Role of Clinical Risk Factors and B-Type Natriuretic Peptide in Assessing the Risk of Asymptomatic Cardiotoxicity in Breast Cancer Patients in Kazakhstan †
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
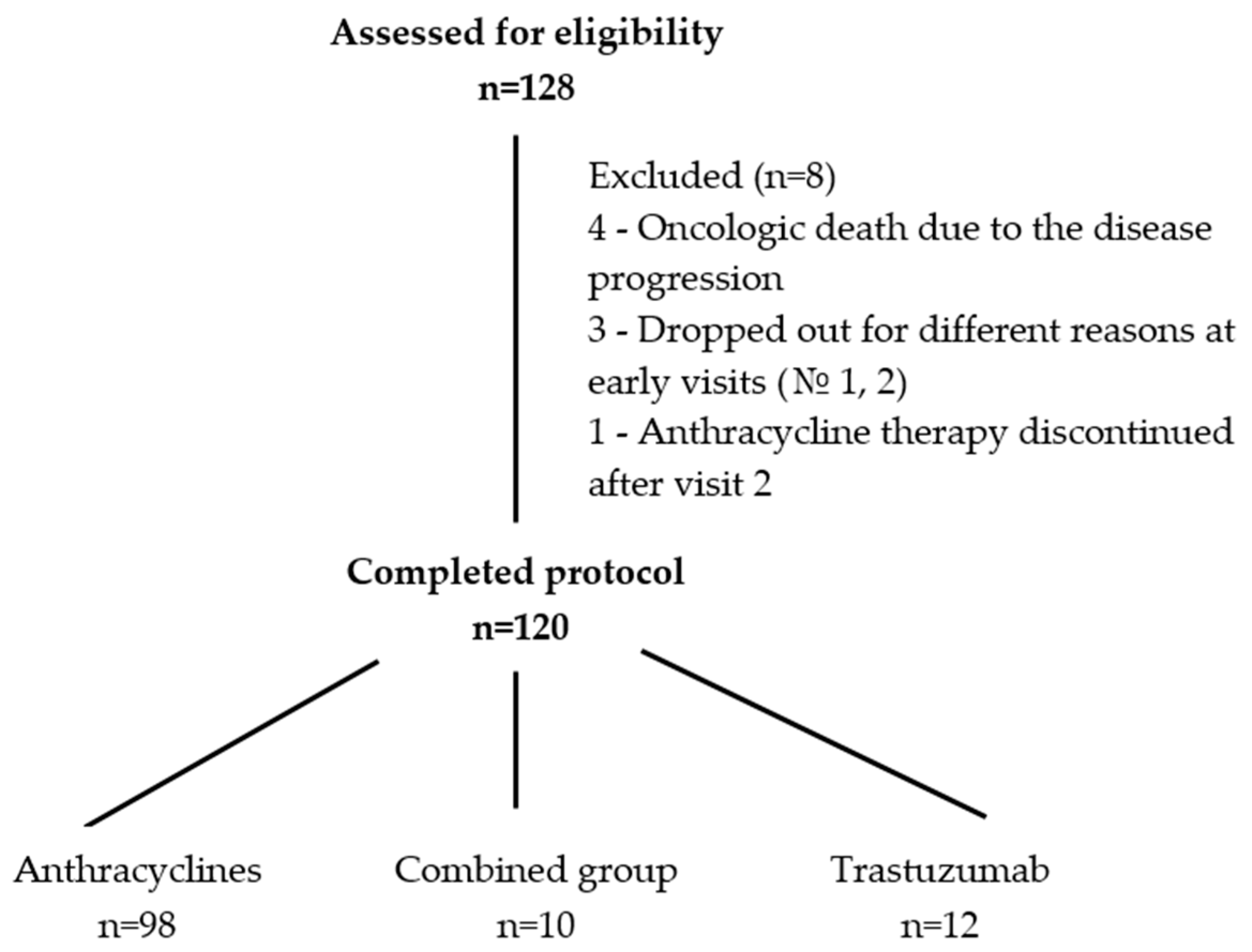
2.2. Patient Selection
2.3. Types of Collected Data and Visit Intervals
2.4. Allocation of Patients by Risk Groups
2.5. Chemotherapy Treatment
2.6. CTRCD Evaluation Criteria and Measurements
- The number of patients who developed cardiotoxic complications, including subclinical (asymptomatic) dysfunction, measured using LVEF monitoring (>10% decline from baseline to a value <53%) and GLS assessment (decrease >15% from baseline) after the chemotherapy onset through all groups;
- Presence of increased values of the tests during chemotherapy treatment: cardiac troponin I (cTnI)—>0.3 ng/mL; brain natriuretic peptide (BNP)—>100 pg/mL; C-reactive protein (CRP)—>5 mg/L; myeloperoxidase (MPO)—>470 pmol/L; galectin-3 (Gal-3)—>28.7 ng/mL; and D-dimer—>0.5 mg/L (presented values were taken from the immunofluorescence analyzers’ manuals from the involved labs by request);
- Time trends in the onset of increasing biomarker values;
- The positive predictive value (PPV) and the negative predictive value (PVN) for all developed statistical models.
