Tumor Progression from a Fibroblast Activation Protein Perspective: Novel Diagnostic and Therapeutic Scenarios for Colorectal Cancer
Abstract
:1. Introduction
- To investigate the immunophenotypic expression of FAP, fibronectin ED-B, and CXCR4 in primary tumors of CRC and their liver, lung, and peritoneum synchronous and/or metachronous metastases.
- To preliminarily establish correlations between the tissue expression of the aforementioned stromal markers and the data potentially obtained from future PET imaging studies.
2. Materials and Methods
- 50 primary tumors;
- 50 1st metastases;
- 17 2nd metastases;
- 7 3rd metastases;
- 1 case of 4th metastasis.
2.1. Statement of Ethics
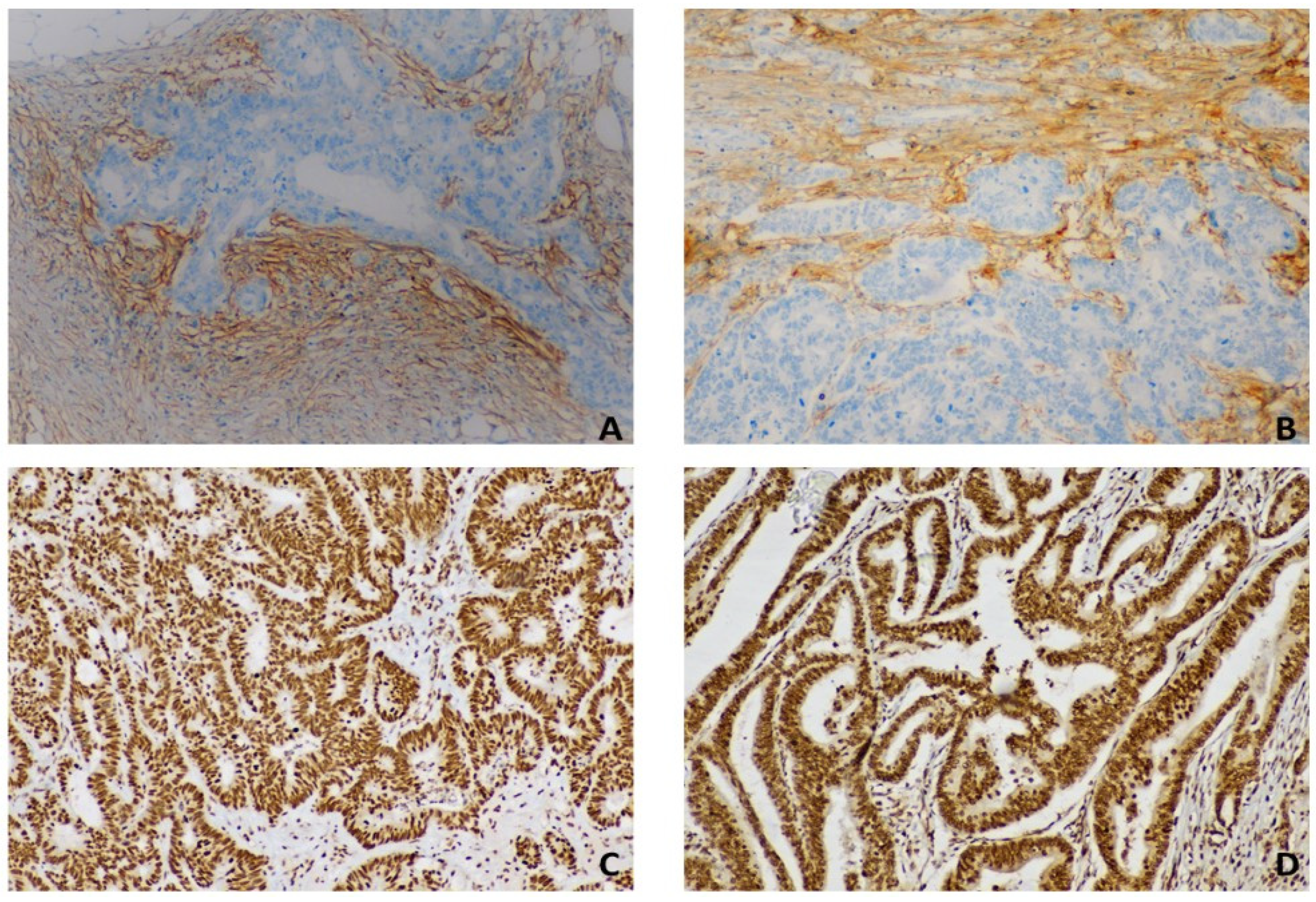
2.2. Immunohistochemistry
2.3. Semiquantitative Analysis of Immunohistochemistry
2.4. Aims
- (1)
- The correlation between the expression of fibronectin and CXCR4 in both the primary tumor and metastatic tissues in terms of intensity, number of positive cells, and intensity score.
- (2)
- The correlation between the expression of FAP, in terms of intensity % and intensity score, in the primary tumor and 1st metastasis.
- (3)
- The differences in terms of expression intensity of FAP and CXCR4 in the subgroups of patients with and without the mutations in BRAF, KRAS, and NRAS.
