The Role of an Artificial Intelligence Method of Improving the Diagnosis of Neoplasms by Colonoscopy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Randomization
2.3. Study Progress
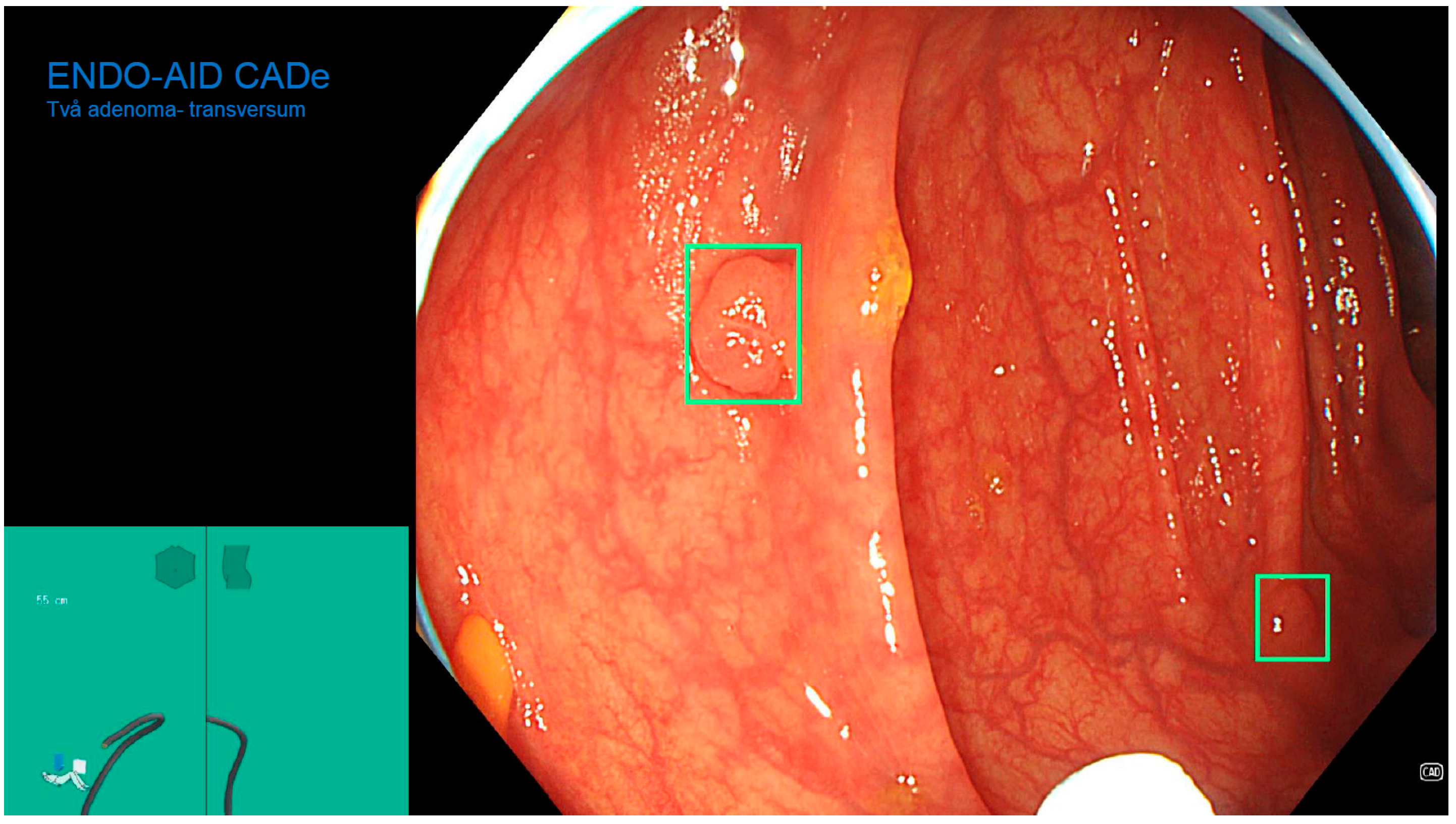
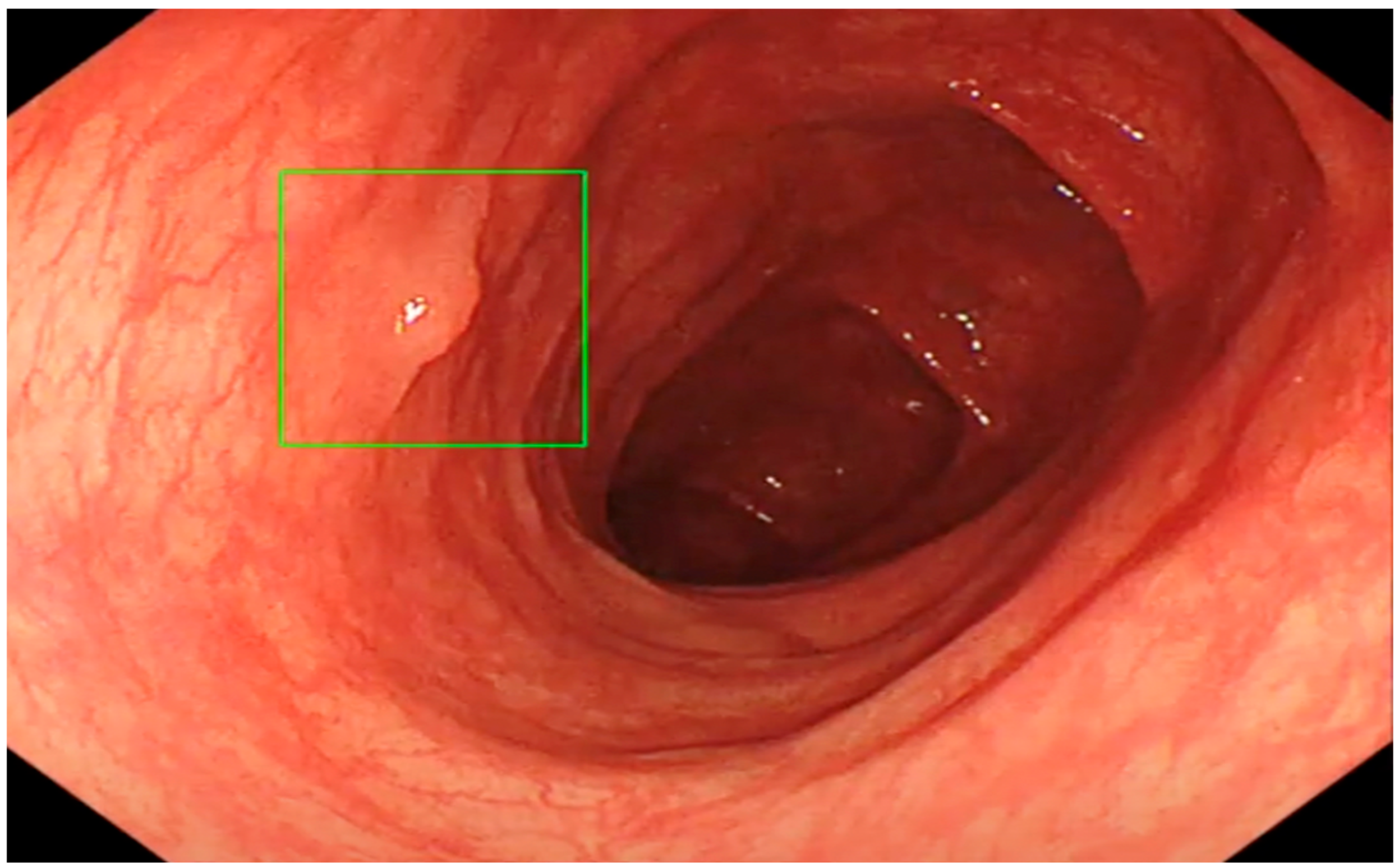
2.4. Colonoscopy Procedure
2.5. Morphological Diagnosis of Lesions Found during Colonoscopy
2.6. Statistical Analysis
3. Results
3.1. Polyp Detection Rates (PDR)
3.2. Adenoma Detection Rate (ADR)
4. Discussion
The Role of Artificial Intelligence in Colonoscopy Examinations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Boyle, P. Global public health—Challenges and leadership. J. Health Inequalities 2018, 4, 55–61. [Google Scholar] [CrossRef]
- World Cancer Research Fund International. Colorectal Cancer Statistics. Available online: https://www.wcrf.org/cancer-trends/colorectal-cancer-statistics/ (accessed on 10 April 2022).