2.7. Cardiac Imaging
2.8. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Evaluation of Imaging Methods’ Dynamics toward Asymptomatic Cardiotoxicity
3.3. Dynamics of Biomarkers
3.3.1. Key Findings on BNP Levels across the CTRCD Groups (Graph A)
3.3.2. Key Findings on CRP Levels across the CTRCD Groups (Graph B)
3.3.3. Key Findings on D-Dimer Levels across the CTRCD Groups (Graph C)
3.3.4. Key Findings on Gal-3 Levels across the CTRCD Groups (Graph D)
3.3.5. Key Findings on cTnI Levels across the CTRCD Groups (Graph E)
3.3.6. Key Findings on MPO Levels across the CTRCD Groups (Graph F)
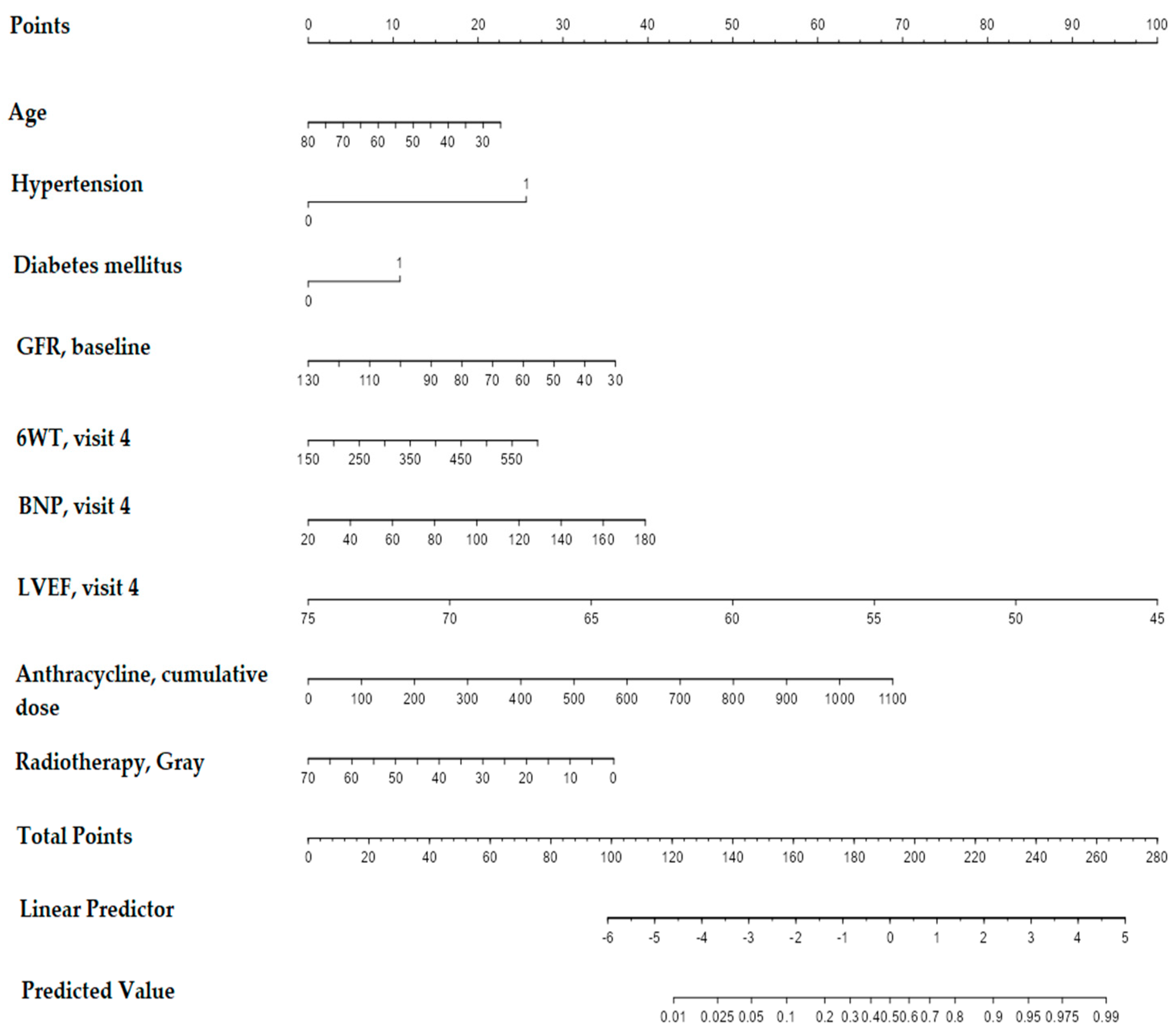
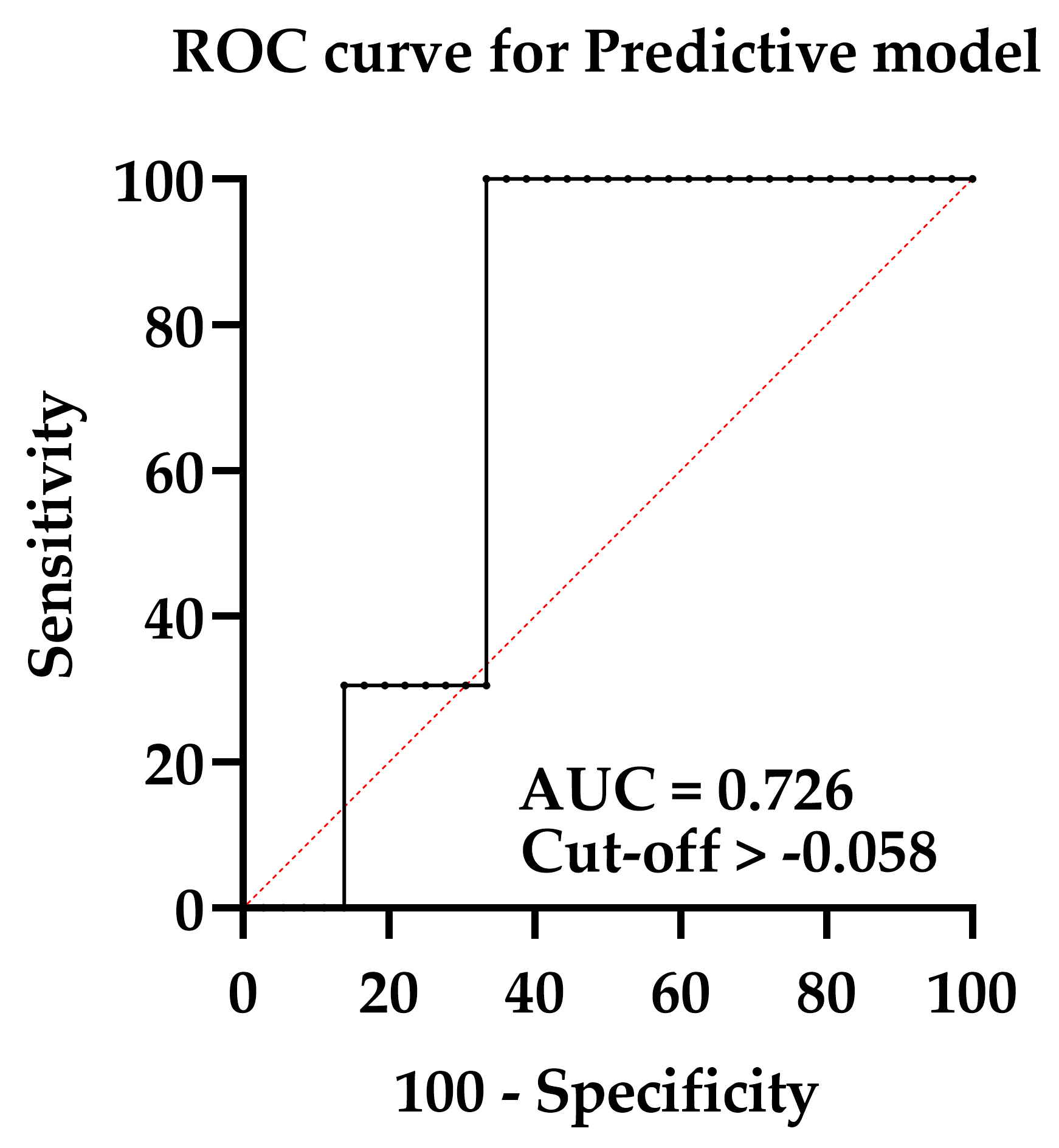
3.4. Designing a Predictive Model for CTRCD Risk Evaluation during or by the End of Treatment before Follow-Up
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Arnold, M.; Morgan, E.; Rumgay, H.; Mafra, A.; Singh, D.; Laversanne, M.; Vignat, J.; Gralow, J.R.; Cardoso, F.; Siesling, S.; et al. Current and future burden of breast cancer: Global statistics for 2020 and 2040. Breast 2022, 66, 15–23. [Google Scholar] [CrossRef]
- Midlenko, A.; Mussina, K.; Zhakhina, G.; Sakko, Y.; Rashidova, G.; Saktashev, B.; Adilbay, D.; Shatkovskaya, O.; Gaipov, A. Prevalence, incidence, and mortality rates of breast cancer in Kazakhstan: Data from the Unified National Electronic Health System, 2014–2019. Front. Public Health 2023, 11, 1132742. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Devasia, T.; Mariotto, A.B.; Yabroff, K.R.; Jemal, A.; Kramer, J.; Siegel, R.L. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 2022, 72, 409–436. [Google Scholar] [CrossRef]
- Berry, L.L.; Davis, S.W.; Godfrey Flynn, A.; Landercasper, J.; Deming, K.A. Is it time to reconsider the term “cancer survivor”? J. Psychosoc. Oncol. 2019, 37, 413–426. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.; Austin, P.C.; Lee, D.S.; Amir, E.; Tu, J.V.; Thavendiranathan, P.; Fung, K.; Anderson, G.M. A population-based study of cardiovascular mortality following early-stage breast cancer. JAMA Cardiol. 2017, 2, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; He, T.; Wang, Z.; Zheng, D.; Shen, Z. Relative Risk of Cardiovascular Mortality in Breast Cancer Patients: A Population-Based Study. Rev. Cardiovasc. Med. 2022, 23, 120. [Google Scholar] [CrossRef]
- Herrmann, J.; Lenihan, D.; Armenian, S.; Barac, A.; Blaes, A.; Cardinale, D.; Carver, J.; Dent, S.; Ky, B.; Lyon, A.R.; et al. Defining cardiovascular toxicities of cancer therapies: An International Cardio-Oncology Society (IC-OS) consensus statement. Eur. Heart J. 2022, 43, 280–299. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.; Colombo, A.; Bacchiani, G.; Tedeschi, I.; Meroni, C.A.; Veglia, F.; Civelli, M.; Lamantia, G.; Colombo, N.; Curigliano, G.; et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation 2015, 131, 1981–1988. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.M.; Whaley, F.S.; Ewer, M.S. Congestive heart failure in patients treated with doxorubicin: A retrospective analysis of three trials. Cancer 2003, 97, 2869–2879. [Google Scholar] [CrossRef] [PubMed]
- Ewer, M.; Lippman, S. Type II chemotherapy-related cardiac dysfunction: Time to recognize a new entity. J. Clin. Oncol. 2005, 23, 2900–2902. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, J.; Lerman, A.; Sandhu, N.P.; Villarraga, H.R.; Mulvagh, S.L.; Kohli, M. Evaluation and Management of Patients With Heart Disease and Cancer: Cardio-Oncology. Mayo Clin. Proc. 2014, 89, 1287–1306. [Google Scholar] [CrossRef] [PubMed]
- Strauss, M.H.; Hall, A.S.; Narkiewicz, K. The Combination of Beta-Blockers and ACE Inhibitors Across the Spectrum of Cardiovascular Diseases. Cardiovasc. Drugs Ther. 2023, 37, 757–770. [Google Scholar] [CrossRef] [PubMed]