- (4)
- The correlations between BRAF, KRAS, NRAS mutations, and FAP and CXCR4 in both the primary tumor and the 1st metastasis.
3. Results
Statistical Analysis
- (1)
- Due to the sample size of the investigated classes, the comparison focused on the primary tumor vs. the first metastasis. No statistically significant correlations emerged in comparing fibronectin with CXCR4 between the primary tumor and the first metastasis.
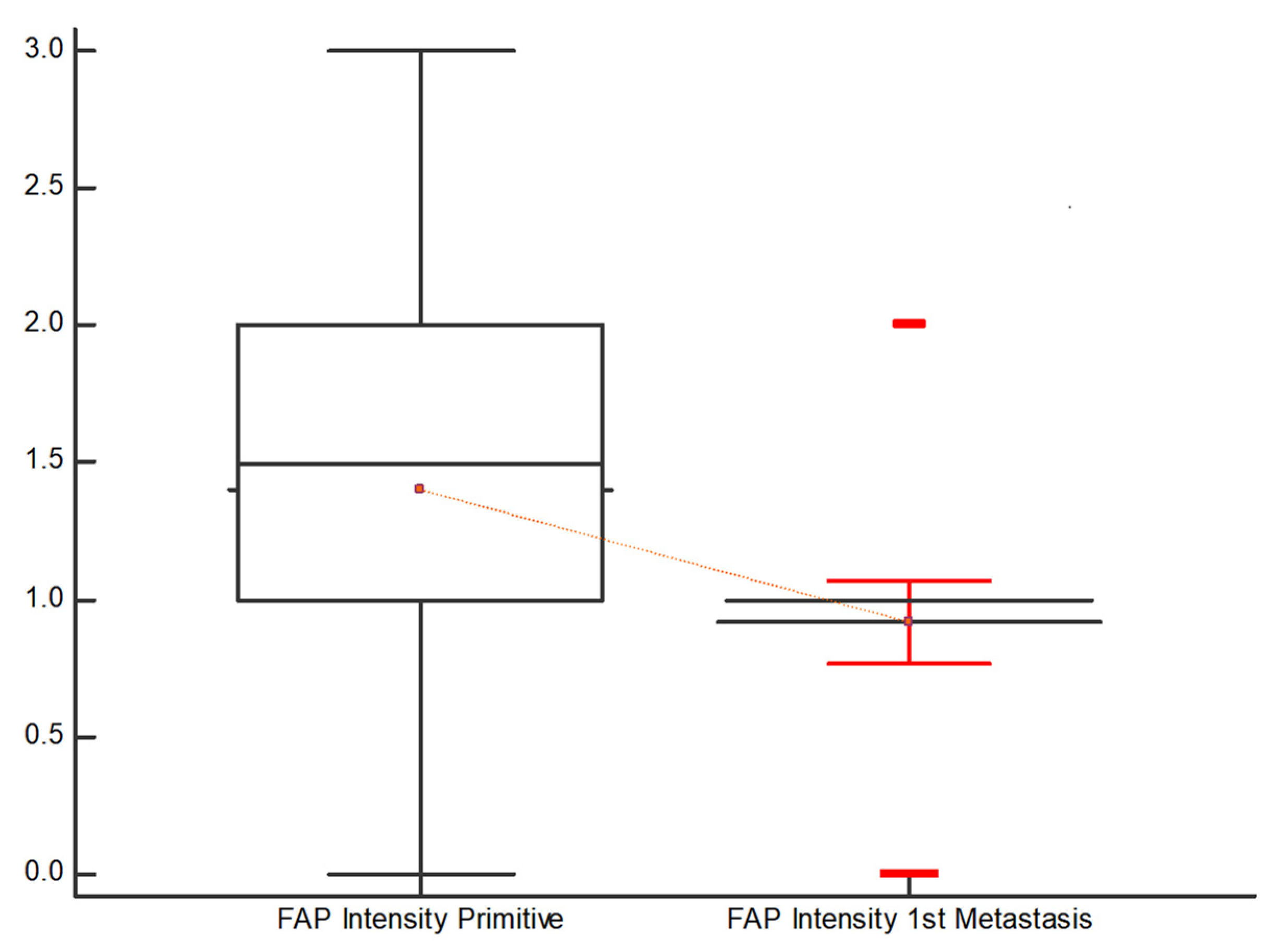
- (2)
- However, the evaluation of FAP in terms of “Intensity %” resulted in a Kendall’s Tau of 0.293 with a significance level of p = 0.0028, and FAP “Intensity score” revealed a Kendall’s Tau of 0.31 with a significance level of p = 0.0016. In both cases, a medium association was indicated.