- Lewandowska, A.; Religioni, U.; Czerw, A.; Deptała, A.; Karakiewicz, B.; Partyka, O.; Pajewska, M.; Sygit, K.; Cipora, E.; Kmieć, K.; et al. Nutritional Treatment of Patients with Colorectal Cancer. Int. J. Environ. Res. Public Health 2022, 19, 6881. [Google Scholar] [CrossRef] [PubMed]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Boland, R.C.; Dominitz, J.A.; Giardiello, F.M.; Johnson, D.A.; Kaltenbach, T.; Levin, T.R.; Lieberman, D.; Robertson, D.J. Colorectal Cancer Screening: Recommendations for Physicians and Patients from the U.S. Multi-Society Task Force on Colorectal Cancer. Am. J. Gastroenterol. 2017, 112, 1016–1030. [Google Scholar] [CrossRef]
- Doubeni, C.A.; Corley, D.A.; Quinn, V.P.; Jensen, C.D.; Zauber, A.G.; Goodman, M.; Johnson, J.R.; Mehta, S.J.; Becerra, T.A.; Zhao, W.K.; et al. Effectiveness of screening colonoscopy in reducing the risk of death from right and left colon cancer: A large community-based study. Gut 2018, 67, 291–298. [Google Scholar] [CrossRef]
- Kuipers, E.J.; Grady, W.M.; Lieberman, D.; Seufferlein, T.; Sung, J.J.; Boelens, P.G. Colorectal cancer. Nat. Rev. Dis. Prim. 2015, 1, 15065. [Google Scholar] [CrossRef]
- Xu, D.; Liu, Y.; Tang, W.; Xu, L.; Liu, T.; Jiang, Y.; Zhou, S.; Qin, X.; Li, J.; Zhao, J.; et al. Regorafenib in Refractory Metastatic Colorectal Cancer: A Multi-Center Retrospective Study. Front. Oncol. 2022, 12, 838870. [Google Scholar] [CrossRef]
- Mehta, R.; Frakes, J.; Kim, J.; Nixon, A.; Liu, Y.; Howard, L.; Jimenez, M.E.M.; Carballido, E.; Imanirad, I.; Sanchez, J.; et al. Phase I Study of Lenvatinib and Capecitabine with External Radiation Therapy in Locally Advanced Rectal Adenocarcinoma. Oncologist 2022, 27, e617–e621. [Google Scholar] [CrossRef]
- Gupta, S. Screening for Colorectal Cancer. Hematol. Oncol. Clin. North Am. 2022, 36, 393–414. [Google Scholar] [CrossRef]
- Bae, J.M.; Kim, J.H.; Kang, G.H. Molecular Subtypes of Colorectal Cancer and Their Clinicopathologic Features, with an Emphasis on the Serrated Neoplasia Pathway. Arch. Pathol. Lab Med. 2016, 140, 406–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corley, D.A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Lee, J.K.; Doubeni, C.A.; Zauber, A.G.; de Boer, J.; Fireman, B.H.; Schottinger, J.E.; et al. Adenoma detection rate and risk of colorectal cancer and death. N. Engl. J. Med. 2014, 370, 1298–1306. [Google Scholar] [CrossRef]
- Marcondes, F.O.; Gourevitch, R.A.; Schoen, R.E.; Crockett, S.D.; Morris, M.; Mehrotra, A. Adenoma Detection Rate Falls at the End of the Day in a Large Multi-site Sample. Dig. Dis. Sci. 2018, 63, 856–859. [Google Scholar] [CrossRef]
- Pu, L.Z.C.T.; Lu, K.; Ovenden, A.; Rana, K.; Singh, G.; Krishnamurthi, S.; Edwards, S.; Wilson, B.; Nakamura, M.; Yamamura, T.; et al. Effect of time of day and specialty on polyp detection rates in Australia. J. Gastroenterol. Hepatol. 2019, 34, 899–906. [Google Scholar]
- Chan, M.Y.; Cohen, H.; Spiegel, B.M. Fewer polyps detected by colonoscopy as the day progresses at a Veteran’s Administration teaching hospital. Clin. Gastroenterol. Hepatol. 2009, 7, 1217–1223. [Google Scholar] [CrossRef]