- Zhong, G.; Chen, C.; Wu, S.; Chen, J.; Han, Y.; Zhu, Q.; Xu, M.; Nie, Q.; Wang, L. Mechanism of angiotensin-converting enzyme inhibitors in the treatment of dilated cardiomyopathy based on a protein interaction network and molecular docking. Cardiovasc. Diagn. Ther. 2023, 13, 534–549. [Google Scholar] [CrossRef] [PubMed]
- Gales, L.; Forsea, L.; Mitrea, D.; Stefanica, I.; Stanculescu, I.; Mitrica, R.; Georgescu, M.; Trifanescu, O.; Anghel, R.; Serbanescu, L. Antidiabetics, Anthelmintics, Statins, and Beta-Blockers as Co-Adjuvant Drugs in Cancer Therapy. Medicina 2022, 58, 1239. [Google Scholar] [CrossRef] [PubMed]
- Cantini, L.; Pecci, F.; Hurkmans, D.P.; Belderbos, R.A.; Lanese, A.; Copparoni, C.; Aerts, S.; Cornelissen, R.; Dumoulin, D.W.; Fiordoliva, I.; et al. High-intensity statins are associated with improved clinical activity of PD-1 inhibitors in malignant pleural mesothelioma and advanced non-small cell lung cancer patients. Eur. J. Cancer 2021, 144, 41–48. [Google Scholar] [CrossRef]
- Lyon, A.R.; López-Fernández, T.; Couch, L.S.; Asteggiano, R.; Aznar, M.C.; Bergler-Klein, J.; Boriani, G.; Cardinale, D.; Cordoba, R.; Cosyns, B.; et al. ESC Scientific Document Group, 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS): Developed by the task force on cardio-oncology of the European Society of Cardiology (ESC). Eur. Heart J. 2022, 43, 4229–4361. [Google Scholar] [CrossRef]
- Semeraro, G.C.; Cipolla, C.M.; Cardinale, D.M. Role of Cardiac Biomarkers in Cancer Patients. Cancers 2021, 13, 5426. [Google Scholar] [CrossRef]
- Pudil, R.; Mueller, C.; Ĉelutkiene, J.; Henriksen, P.A.; Lenihan, D.; Dent, S.; Barac, A.; Stanway, S.; Moslehi, J.; Suter, T.M.; et al. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: A position statement from the Cardio-Oncology Study Group of the Heart Failure Association and the Cardio-Oncology Council of the European Society of Cardiology. Eur. J. Heart Fail. 2020, 22, 1966–1983. [Google Scholar] [CrossRef]
- Demissei, B.G.; Hubbard, R.A.; Zhang, L.; Smith, A.M.; Sheline, K.; McDonald, C.; Narayan, V.; Domchek, S.M.; DeMichele, A.; Shah, P.; et al. Changes in cardiovascular biomarkers with breast cancer therapy and associations with cardiac dysfunction. J. Am. Heart Assoc. 2020, 9, e014708. [Google Scholar] [CrossRef]
- Meessen, J.M.; Cardinale, D.; Ciceri, F.; Sandri, M.T.; Civelli, M.; Bottazzi, B.; Cucchi, G.; Menatti, E.; Mangiavacchi, M.; Condorelli, G.; et al. Circulating biomarkers and cardiac function over 3 years after chemotherapy with anthracyclines: The ICOS-ONE trial. ESC Heart Fail. 2020, 7, 1452–1466. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.M.; Zaninotto, M.; Cipolla, C.M.; Passino, C.; Plebani, M.; Clerico, A. Cardiotoxic effects and myocardial injury: The search for a more precise definition of drug cardiotoxicity. Clin. Chem. Lab. Med. 2020, 59, 51–57. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, Y.; Zhang, Y.; Fang, F.; Liu, J.; Xia, Y.; Liu, Y. Cardiac Biomarkers for the Detection and Management of Cancer Therapy-Related Cardiovascular Toxicity. J. Cardiovasc. Dev. Dis. 2022, 9, 372. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Wang, X.; Li, S.; Liu, Y.; Cui, Y.; Deng, X. Advances in Biomarkers for Detecting Early Cancer Treatment-Related Cardiac Dysfunction. Front. Cardiovasc. Med. 2021, 8, 753313. [Google Scholar] [CrossRef] [PubMed]
- Tuegel, C.; Katz, R.; Alam, M.; Bhat, Z.; Bellovich, K.; de Boer, I.; Brosius, F.; Gadegbeku, C.; Gipson, D.; Hawkins, J.; et al. GDF-15, Galectin 3, Soluble ST2, and Risk of Mortality and Cardiovascular Events in CKD. Am. J. Kidney Dis. 2018, 72, 519–528. [Google Scholar] [CrossRef] [PubMed]
- Pauklin, P.; Zilmer, M.; Eha, J.; Tootsi, K.; Kals, M.; Kampus, P. Markers of Inflammation, Oxidative Stress, and Fibrosis in Patients with Atrial Fibrillation. Oxid. Med. Cell. Longev. 2022, 2022, 4556671. [Google Scholar] [CrossRef]