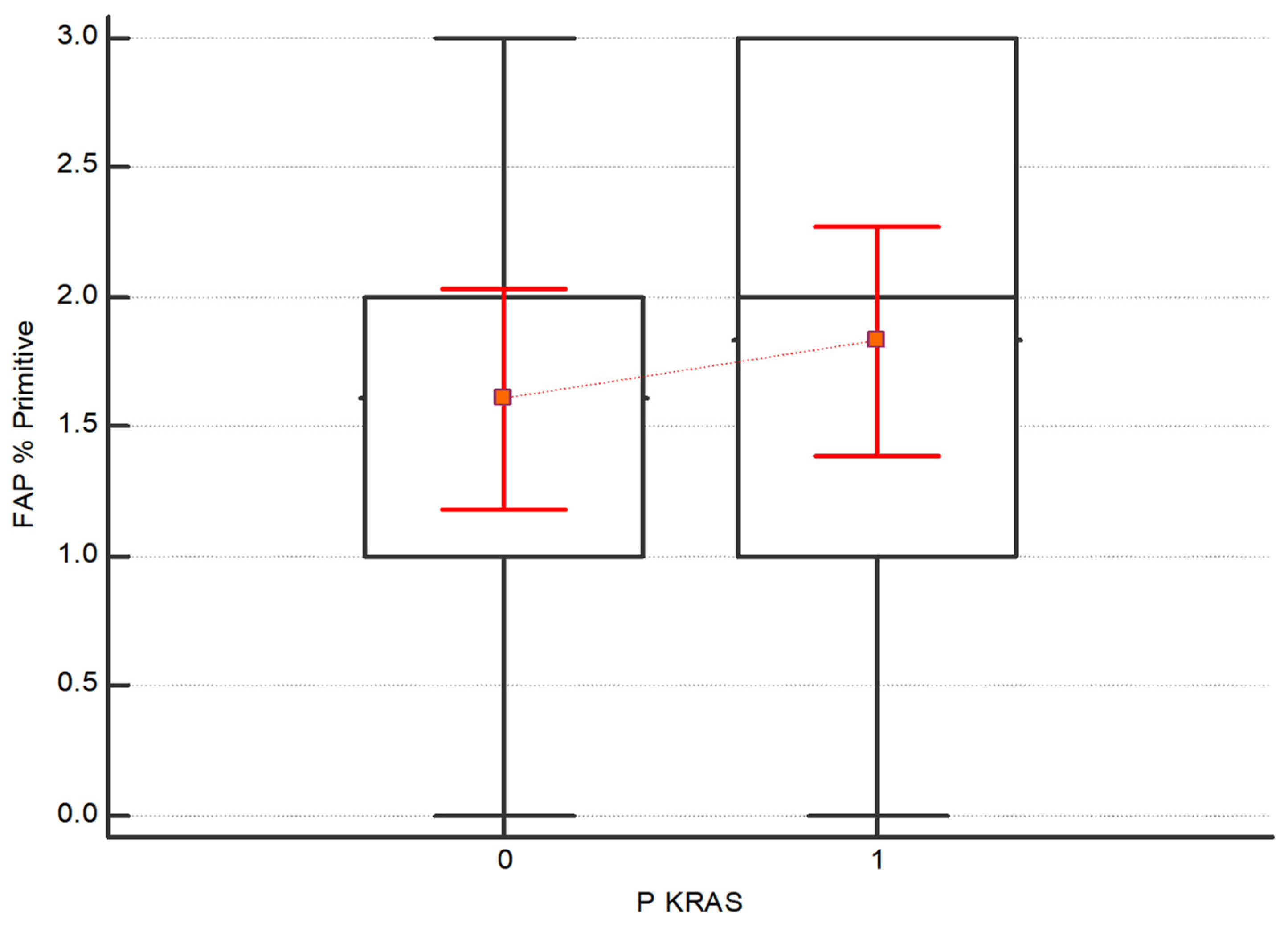
- (3)
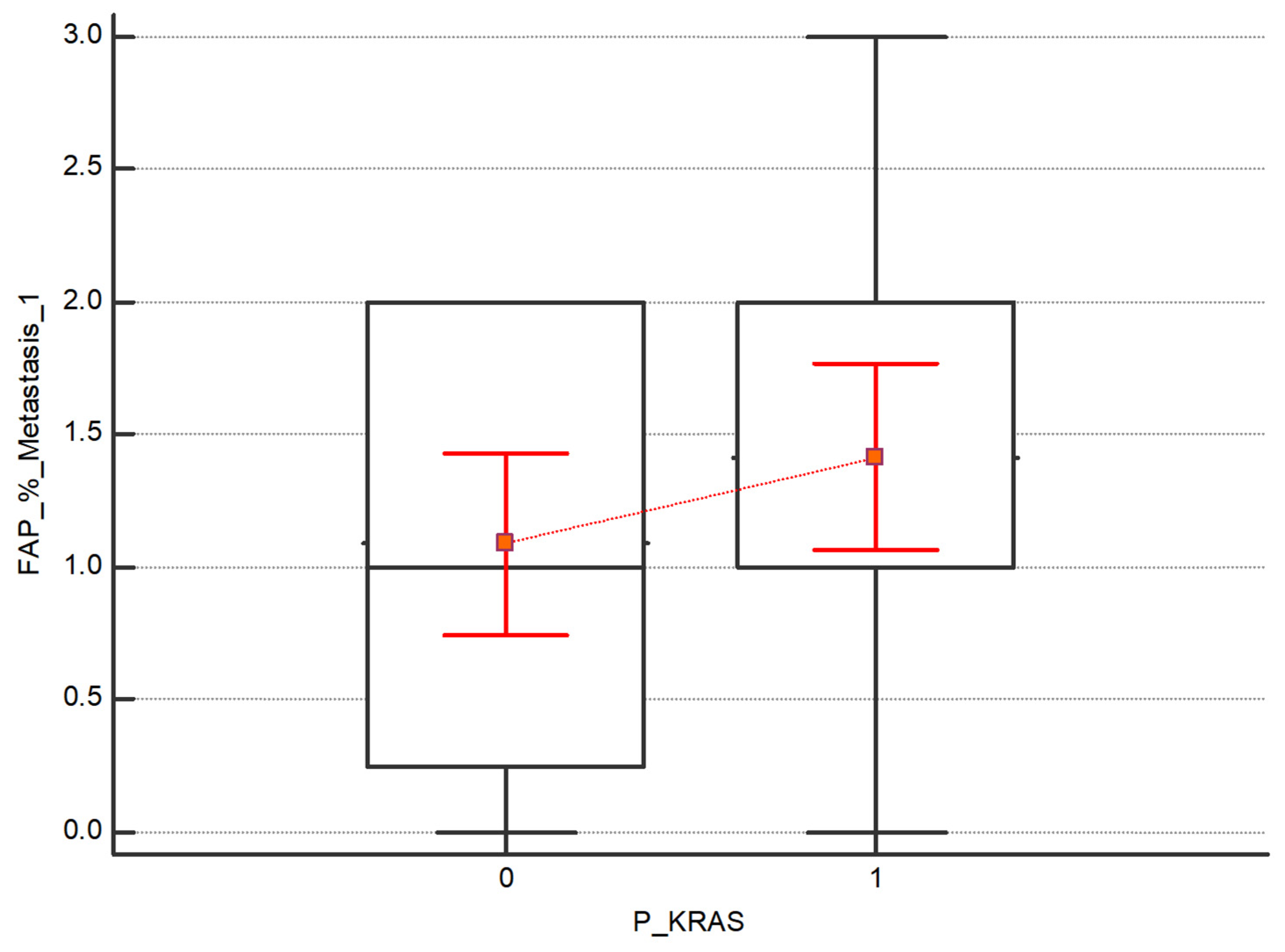
- The Kruskal–Wallis test was also performed to assess the difference in expression intensity of FAP and CXCR4 in the subgroups of patients with and without the mutations in BRAF, KRAS and NRAS. No statistical differences between the subgroups have been detected. Regarding FAP, although no statistically significant, higher values emerge in both the primary tumor and the 1st metastasis in patients with KRAS mutation respecting those without mutations, as shown in Figure 5 and Figure 6.
- (4)
- Furthermore, the correlation between BRAF, KRAS, NRAS mutations, and FAP and CXCR4 in both the primary tumor and the first metastasis were examined. Due to the limited sample, the mutational assessment highlighted just the presence without specifying the mutation type. No statistically significant values emerged in associating CXCR4 or FAP expression with BRAF, KRAS, and NRAS mutations.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wen, R.; Zhou, L.; Peng, Z.; Fan, H.; Zhang, T.; Jia, H.; Gao, X.; Hao, L.; Lou, Z.; Cao, F.; et al. Single-cell sequencing technology in colorectal cancer: A new technology to disclose the tumor heterogeneity and target precise treatment. Front. Immunol. 2023, 14, 1175343. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer (IARC). Globocan 2020: Cancer Fact Sheets—Colorectal Cancer. Available online: https://gco.iarc.fr/today/data/factsheets/cancers/10_8_9-Colorectum-fact-sheet.pdf (accessed on 1 October 2023).
- Keum, N.; Giovannucci, E. Global burden of colorectal cancer: Emerging trends, risk factors and prevention strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.; Arnold, M.; Gini, A.; Lorenzoni, V.; Cabasag, C.J.; Laversanne, M.; Vignat, J.; Ferlay, J.; Murphy, N.; Bray, F. Global burden of colorectal cancer in 2020 and 2040: Incidence and mortality estimates from GLOBOCAN. Gut 2023, 72, 338–344. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.; Zhao, Z.; Deng, Y.; Zheng, Z.; Huang, Y.; Huang, S.; Chi, P. The global, regional, and national early-onset colorectal cancer burden and trends from 1990 to 2019: Results from the Global Burden of Disease Study. BMC Public Health 2022, 22, 1896. [Google Scholar] [CrossRef]
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics. CA A Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef]
- Xi, Y.; Xu, P. Global colorectal cancer burden in 2020 and projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Li, J.; Chen, D.; Shen, M. Tumor Microenvironment Shapes Colorectal Cancer Progression, Metastasis, and Treatment Responses. Front. Med. 2022, 9, 869010. [Google Scholar] [CrossRef]