- Mahmud, N.; Cohen, J.; Tsourides, K.; Berzin, T.M. Computer vision and augmented reality in gastrointestinal endoscopy. Gastroenterol. Rep. 2015, 3, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, H.R.; Shieh, F.K.; Chan, F.W.; Ciarleglio, M.; Deng, Y.; Rogart, J.N.; Jamidar, P.A.; Siddiqui, U.D. Nurse observation during colonoscopy increases polyp detection: A randomized prospective study. Am. J. Gastroenterol. 2013, 108, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Misawa, M.; Kudo, S.-E.; Mori, Y.; Cho, T.; Kataoka, S.; Yamauchi, A.; Ogawa, Y.; Maeda, Y.; Takeda, K.; Ichimasa, K.; et al. Artificial Intelligence-Assisted Polyp Detection for Colonoscopy: Initial Experience. Gastroenterology 2018, 154, 2027–2029. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.F.; Chapados, N.; Soudan, F.; Oertel, C.; Pérez, M.L.; Kelly, R.; Iqbal, N.; Chandelier, F.; Rex, D.K. Real-time differentiation of adenomatous and hyperplastic diminutive colorectal polyps during analysis of unaltered videos of standard colonoscopy using a deep learning model. Gut 2019, 68, 94–100. [Google Scholar] [CrossRef]
- Klare, P.; Sander, C.; Prinzen, M.; Haller, B.; Nowack, S.; Abdelhafez, M.; Poszler, A.; Brown, H.; Wilhelm, D.; Schmid, R.M.; et al. Automated polyp detection in the colorectum: A prospective study (with videos). Gastrointest. Endosc. 2019, 89, 576–582.e1. [Google Scholar] [CrossRef]
- Participants in the Paris Workshop. The Paris endoscopic classification of superficial neoplastic lesions: Esophagus, stomach, and colon: 30 November to 1 December 2002. Gastrointest. Endosc. 2003, 58, S3–S43. [Google Scholar] [CrossRef]
- Hewett, D.; Kaltenbach, T.; Sano, Y.; Tanaka, S.; Saunders, B.P.; Ponchon, T.; Soetikno, R.; Rex, D.K. Validation of a simple classification system for endoscopic diagnosis of small colorectal polyps using narrow-band imaging. Gastroenterology 2012, 143, 599–607.e1. [Google Scholar] [CrossRef] [PubMed]
- Ahadi, M.; Sokolova, A.; Brown, I.; Chou, A.; Gill, A.J. The 2019 World Health Organization Classification of appendiceal, colorectal and anal canal tumours: An update and critical assessment. Pathology 2021, 53, 454–461. [Google Scholar] [CrossRef]
- IBM Corp. Released IBM SPSS Statistics for Windows, Version 28.0; IBM Corp.: Armonk, NY, USA, 2021. [Google Scholar]
- Burke, C.; Kaul, V.; Pohl, H. Polyp Resection and Removal Procedures: Insights From the 2017 Digestive Disease Week. Gastroenterol. Hepatol. 2017, 13, 1–24. [Google Scholar]
- Cohen, J.; Grunwald, D.; Grossberg, L.B.; Sawhney, M. The Effect of Right Colon Retroflexion on Adenoma Detection: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2017, 51, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Chin, M.; Karnes, W.; Jamal, M.M.; Lee, J.G.; Lee, R.; Samarasena, J.; Bechtold, M.L.; Nguyen, D.L. Use of the Endocuff during routine colonoscopy examination improves adenoma detection: A meta-analysis. World J. Gastroenterol. 2016, 22, 9642–9649. [Google Scholar] [CrossRef]
- Rex, D.K. Maximizing detection of adenomas and cancers during colonoscopy. Am. J. Gastroenterol. 2006, 101, 2866–2877. [Google Scholar] [CrossRef]
- Memmert, D.; Unkelbach, C.; Ganns, S. The impact of regulatory fit on performance in an inattentional blindness paradigm. J. Gen. Psychol. 2010, 137, 129–139. [Google Scholar] [CrossRef]
- Alagappan, M. Artificial intelligence in gastrointestinal endoscopy: The future is almost here. World J. Gastrointest. Endosc. 2018, 10, 239–249. [Google Scholar] [CrossRef]