- Suzuki, K.; Terakawa, T.; Furukawa, J.; Harada, K.; Hinata, N.; Nakano, Y.; Fujisawa, M. C-reactive protein and the neutrophil-to-lymphocyte ratio are prognostic biomarkers in metastatic renal cell carcinoma patients treated with nivolumab. Int. J. Clin. Oncol. 2020, 25, 135–144. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Kato, T.; Morimoto, T.; Yaku, H.; Inuzuka, Y.; Tamaki, Y.; Yamamoto, E.; Yoshikawa, Y.; Kitai, T.; Taniguchi, R.; et al. C-reactive protein at discharge and 1-year mortality in hospitalised patients with acute decompensated heart failure: An observational study. BMJ Open 2020, 10, e041068. [Google Scholar] [CrossRef]
- Kuster, N.; Huet, F.; Dupuy, A.-M.; Akodad, M.; Battistella, P.; Agullo, A.; Leclercq, F.; Kalmanovich, E.; Meilhac, A.; Aguilhon, S.; et al. Multimarker approach including CRP, sST2 and GDF-15 for prognostic stratification in stable heart failure. ESC Heart Fail. 2020, 7, 2230–2239. [Google Scholar] [CrossRef]
- Suthahar, N.; Meijers, W.C.; Silljé, H.H.W.; Ho, J.E.; Liu, F.-T.; de Boer, R.A. Galectin-3 activation and inhibition in heart failure and cardiovascular disease: An update. Theranostics 2018, 8, 593–609. [Google Scholar] [CrossRef] [PubMed]
- Ndrepepa, G. Myeloperoxidase—A bridge linking inflammation and oxidative stress with cardiovascular disease. Clin. Chim. Acta 2019, 493, 36–51. [Google Scholar] [CrossRef] [PubMed]
- Posch, F.; Niedrist, T.; Glantschnig, T.; Firla, S.; Moik, F.; Kolesnik, E.; Wallner, M.; Verheyen, N.; Jost, P.J.; Zirlik, A. Left ventricular ejection fraction and cardiac biomarkers for dynamic prediction of cardiotoxicity in early breast cancer. Front. Cardiovasc. Med. 2022, 9, 933428. [Google Scholar] [CrossRef] [PubMed]
- Tlegenova, Z.; Balmagambetova, S.; Zholdin, B.; Kurmanalina, G.; Talipova, I.; Koyshybaev, A.; Urazova, A.; Nurmanova, D.; Urazayev, O.; Sultanbekova, G.; et al. A first approach to identifying cardiotoxic effects of Breast cancer chemotherapeutic treatment in Kazakhstan. J. Clin. Med. Kazakhstan 2022, 19, 28–35. [Google Scholar] [CrossRef]
- Balmagambetova, S.; Tlegenova, Z.; Zholdin, B.; Kurmanalina, G.; Talipova, I.; Koyshybaev, A.; Nurmanova, D.; Sultanbekova, G.; Baspayeva, M.; Kubenova, K.; et al. Early Diagnosis of Chemotherapy-Linked Cardiotoxicity in Breast Cancer Patients Using Conventional Biomarker Panel: A Prospective Study Protocol. Diagnostics 2022, 12, 2714. [Google Scholar] [CrossRef] [PubMed]
- Genuino, A.J.; Chaikledkaew, U.; The, D.O.; Reungwetwattana, T.; Thakkinstian, A. Adjuvant trastuzumab regimen for HER2-positive early-stage breast cancer: A systematic review and meta-analysis. Expert Rev. Clin. Pharmacol. 2019, 12, 815–824. [Google Scholar] [CrossRef]
- Lyon, A.R.; Dent, S.; Stanway, S.; Earl, H.; Brezden-Masley, C.; Cohen-Solal, A.; Tocchetti, C.G.; Moslehi, J.J.; Groarke, J.D.; Bergler-Klein, J.; et al. Baseline cardiovascular risk assessment in cancer patients scheduled to receive cardiotoxic cancer therapies: A position statement and new risk assessment tools from the Cardio-Oncology Study Group of the Heart Failure Association of the European Society of Cardiology in collaboration with the International Cardio-Oncology Society. Eur. J. Heart Fail. 2020, 22, 1945–1960. [Google Scholar] [CrossRef]
- Ĉelutkiene, J.; Pudil, R.; López-Fernández, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524, Erratum in Eur. J. Heart Fail. 2021, 23, 345. [Google Scholar] [CrossRef]
- Duncan, A.E.; Alfirevic, A.; Sessler, D.I.; Popovic, Z.B.; Thomas, J.D. Perioperative assessment of myocardial deformation. Anesth. Analg. 2014, 118, 525–544. [Google Scholar] [CrossRef]
- Si, M.; Jiang, H.; Zhao, Y.; Qi, X.; Li, R.; Long, X.; Qiao, J. Nomogram for Predicting Live Birth after the First Fresh Embryo Transfer in Patients with PCOS Undergoing IVF/ICSI Treatment with the GnRH-Ant Protocol. Diagnostics 2023, 13, 1927. [Google Scholar] [CrossRef]
