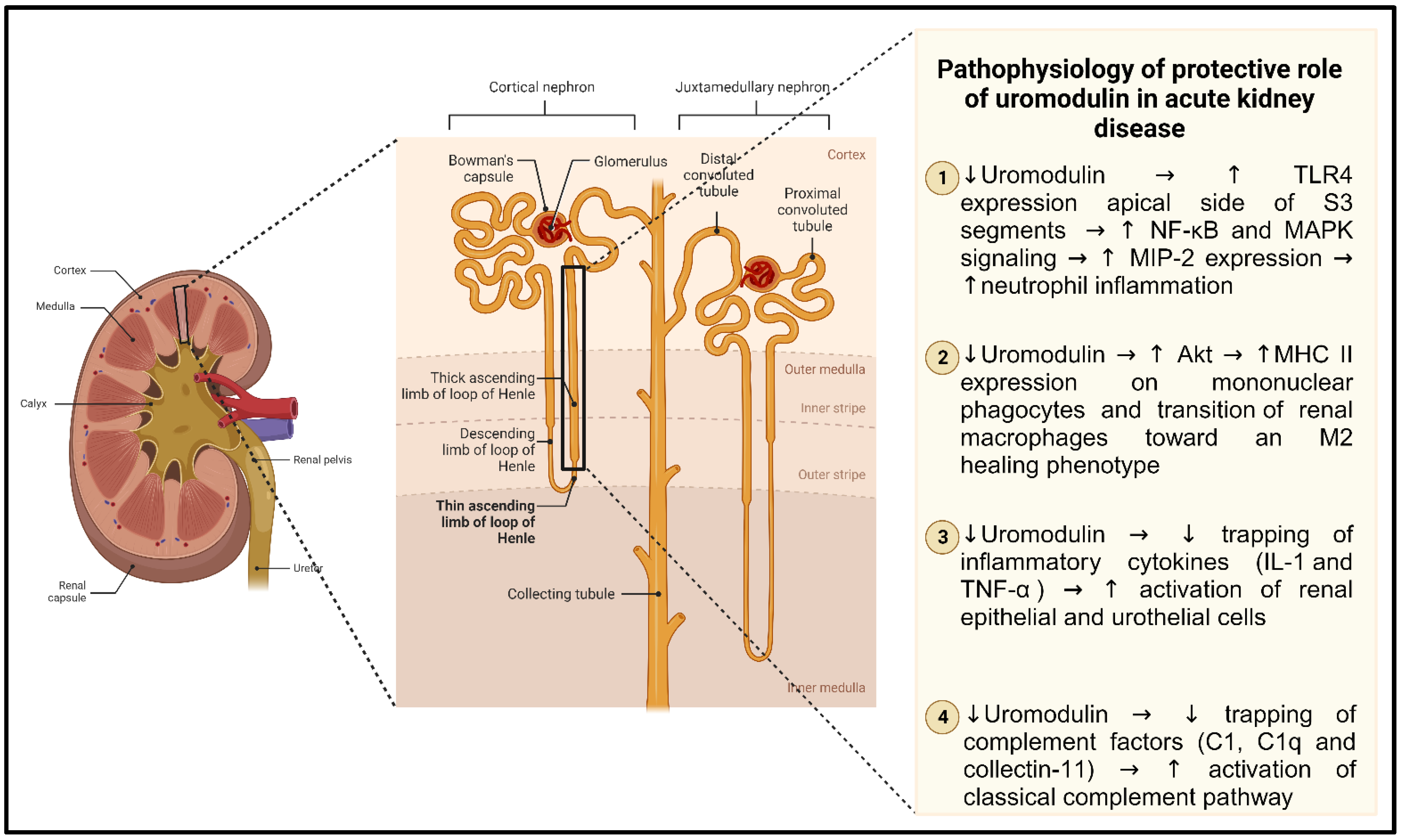
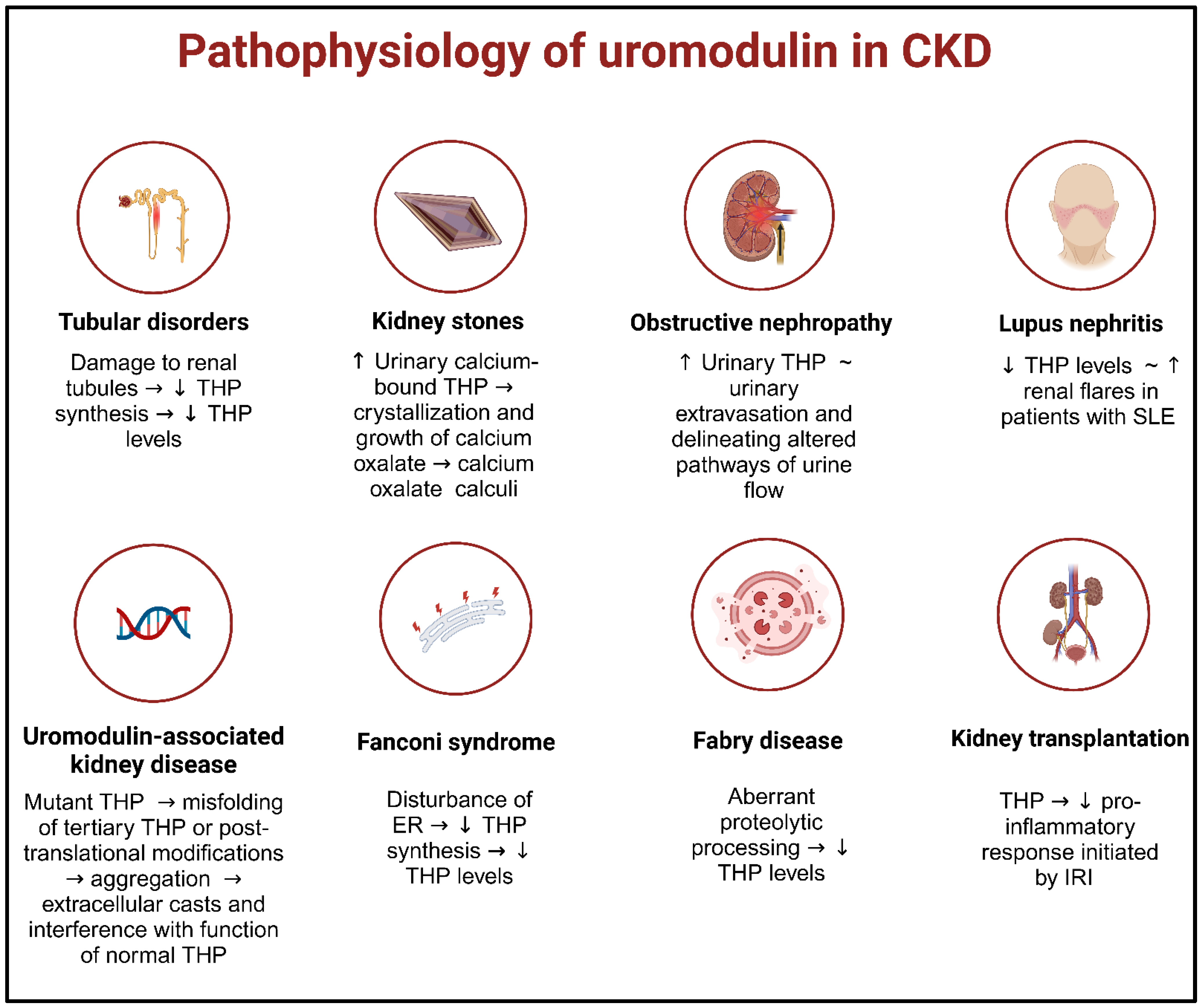
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thielemans, R.; Speeckaert, R.; Delrue, C.; De Bruyne, S.; Oyaert, M.; Speeckaert, M.M. Unveiling the Hidden Power of Uromodulin: A Promising Potential Biomarker for Kidney Diseases. Diagnostics 2023, 13, 3077. https://doi.org/10.3390/diagnostics13193077
Thielemans R, Speeckaert R, Delrue C, De Bruyne S, Oyaert M, Speeckaert MM. Unveiling the Hidden Power of Uromodulin: A Promising Potential Biomarker for Kidney Diseases. Diagnostics. 2023; 13(19):3077. https://doi.org/10.3390/diagnostics13193077
Chicago/Turabian StyleThielemans, Raïsa, Reinhart Speeckaert, Charlotte Delrue, Sander De Bruyne, Matthijs Oyaert, and Marijn M. Speeckaert. 2023. "Unveiling the Hidden Power of Uromodulin: A Promising Potential Biomarker for Kidney Diseases" Diagnostics 13, no. 19: 3077. https://doi.org/10.3390/diagnostics13193077