- Chandra, R.; Karalis, J.D.; Liu, C.; Murimwa, G.Z.; Park, J.V.; Heid, C.A.; Reznik, S.I.; Huang, E.; Minna, J.D.; Brekken, R.A. The Colorectal Cancer Tumor Microenvironment and Its Impact on Liver and Lung Metastasis. Cancers 2021, 13, 6206. [Google Scholar] [CrossRef]
- Kalluri, R.; Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 2006, 6, 392–401. [Google Scholar] [CrossRef]
- Östman, A.; Augsten, M. Cancer-associated fibroblasts and tumor growth—bystanders turning into key players. Curr. Opin. Genet. Dev. 2009, 19, 67–73. [Google Scholar] [CrossRef]
- Peng, Z.; Ye, M.; Ding, H.; Feng, Z.; Hu, K. Spatial transcriptomics atlas reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment components in colorectal cancer. J. Transl. Med. 2022, 20, 302. [Google Scholar] [CrossRef]
- Peddareddigari, V.G.; Wang, D.; DuBois, R.N. The Tumor Microenvironment in Colorectal Carcinogenesis. Cancer Microenviron. 2010, 3, 149–166. [Google Scholar] [CrossRef]
- Whiteside, T.L. The tumor microenvironment and its role in promoting tumor growth. Oncogene 2008, 27, 5904–5912. [Google Scholar] [CrossRef]
- Wu, Y.; Jia, H.; Zhou, H.; Liu, X.; Sun, J.; Zhou, X.; Zhao, H. Immune and stromal related genes in colon cancer: Analysis of tumour microenvironment based on the cancer genome atlas (TCGA) and gene expression omnibus (GEO) databases. Scand. J. Immunol. 2022, 95, e13119. [Google Scholar] [CrossRef]
- Wu, X.; Yan, H.; Qiu, M.; Qu, X.; Wang, J.; Xu, S.; Zheng, Y.; Ge, M.; Yan, L.; Liang, L. Comprehensive characterization of tumor microenvironment in colorectal cancer via molecular analysis. eLife 2023, 12, e86032. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, X.; Wu, C. The Role of the Tumor Microenvironment and Treatment Strategies in Colorectal Cancer. Front. Immunol. 2021, 12, 792691. [Google Scholar] [CrossRef]
- Bates, R.C.; Pursell, B.M.; Mercurio, A.M. Epithelial-Mesenchymal Transition and Colorectal Cancer: Gaining Insights into Tumor Progression Using LIM 1863 Cells. Cells Tissues Organs 2007, 185, 29–39. [Google Scholar] [CrossRef]
- Bataille, F.; Rohrmeier, C.; Bates, R.; Weber, A.; Rieder, F.; Brenmoehl, J.; Strauch, U.; Farkas, S.; Fürst, A.; Hofstädter, F.; et al. Evidence for a role of epithelial mesenchymal transition during pathogenesis of fistulae in Crohnʼs disease. Inflamm. Bowel Dis. 2008, 14, 1514–1527. [Google Scholar] [CrossRef]
- Zafari, N.; Khosravi, F.; Rezaee, Z.; Esfandyari, S.; Bahiraei, M.; Bahramy, A.; Ferns, G.A.; Avan, A. The role of the tumor microenvironment in colorectal cancer and the potential therapeutic approaches. J. Clin. Lab. Anal. 2022, 36, e24585. [Google Scholar] [CrossRef]