- Tian, Y.; He, Y.; Li, X.; Liu, X. Novel nomograms to predict lymph node metastasis and distant metastasis in resected patients with early-stage non-small cell lung cancer. Ann. Palliat. Med. 2021, 10, 2548–2566. [Google Scholar] [CrossRef]
- MedCalcSoftware Ltd. Diagnostic Test Evaluation Calculator. Version 22.014. Available online: http://www.medcalc.org/calc/diagnostic_test.php (accessed on 8 October 2023).
- Trevethan, R. Sensitivity, Specificity, and Predictive Values: Foundations, Pliabilities, and Pitfalls in Research and Practice. Front. Public Health 2017, 5, 307. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.Z.I.; Turin, T.C. Variable selection strategies and its importance in clinical prediction modelling. Fam. Med. Community Health 2020, 8, e000262. [Google Scholar] [CrossRef] [PubMed]
- Valiyaveettil, D.; Joseph, D.; Malik, M. Cardiotoxicity in breast cancer treatment: Causes and mitigation. Cancer Treat. Res. Commun. 2023, 37, 100760. [Google Scholar] [CrossRef] [PubMed]
- Perez, I.E.; Taveras Alam, S.; Hernandez, G.A.; Sancassani, R. Cancer Therapy-Related Cardiac Dysfunction: An Overview for the Clinician. Clin. Med. Insights Cardiol. 2019, 13, 1179546819866445. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Jang, H.; Jeon, M.; Fadol, A.P.; Kim, S. Cancer treatment-related cardiac dysfunction in breast cancer survivors: A retrospective descriptive study using electronic health records from a Korean tertiary hospital. Eur. J. Oncol. Nurs. 2022, 59, 102163. [Google Scholar] [CrossRef]
- Cardinale, D.; Iacopo, F.; Cipolla, C.M. Cardiotoxicity of Anthracyclines. Front. Cardiovasc. Med. 2020, 7, 512096. [Google Scholar] [CrossRef]
- Esteban-Fernández, A.; Carvajal Estupiñan, J.F.; Gavira-Gómez, J.J.; Pernas, S.; Moliner, P.; Garay, A.; Sánchez-González, Á.; Fernández-Rozas, I.; González-Costello, J. Clinical Profile and Prognosis of a Real-World Cohort of Patients With Moderate or Severe Cancer Therapy-Induced Cardiac Dysfunction. Front. Cardiovasc. Med. 2021, 8, 721080. [Google Scholar] [CrossRef]
- Kobat, H.; Elkonaissi, I.; Foreman, E.; Davidson, M.; Idaikkadar, P.; O’Brien, M.; Nabhani-Gebara, S. Smoking, diabetes mellitus and previous cardiovascular disease as predictors of anti-cancer treatment-induced cardiotoxicity in non-small cell lung cancer: A real-world study. Clin. Lung Cancer 2023. In press. [Google Scholar] [CrossRef]
- Ruane, L.; Prasad, S.; Atherton, J. Straining for More Evidence. J. Am. Coll. Cardiol. CardioOnc. 2023, 5, 711–714. [Google Scholar] [CrossRef]
- Xu, A.; Yuan, M.; Zhan, X.; Zhao, G.; Mu, G.; Wang, T.; Hu, H.; Fu, H. Early detection of immune checkpoint inhibitor-related subclinical cardiotoxicity: A pilot study by using speckle tracking imaging and three-dimensional echocardiography. Front. Cardiovasc. Med. 2022, 9, 1087287. [Google Scholar] [CrossRef]
- Ardelean, A.M.; Olariu, I.C.; Isac, R.; Jurac, R.; Stolojanu, C.; Murariu, M.; Toma, A.-O.; Braescu, L.; Mavrea, A.; Doros, G. Correlation of Speckle-Tracking Echocardiography with Traditional Biomarkers in Predicting Cardiotoxicity among Pediatric Hemato-Oncology Patients: A Comprehensive Evaluation of Anthracycline Dosages and Treatment Protocols. Children 2023, 10, 1479. [Google Scholar] [CrossRef]
- Lu, X.; Zhao, Y.; Chen, C.; Han, C.; Xue, L.; Xing, D.; Huang, O.; Tao, M. BNP as a marker for early prediction of anthracycline-induced cardiotoxicity in patients with breast cancer. Oncol. Lett. 2019, 18, 4992–5001. [Google Scholar] [CrossRef] [PubMed]
- Leya, J.; Haiek, S. Role of Troponin and BNP in the Management of Patients with Cancer. Expert Analysis. American College of Cardiology. 2019. Available online: http://www.acc.org/latest-in-cardiology/articles/2019/12/10/15/15/role-of-troponin-and-bnp-in-the-management-of-patients-with-cancer (accessed on 11 December 2019).