- Wang, P.; Berzin, T.M.; Brown, J.R.G.; Bharadwaj, S.; Becq, A.; Xiao, X.; Liu, P.; Li, L.; Song, Y.; Zhang, D.; et al. Real-time automatic detection system increases colonoscopic polyp and adenoma detection rates: A prospective randomised controlled study. Gut 2019, 68, 1813–1819. [Google Scholar] [CrossRef] [PubMed]
- Krupinski, E.A. Current perspectives in medical image perception. Atten. Percept. Psychophys. 2010, 72, 1205–1217. [Google Scholar] [CrossRef] [PubMed]
- Travis, S.P.L.; Schnell, D.; Krzeski, P.; Abreu, M.T.; Altman, D.G.; Colombel, J.-F.; Feagan, B.G.; Hanauer, S.B.; Lémann, M.; Lichtenstein, G.R.; et al. Developing an instrument to assess the endoscopic severity of ulcerative colitis: The Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut 2012, 61, 535–542. [Google Scholar] [CrossRef]
- Murphy, K.P. Machine Learning: A Probabilistic Perspective; MIT Press: Cambridge, MA, USA, 2013. [Google Scholar]
- Su, J.R.; Li, Z.; Shao, X.J.; Ji, C.R.; Ji, R.; Zhou, R.C.; Li, G.C.; Liu, G.Q.; He, Y.S.; Zuo, X.L.; et al. Impact of a real-time automatic quality control system on colorectal polyp and adenoma detection: A prospective randomized controlled study (with videos). Gastrointest. Endosc. 2020, 91, 415–424.e4. [Google Scholar] [CrossRef] [PubMed]
- Deliwala, S.S.; Hamid, K.; Barbarawi, M.; Lakshman, H.; Zayed, Y.; Kandel, P.; Malladi, S.; Singh, A.; Bachuwa, G.; Gurvits, G.E.; et al. Artificial intelligence (AI) real-time detection vs. routine colonoscopy for colorectal neoplasia: A meta-analysis and trial sequential analysis. Int. J. Colorectal. Dis. 2021, 36, 2291–2303. [Google Scholar]
- Gates, M.; Wingert, A.; Featherstone, R.; Samuels, C.; Simon, C.; Dyson, M.P. Impact of fatigue and insufficient sleep on physician and patient outcomes: A systematic review. BMJ Open 2018, 8, e021967. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, K.; Xu, L.; Yuan, X.; Wu, Y.; Chen, P. Diagnostic yield is not influenced by the timing of screening endoscopy: Morning versus afternoon. Scand J. Gastroenterol. 2018, 53, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Jensen, C.D.; Marks, A.R.; Zhao, W.K.; Doubeni, C.A.; Zauber, A.G.; Quinn, V.P.; Levin, T.R.; Corley, D.A. Endoscopist fatigue estimates and colonoscopic adenoma detection in a large community-based setting. Gastrointest. Endosc. 2017, 85, 601–610. [Google Scholar] [CrossRef] [PubMed]
- Rex, D.K.; Schoenfeld, P.S.; Cohen, J.; Pike, I.M.; Adler, D.G.; Fennerty, M.B.; Lieb, J.G., 2nd; Park, W.G.; Rizk, M.K.; Sawhney, M.S.; et al. Quality indicators for colonoscopy. Gastrointest. Endosc. 2015, 81, 31–53. [Google Scholar] [CrossRef]
- Harewood, G.C.; Lieberman, D.A. Colonoscopy practice patterns since introduction of medicare coverage for average-risk screening. Clin. Gastroenterol. Hepatol. 2004, 2, 72–77. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, J.; Chen, Y.; Sun, H.; Li, B.; Zhang, Q.; Sun, K.; Wang, Z.; Qian, X.; Zhan, T.; et al. Negative Effects of Endoscopists’ Fatigue on Colonoscopy Quality on 34,022 Screening Colonoscopies. J. Gastrointestin. Liver Dis. 2021, 30, 358–365. [Google Scholar] [CrossRef]
- Singh, S.; Dhawan, M.; Chowdhry, M.; Babich, M.; Aoun, E. Differences between morning and afternoon colonoscopies for adenoma detection in female and male patients. Ann. Gastroenterol. 2016, 29, 497–501. [Google Scholar] [CrossRef]