- Li, F.; Cao, Y.; Townsend, C.M.; Ko, T.C. TGF-β Signaling in Colon Cancer Cells. World J. Surg. 2005, 29, 306–311. [Google Scholar] [CrossRef]
- Reinacher-Schick, A.; Baldus, S.E.; Romdhana, B.; Landsberg, S.; Zapatka, M.; Mönig, S.P.; Hölscher, A.H.; Dienes, H.P.; Schmiegel, W.; Schwarte-Waldhoff, I. Loss of Smad4 correlates with loss of the invasion suppressor E-cadherin in advanced colorectal carcinomas. J. Pathol. 2004, 202, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Berral-González, A.; López-Cade, I.; Galindo-Pumariño, C.; Bueno-Fortes, S.; Martín-Merino, M.; Carrato, A.; Ocaña, A.; De La Pinta, C.; López-Alfonso, A.; et al. Cancer-associated fibroblast-derived gene signatures determine prognosis in colon cancer patients. Mol. Cancer 2021, 20, 73. [Google Scholar] [CrossRef] [PubMed]
- Popova, N.V.; Jücker, M. The Functional Role of Extracellular Matrix Proteins in Cancer. Cancers 2022, 14, 238. [Google Scholar] [CrossRef] [PubMed]
- Henry, L.R.; Lee, H.-O.; Lee, J.S.; Klein-Szanto, A.; Watts, P.; Ross, E.A.; Chen, W.-T.; Cheng, J.D. Clinical Implications of Fibroblast Activation Protein in Patients with Colon Cancer. Clin. Cancer Res. 2007, 13, 1736–1741. [Google Scholar] [CrossRef]
- Wu, C.; Gu, J.; Gu, H.; Zhang, X.; Zhang, X.; Ji, R. The recent advances of cancer associated fibroblasts in cancer progression and therapy. Front. Oncol. 2022, 12, 1008843. [Google Scholar] [CrossRef]
- Mhaidly, R.; Mechta-Grigoriou, F. Role of cancer-associated fibroblast subpopulations in immune infiltration, as a new means of treatment in cancer. Immunol. Rev. 2021, 302, 259–272. [Google Scholar] [CrossRef]
- Loeffler, M.; Krüger, J.A.; Niethammer, A.G.; Reisfeld, R.A. Targeting tumor-associated fibroblasts improves cancer chemotherapy by increasing intratumoral drug uptake. J. Clin. Investig. 2006, 116, 1955–1962. [Google Scholar] [CrossRef]
- Banerjee, A.; Chabria, Y.; Kanna NR, R.; Gopi, J.; Rowlo, P.; Sun, X.-F.; Pathak, S. Role of Tumor Specific niche in Colon Cancer Progression and Emerging Therapies by Targeting Tumor Microenvironment. In Advances in Experimental Medicine and Biology; Springer: Berlin/Heidelberg, Germany, 2021; pp. 177–192. [Google Scholar] [CrossRef]
- Fitzgerald, A.A.; Weiner, L.M. The role of fibroblast activation protein in health and malignancy. Cancer Metastasis Rev. 2020, 39, 783–803. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, S.; Kelly, T. Seprase Promotes Rapid Tumor Growth and Increased Microvessel Density in a Mouse Model of Human Breast Cancer. Cancer Res. 2004, 64, 2712–2716. [Google Scholar] [CrossRef]