- Di Lici, D.; Manno, G.; Novo, G. Subclinical Cardiotoxicity: The Emerging Role of Myocardial Work and Other Imaging Techniques. Curr. Probl. Cardiol. 2021, 46, 100818. [Google Scholar] [CrossRef] [PubMed]
- Papendick, C. High Sensitivity Troponin vs. Conventional Troponin: An Expert View. Available online: http://cardiothinklab.com/high-sensitivity-troponin-vs-conventional-troponin-an-expert-view/ (accessed on 7 December 2022).
- Tamura, Y.; Tamura, Y.; Takemura, R.; Yamada, K.; Taniguchi, H.; Iwasawa, J.; Yada, H.; Kawamura, A. Longitudinal Strain and Troponin I Elevation in Patients Undergoing Immune Checkpoint Inhibitor Therapy. JACC: CardioOncology 2022, 4, 673–685. [Google Scholar] [CrossRef]
- Lv, X.; Pan, C.; Guo, H.; Chang, J.; Gao, X.; Wu, X.; Zhi, X.; Ren, C.; Chen, Q.; Jiang, H.; et al. Early diagnostic value of high-sensitivity cardiac troponin T for cancer treatment-related cardiac dysfunction: A meta-analysis. ESC Heart Fail. 2023, 10, 2170–2182. [Google Scholar] [CrossRef]
- Ananthan, K.; Lyon, A.R. The Role of Biomarkers in Cardio-Oncology. J. Cardiovasc. Trans. Res. 2020, 13, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Gao, D.; Xue, J.; Zuo, Z. Galectin-3 and Myeloperoxidase May Monitor Cancer-Therapy-Related Cardiotoxicity? A Systematic Review and Meta-Analysis. Biomolecules 2022, 12, 1788. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Hirshfield, K.M.; Jabbour, S.K.; Toppmeyer, D.; Haffty, B.G.; Khan, A.J.; Goyal, S. Serum biomarkers for the detection of cardiac toxicity after chemotherapy and radiation therapy in breast cancer patients. Front. Oncol. 2014, 4, 277. [Google Scholar] [CrossRef] [PubMed]
- Oikawa, M.; Yaegashi, D.; Yokokawa, T.; Misaka, T.; Sato, T.; Kaneshiro, T.; Kobayashi, A.; Yoshihisa, A.; Nakazato, K.; Ishida, T.; et al. D-Dimer Is a Predictive Factor of Cancer Therapeutics-Related Cardiac Dysfunction in Patients Treated With Cardiotoxic Chemotherapy. Front. Cardiovasc. Med. 2022, 8, 807754. [Google Scholar] [CrossRef]
- Leerink, J.M.; de Baat, E.C.; Feijen, E.A.; Bellersen, L.; van Dalen, E.C.; Grotenhuis, H.B.; Kapusta, L.; Kok, W.E.; Loonen, J.; van der Pal, H.J.; et al. Cardiac disease in childhood cancer survivors: Risk prediction, prevention, and surveillance: Cardiopulmonary state-of-the-art review. J. Am. Coll. Cardiol. CardioOnc. 2020, 2, 363–378. [Google Scholar] [CrossRef]


| Variables | Total, n = 120 | Cardiotoxicity (−) n = 86 | Cardiotoxicity (+) n = 34 | p-Value |
|---|---|---|---|---|
| Age, years | 55.5 ± 11.1 | 52.5 ± 11.5 | 57.9 ± 8.9 | 0.018 |
| BMI*, kg/m2 | 27.3 (23.2; 30.9) | 26.1 (23.0; 30.9) | 28.6 (25.6; 31.3) | 0.056 |
| SBP*, mmHg | 120.0 ± 17.9 | 117.0 ± 17.0 | 127.3 ± 18.0 | 0.002 |
| Charlson comorbidity index, scores | 5 (3; 6) | 5 (3; 6) | 5 (4; 6) | 0.047 |
| Glucose, mmol/L | 5.2 (4.7; 5.6) | 5.2 (4.7; 5.5) | 5.5 (4.9; 6.0) | 0.018 |
| Cholesterol, mmol/L | 5.3 ± 0.85 | 5.3 ± 0.86 | 5.1 ± 0.84 | 0.297 |
| Creatinine, µmol/L | 67.6 (59.2; 77.2) | 67.4 (58.8;76.5) | 67.7 (62.0;78.4) | 0.467 |
| Egfr*, mL/min/1.73 m2 | 90.7 ± 17.6 | 92.7 ± 17.8 | 85.6 ± 16.1 | 0.070 |
| Baseline 6WT | 423.8 ± 60.5 | 431.8 ± 59.1 | 403.5 ± 59.9 | 0.038 |
| Baseline LVEF, % | 59.8 ± 3.6 | 60.1 ± 3.7 | 58.8 ± 3.3 | 0.074 |
| Baseline GLS, % | −18.2 (−16.8; −19.5) | −18.2 −16.9; −19.5) | −17.9 (−16.5; −19.4) | 0.835 |
| Baseline cTnI, ng/mL | 0.10 (0.10;0.10) | 0.10 (0.10; 0.10) | 0.10 (0.10; 0.22) | 0.337 |
| Baseline BNP, pg/mL | 48.9 (35.5; 65.1) | 49.3 (35.8; 65.5) | 48.9 (35.3; 60.5) | 0.628 |
| Baseline CRP, mg/L | 5.1 (2.8; 11.5) | 4.8 (2.7; 10.3) | 5.3 (3.3; 12.1) | 0.389 |
| Baseline D dimer, mg/L | 0.90 (0.53; 2.08) | 1.02 (0.54; 2.25) | 0.73 (0.52; 1.49) | 0.133 |