- Liu, A.; Wang, H.; Lin, Y.; Fu, L.; Liu, Y.; Yan, S.; Chen, H. Gastrointestinal endoscopy nurse assistance during colonoscopy and polyp detection: A PRISMA-compliant meta-analysis of randomized control trials. Medicine (Baltimore) 2020, 99, e21278. [Google Scholar] [CrossRef] [PubMed]
- Teng, T.Y.; Khor, S.N.; Kailasam, M.; Cheah, W.K.; Lau, C.C.L. Morning colonoscopies are associated with improved adenoma detection rates. Surg. Endosc. 2016, 30, 1796–1803. [Google Scholar] [CrossRef] [PubMed]
- Jaho, F.; Kroijer, R.; Ploug, M. Time-of-day variation in the diagnostic quality of screening colonoscopies: A registry-based study. Ann. Gastroenterol. 2021, 34, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, S.-B.; Wang, S.-L.; Fang, J.; Xia, T.; Su, X.-J.; Xu, C.; Li, Z.-S.; Bai, Y. Comparison of efficacy of colonoscopy between the morning and afternoon: A systematic review and meta-analysis. Dig. Liver Dis. 2018, 50, 661–667. [Google Scholar] [CrossRef]



| Without AI | With AI | Overall | |
|---|---|---|---|
| Females | 104 | 103 | 207 |
| Males | 102 | 91 | 193 |
| DR1 | |||
| Patients | 98 | 91 | 189 |
| Morning | 42 | 43 | 85 |
| Afternoon | 56 | 48 | 104 |
| DR2 | |||
| Patients | 108 | 103 | 211 |
| Morning | 62 | 57 | 119 |
| Afternoon | 46 | 46 | 92 |
| Overall | |||
| Patients | 206 | 194 | 400 |
| Morning Time | p-Value | Afternoon | p-Value | |||
|---|---|---|---|---|---|---|
| Without AI | With AI | Without AI | With AI | |||
| Colonoscopies | 42 | 43 | 56 | 48 | ||
| Polyps found | 14 | 14 | 0.939 | 16 | 18 | 0.333 |
| PDR | 33.3% | 32.6% | 28.5% | 37.5% | ||
| Adenomas found | 7 | 12 | 0.214 | 8 | 11 | 0.256 |
| ADR | 16.3% | 27.9% | 14.3% | 22.9% | ||
| Morning Time | p-Value | Afternoon | p-Value | |||
|---|---|---|---|---|---|---|
| Without AI | With AI | Without AI | With AI | |||
| Colonoscopies | 62 | 57 | 46 | 46 | ||
| Polyps found | 37 | 31 | 0.56 | 18 | 23 | 0.294 |
| PDR | 59.70% | 54.40% | 39.10% | 50% | ||
| Adenomas found | 18 | 20 | 0.479 | 10 | 16 | 0.165 |
| ADR | 29% | 35.10% | 21.70% | 34.80% | ||
| Morning Time | p-Value | Afternoon | p-Value | |||
|---|---|---|---|---|---|---|
| Without AI | With AI | Without AI | With AI | |||
| Colonoscopies | 104 | 100 | 102 | 94 | ||
| Polyps found | 51 | 45 | 0.563 | 34 | 41 | 0.139 |
| PDR | 49.04% | 45.00% | 33.33% | 43.62% | ||
| Adenomas found | 25 | 32 | 0.205 | 18 | 27 | 0.065 |
| ADR | 24.04% | 32.00% | 17.65% | 28.72% | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vilkoite, I.; Tolmanis, I.; Meri, H.A.; Polaka, I.; Mezmale, L.; Anarkulova, L.; Leja, M.; Lejnieks, A. The Role of an Artificial Intelligence Method of Improving the Diagnosis of Neoplasms by Colonoscopy. Diagnostics 2023, 13, 701. https://doi.org/10.3390/diagnostics13040701
Vilkoite I, Tolmanis I, Meri HA, Polaka I, Mezmale L, Anarkulova L, Leja M, Lejnieks A. The Role of an Artificial Intelligence Method of Improving the Diagnosis of Neoplasms by Colonoscopy. Diagnostics. 2023; 13(4):701. https://doi.org/10.3390/diagnostics13040701
Chicago/Turabian StyleVilkoite, Ilona, Ivars Tolmanis, Hosams Abu Meri, Inese Polaka, Linda Mezmale, Linda Anarkulova, Marcis Leja, and Aivars Lejnieks. 2023. "The Role of an Artificial Intelligence Method of Improving the Diagnosis of Neoplasms by Colonoscopy" Diagnostics 13, no. 4: 701. https://doi.org/10.3390/diagnostics13040701