- Lindner, T.; Loktev, A.; Giesel, F.; Kratochwil, C.; Altmann, A.; Haberkorn, U. Targeting of activated fibroblasts for imaging and therapy. EJNMMI Radiopharm. Chem. 2019, 4, 16. [Google Scholar] [CrossRef]
- Wang, J.P.; Hielscher, A. Fibronectin: How Its Aberrant Expression in Tumors May Improve Therapeutic Targeting. J. Cancer 2017, 8, 674–682. [Google Scholar] [CrossRef]
- Yi, W.; Xiao, E.; Ding, R.; Luo, P.; Yang, Y. High expression of fibronectin is associated with poor prognosis, cell proliferation and malignancy via the NF-κB/p53-apoptosis signaling pathway in colorectal cancer. Oncol. Rep. 2016, 36, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
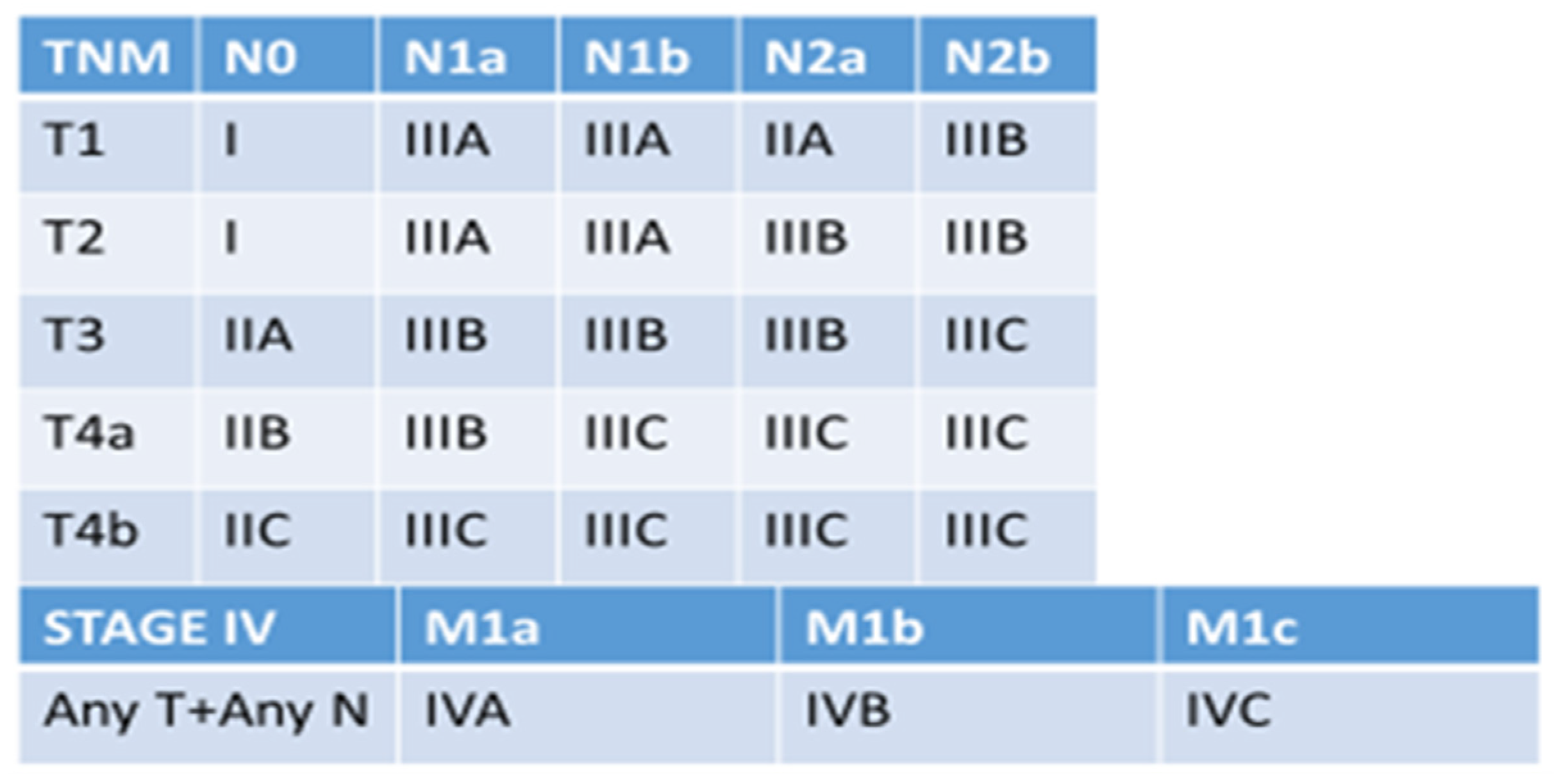
- Dominguez, O.H.; Yilmaz, S.; Steele, S.R. Stage IV Colorectal Cancer Management and Treatment. J. Clin. Med. 2023, 12, 2072. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.-P.; Zhang, C.-D.; Fu, X.; Ba, Y.; Yue, S.; Zhao, Z.-M.; Dai, D.-Q. A Novel TNM Classification for Colorectal Cancers based on the Metro-ticket Paradigm. J. Cancer 2021, 12, 3299–3306. [Google Scholar] [CrossRef]
- Bentzen, S.M.; Atasoy, B.M.; Daley, F.M.; Dische, S.; Richman, P.I.; Saunders, M.I.; Trott, K.R.; Wilson, G.D. Epidermal Growth Factor Receptor Expression in Pretreatment Biopsies From Head and Neck Squamous Cell Carcinoma As a Predictive Factor for a Benefit From Accelerated Radiation Therapy in a Randomized Controlled Trial. J. Clin. Oncol. 2005, 23, 5560–5567. [Google Scholar] [CrossRef]
- Neophytou, C.M.; Panagi, M.; Stylianopoulos, T.; Papageorgis, P. The Role of Tumor Microenvironment in Cancer Metastasis: Molecular Mechanisms and Therapeutic Opportunities. Cancers 2021, 13, 2053. [Google Scholar] [CrossRef] [PubMed]
- Maia, A.; Wiemann, S. Cancer-Associated Fibroblasts: Implications for Cancer Therapy. Cancers 2021, 13, 3526. [Google Scholar] [CrossRef]
- Yang, X.; Lin, Y.; Shi, Y.; Li, B.; Liu, W.; Yin, W.; Dang, Y.; Chu, Y.; Fan, J.; He, R. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3–CCL2 Signaling. Cancer Res. 2016, 76, 4124–4135. [Google Scholar] [CrossRef]
- Teichgräber, V.; Monasterio, C.; Chaitanya, K.; Boger, R.; Gordon, K.; Dieterle, T.; Jäger, D.; Bauer, S. Specific inhibition of fibroblast activation protein (FAP)-alpha prevents tumor progression in vitro. Adv. Med. Sci. 2015, 60, 264–272. [Google Scholar] [CrossRef]
- Zi, F.; He, J.; He, D.; Li, Y.; Yang, L.; Cai, Z. Fibroblast activation protein α in tumor microenvironment: Recent progression and implications (Review). Mol. Med. Rep. 2015, 11, 3203–3211. [Google Scholar] [CrossRef]