| Baseline MPO, pmol/L | 120 (100; 301) | 120 (100; 164) | 120 (120; 301) | 0.560 |
| Baseline Gal-3, ng/L | 14.7 (11.4; 21.1) | 14.7 (11.4; 20.5) | 14.7 (11.4; 23.3) | 0.596 |
| Anthracycline, cumulative doses | 408.9 ± 228.1 | 382.3 ± 233.4 | 476.0 ± 201.8 | 0.045 |
| Radiotherapy, GY* | 33.9 ± 15.9 | 34.5 ± 16.1 | 32.4 ± 15.6 | 0.392 |
| Baseline CV* risk, n (%) | 0.011 | |||
| High | 7 (5.83) | 3 (3.5) | 4 (11.8) | |
| Moderate | 46 (38.33) | 28 (32.5) | 18 (52.9) | |
| Low | 67 (55.83) | 55 (64.0) | 12 (35.3) | |
| Heredity, n (%) | 14 (11.7) | 11 (12.8) | 3 (8.8) | 0.542 |
| Menopause, n (%) | 79 (65.8) | 53 (61.6) | 26 (76.5) | 0.122 |
| Localization: | 0.174 | |||
| Right, n (%) | 52 (43.3) | 35 (50.0) | 17 (50.0) | |
| Left | 65 (54.2) | 51 (50.0) | 14 (41.2) | |
| Both sides | 3 (2.5) | - | 3 (8.8) | |
| Clinical stage, n (%): | 0.971 | |||
| I | 6 (5.0) | 5 (5.8) | 1 (2.9) | |
| IIA | 44 (36.7) | 32 (37.2) | 12 (35.3) | |
| IIB | 54 (45.0) | 37 (43.0) | 17 (50.0) | |
| IIIA | 5 (4.2) | 4 (4.7) | 1 (2.9) | |
| IIIB | 11 (9.1) | 8 (9.3) | 3 (8.8) | |
| Tumor histotype, n (%): | 0.522 | |||
| 1-Invasive carcinoma unspecified | 84 (70.0) | 57 (66.3) | 27 (79.4) | |
| 2-Invasive ductal carcinoma | 31 (25.8) | 25 (29.1) | 6 (17.7) | |
| 3-Invasive lobular cancer | 4 (3.3) | 3 (3.5) | 1 (2.9) | |
| 4-Angiosarcoma | 1 (0.83) | 1 (1.1) | - | |
| Breast cancer types, n (%): | 0.442 | |||
| Nodular cancer | 101 (84.2) | 72 (83.7) | 29 (85.4) | |
| Diffuse forms | 19 (15.8) | 14 (16.3) | 5 (14.6) | |
| Immunohistochemistry, n (%): | 0.065 | |||
| TNBC* | 19 (15.8) | 17 (19.8) | 2 (5.9) | |
| Luminal A type | 33 (27.5) | 23 (26.7) | 10 (29.4) | |
| Luminal B (positive) | 17 (14.2) | 12 (14.0) | 5 (14.7) | |
| Luminal B (negative) | 43 (35.8) | 26 (30.2) | 17 (50.0) | |
| HER2-positive | 8 (6.7) | 8 (9.3) | - | |
| Smoking, n (%) | 16 (13.3) | 11 (12.8) | 5 (14.7) | 0.781 |
| Hypertension, n (%) | 61 (50.8) | 35 (40.7) | 26 (76.5) | <0.001 |
| Diabetes mellitus, n (%) | 16 (13.3) | 6 (7.0) | 10 (29.4) | 0.001 |
| Ischemic heart disease, n (%) | 2 (1.7) | - | 2 (5.9) | 0.023 |
| Cardioprotective medications, if prescribed, n (%) | 61 (50.8) | 39 (45.3) | 22 (64.7) | 0.056 |
| Treatment administered, n (%): | 0.269 | |||
| Anthracyclines | 98 (81.7) | 68 (79.1) | 30 (88.2) | |
| Combined | 10 (8.3) | 7 (8.1) | 3 (8.8) | |
| Trastuzumab | 12 (10) | 11 (12.8) | 1 (2.9) |
| Visits | CTRCD(+), M (IQR), n = 34 | CTRCD(−), M (IQR), n = 86 | p-Value |
|---|---|---|---|
| GLS (%): | |||
| 1 | −18.1 (−19.4; −16.5) | −18.2 (−19.5; −16.9) | 0.835 |
| 2 | −16.6 (−17.6; −15.5) | −17.75 (−18.7; −16.7) | 0.003 |
| 3 | −15.7 (−16.7; −14.1) | −17.6 (−18.8; −16.4) | <0.000 |
| 4 | −14.9 (−16.1; −13.2) | −17.4 (−18.4; −16.4) | <0.000 |
| 5 | −13.4 (−14.7; −12.2) | −17.1 (−18.4; −15.7) | <0.000 |
| ANOVA Chi Sqr. (n = 34, df = 4) 88.846; p < 0.00001; Coeff. of concordance 0.653; aver. Rank r = 0.643. | ANOVA Chi Sqr. (n = 86, df = 4) 16.945; p 0.002; Coeff. of concordance 0.049; aver. Rank r = 0.038. | ||
| LVEF (%): | |||
| 1 | 58.0 (57.0; 61.0) | 60.0 (58.0; 62.0) | 0.075 |
| 2 | 56.0 (55.0; 59.0) | 58.0 (56.0; 60.0) | 0.004 |
| 3 | 56.0 (55.0; 57.0) | 58.0 (56.0; 60.0) | 0.0003 |
| 4 | 56.0 (55.0; 58.0) | 58.0 (56.0; 59.0) | 0.0001 |
| 5 | 56.0 (54.0; 57.0) | 57.0 (56.0; 59.0) | 0.0003 |
| ANOVA Chi Sqr. (n = 34. df = 4) 35.98; p < 0.00000; Coeff. of concordance = 0.264; aver. Rank r = 0.242. | ANOVA Chi Sqr. (n = 86. df = 4) 39.92; p < 0.00000; Coeff. of concordance = 0.116; aver. Rank r = 0.106. | ||
| Visits | CTRCD(+), M (IQR), n = 34 | CTRCD(−), M (IQR), n = 86 | p-Value |