- Liu, F.; Qi, L.; Liu, B.; Liu, J.; Zhang, H.; Che, D.; Cao, J.; Shen, J.; Geng, J.; Bi, Y.; et al. Fibroblast Activation Protein Overexpression and Clinical Implications in Solid Tumors: A Meta-Analysis. PLoS ONE 2015, 10, e0116683. [Google Scholar] [CrossRef]
- Koulouridi, A.; Karagianni, M.; Messaritakis, I.; Sfakianaki, M.; Voutsina, A.; Trypaki, M.; Bachlitzanaki, M.; Koustas, E.; Karamouzis, M.V.; Ntavatzikos, A.; et al. Prognostic Value of KRAS Mutations in Colorectal Cancer Patients. Cancers 2022, 14, 3320. [Google Scholar] [CrossRef]
- De Roock, W.; Claes, B.; Bernasconi, D.; De Schutter, J.; Biesmans, B.; Fountzilas, G.; Kalogeras, K.T.; Kotoula, V.; Papamichael, D.; Laurent-Puig, P.; et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: A retrospective consortium analysis. Lancet Oncol. 2010, 11, 753–762. [Google Scholar] [CrossRef]
- Rohren, E.M.; Turkington, T.G.; Coleman, R.E. Clinical Applications of PET in Oncology. Radiology 2004, 231, 305–332. [Google Scholar] [CrossRef] [PubMed]
- Dassen, A.; Lips, D.; Hoekstra, C.; Pruijt, J.; Bosscha, K. FDG-PET has no definite role in preoperative imaging in gastric cancer. Eur. J. Surg. Oncol. 2009, 35, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Xin, L.; Gao, J.; Zheng, Z.; Chen, Y.; Lv, S.; Zhao, Z.; Yu, C.; Yang, X.; Zhang, R. Fibroblast Activation Protein-α as a Target in the Bench-to-Bedside Diagnosis and Treatment of Tumors: A Narrative Review. Front. Oncol. 2021, 11, 648187. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yu, D. Tumor microenvironment as a therapeutic target in cancer. Pharmacol. Ther. 2020, 221, 107753. [Google Scholar] [CrossRef]
- Taralli, S.; Lorusso, M.; Perrone, E.; Perotti, G.; Zagaria, L.; Calcagni, M.L. PET/CT with Fibroblast Activation Protein Inhibitors in Breast Cancer: Diagnostic and Theranostic Application—A Literature Review. Cancers 2023, 15, 908. [Google Scholar] [CrossRef]
- Cheng, Z.; Wang, S.; Xu, S.; Du, B.; Li, X.; Li, Y. FAPI PET/CT in Diagnostic and Treatment Management of Colorectal Cancer: Review of Current Research Status. J. Clin. Med. 2023, 12, 577. [Google Scholar] [CrossRef]
- Kuyumcu, S.; Sanli, Y.; Subramaniam, R.M. Fibroblast-Activated Protein Inhibitor PET/CT: Cancer Diagnosis and Management. Front. Oncol. 2021, 11, 758958. [Google Scholar] [CrossRef]
- Rowe, S.P.; Pomper, M.G. Molecular imaging in oncology: Current impact and future directions. CA A Cancer J. Clin. 2022, 72, 333–352. [Google Scholar] [CrossRef] [PubMed]

| FAP % Primitive | FAP Intensity Primitive | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | Total (n) | p Value | 0 | 1 | 2 | 3 | Total (n) | p Value | ||