|---|---|---|---|
| 1 | 48.9 (35.3; 60.5) | 49.3 (35.7; 65.5) | 0.629 |
| 2 | 56.8 (42.7; 68.3) | 49.0 (37.2; 64.1) | 0.104 |
| 3 | 65.3 (48.0; 89.7) | 61.7 (47.9; 83.1) | 0.463 |
| 4 | 78.6 (62.5; 97.9) | 64.4 (52.0; 81.5) | 0.006 |
| 5 | 87.8 (71.8; 114.5) | 67.9 (58.9; 89.00) | 0.001 |
| ANOVA Chi Sqr. (n = 34; df = 4) 38.353; p < 0.00000; Coeff. of concordance = 0.282; aver. Rank r = 0.260. | ANOVA Chi Sqr. (n = 86; df =4) 61.455; p< 0.00000; Coeff. of concordance = 0.179; aver. Rank r = 0.169. | ||
| Variables, n 120 | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | p-Value | aOR (95% CI) | p-Value | |
| Age, years | 1.049 (1.008;1.092) | 0.019 | ||
| Body mass index, kg/m2 | 1.059 (0.998;1.124) | 0.051 | ||
| SBP, mmHg | 1.032 (1.009;1.056) | 0.006 | ||
| Charlson comorbidity index, scores | 1.166 (0.960;1.416) | 0.121 | ||
| Cholesterol, mmol/L | 0.798 (0.492;1.295) | 0.361 | ||
| Glucosae, mmol/L | 1.479 (1.030;2.124) | 0.034 | ||
| Creatinine, µmol/L | 1.018 (0.988;1.049) | 0.232 | ||
| eGFR, mL/min/1.73 m2 | 0.977 (0.954;1.00) | 0.048 | ||
| Baseline 6WT, meters | 0.992 (0.985;0.999) | 0.024 | ||
| Baseline cTnI, ng/mL | 7.674 (0.065;903.591) | 0.402 | ||
| Baseline BNP, pg/mL | 1.011 (0.997;1.025) | 0.116 | ||
| Baseline CRP, mg/L | 1.002 (0.983;1.022) | 0.822 | ||
| Baseline D-dimer, mg/L | 0.781 (0.568;1.074) | 0.129 | ||
| Baseline MPO, U/mL | 0.997 (0.986;1.009) | 0.650 | ||
| Baseline Gal-3, ng/mL | 1.023 (0.975;1.075) | 0.351 | ||
| Baseline LVEF,% | 0.901 (0.801;1.013) | 0.080 | ||
| Baseline GLS,% | 1.037 (0.880;1.222) | 0.663 | ||
| Anthracycline, cumulative dose | 1.002 (1.000;1.004) | 0.046 | 1.002 (1.000; 1.004) | 0.030 |
| Baseline cardiotoxicity risk: | ||||
| Low risk | Reference | |||
| Moderate | 2.569 (1.094;6.031) | 0.030 | ||
| High | 5.641 (1.123;28.345) | 0.036 | ||
| Heredity | 0.660 (0.172;2.529) | 0.544 | ||
| Menopause | 2.024 (0.820;4.996) | 0.126 | ||
| Left side of the breast | 0.565 (0.247;1.293) | 0.177 | ||
| Smoking | 1.176 (0.376;3.678) | 0.781 | ||
| Hypertension | 4.736 (1.922;11.667) | 0.001 | 5.178 (2.042; 13.131) | 0.001 |
| Diabetes mellitus | 5.556 (1.831;16.860) | 0.002 | ||
| Cardioprotective medications, if prescribed | 2.21 (0.97;5.02) | 0.058 | ||
| Radiotherapy, total dose in Gy | 0.992 (0.968;1.016) | 0.059 | ||
| Radiotherapy, if received | 0.987 (0.287;3.390) | 0.983 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tlegenova, Z.; Balmagambetova, S.; Zholdin, B.; Kurmanalina, G.; Talipova, I.; Koyshybaev, A.; Sultanbekova, G.; Baspayeva, M.; Madinova, S.; Kubenova, K.; et al. Role of Clinical Risk Factors and B-Type Natriuretic Peptide in Assessing the Risk of Asymptomatic Cardiotoxicity in Breast Cancer Patients in Kazakhstan. Diagnostics 2023, 13, 3557. https://doi.org/10.3390/diagnostics13233557
Tlegenova Z, Balmagambetova S, Zholdin B, Kurmanalina G, Talipova I, Koyshybaev A, Sultanbekova G, Baspayeva M, Madinova S, Kubenova K, et al. Role of Clinical Risk Factors and B-Type Natriuretic Peptide in Assessing the Risk of Asymptomatic Cardiotoxicity in Breast Cancer Patients in Kazakhstan. Diagnostics. 2023; 13(23):3557. https://doi.org/10.3390/diagnostics13233557
Chicago/Turabian StyleTlegenova, Zhenisgul, Saule Balmagambetova, Bekbolat Zholdin, Gulnara Kurmanalina, Iliada Talipova, Arip Koyshybaev, Gulmira Sultanbekova, Mira Baspayeva, Saule Madinova, Kulparshan Kubenova, and et al. 2023. "Role of Clinical Risk Factors and B-Type Natriuretic Peptide in Assessing the Risk of Asymptomatic Cardiotoxicity in Breast Cancer Patients in Kazakhstan" Diagnostics 13, no. 23: 3557. https://doi.org/10.3390/diagnostics13233557