| BRAF | 0.1165 | BRAF | 0.7038 | ||||||||||
| Negative (%) | 18.6 | 18.6 | 37.2 | 25.6 | 43 | Negative (%) | 18.6 | 32.6 | 41.9 | 7 | 43 | ||
| Positive (%) | 0 | 0 | 100 | 0 | 4 | Positive (%) | 0 | 50 | 50 | 0 | 4 | ||
| KRAS | 0.7297 | KRAS | 0.9577 | ||||||||||
| Negative (%) | 17.4 | 21.7 | 43.5 | 17.4 | 23 | Negative (%) | 17.4 | 34.8 | 43.5 | 4.3 | 23 | ||
| Positive (%) | 16.7 | 12.5 | 41.7 | 29.2 | 24 | Positive (%) | 16.7 | 33.3 | 41.7 | 8.3 | 24 | ||
| NRAS | 0.7127 | NRAS | 0.4202 | ||||||||||
| Negative (%) | 17.8 | 17.8 | 42.2 | 22.2 | 45 | Negative (%) | 17.8 | 35.6 | 40 | 6.7 | 45 | ||
| Positive (%) | 0 | 0 | 50 | 50 | 2 | Positive (%) | 0 | 0 | 100 | 0 | 2 | ||
| FAP % Metastasis 1 | FAP Intensity Metastasis 1 | ||||||||||||
| 0 | 1 | 2 | 3 | Total (n) | p Value | 0 | 1 | 2 | 3 | Total (n) | p Value | ||
| BRAF | 0.6521 | BRAF | 0.4335 | ||||||||||
| Negative (%) | 18.5 | 46.5 | 27.9 | 7 | 43 | Negative (%) | 18.6 | 69.8 | 11.6 | 0 | 43 | ||
| Positive (%) | 0 | 50 | 50 | 0 | 4 | Positive (%) | 0 | 100 | 0 | 0 | 4 | ||
| KRAS | 0.119 | KRAS | 0.2657 | ||||||||||
| Negative (%) | 26.1 | 39.1 | 34.8 | 0 | 23 | Negative (%) | 26.1 | 65.2 | 8.7 | 0 | 23 | ||
| Positive (%) | 8.3 | 54.2 | 25 | 12.5 | 24 | Positive (%) | 8.3 | 79.2 | 12.5 | 0 | 24 | ||
| NRAS | 0.8543 | NRAS | 0.1702 | ||||||||||
| Negative (%) | 17.8 | 46.7 | 28.9 | 6.7 | 45 | Negative (%) | 17.8 | 73.3 | 8.9 | 0 | 45 | ||
| Positive (%) | 0 | 50 | 50 | 0 | 2 | Positive (%) | 0 | 50 | 50 | 0 | 2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rossetti, M.; Stanca, S.; Del Frate, R.; Bartoli, F.; Marciano, A.; Esposito, E.; Fantoni, A.; Erba, A.P.; Lippolis, P.V.; Faviana, P. Tumor Progression from a Fibroblast Activation Protein Perspective: Novel Diagnostic and Therapeutic Scenarios for Colorectal Cancer. Diagnostics 2023, 13, 3199. https://doi.org/10.3390/diagnostics13203199
Rossetti M, Stanca S, Del Frate R, Bartoli F, Marciano A, Esposito E, Fantoni A, Erba AP, Lippolis PV, Faviana P. Tumor Progression from a Fibroblast Activation Protein Perspective: Novel Diagnostic and Therapeutic Scenarios for Colorectal Cancer. Diagnostics. 2023; 13(20):3199. https://doi.org/10.3390/diagnostics13203199
Chicago/Turabian StyleRossetti, Martina, Stefano Stanca, Rossella Del Frate, Francesco Bartoli, Andrea Marciano, Enrica Esposito, Alessandra Fantoni, Anna Paola Erba, Piero Vincenzo Lippolis, and Pinuccia Faviana. 2023. "Tumor Progression from a Fibroblast Activation Protein Perspective: Novel Diagnostic and Therapeutic Scenarios for Colorectal Cancer" Diagnostics 13, no. 20: 3199. https://doi.org/10.3390/diagnostics13203199







