The Adaptive Force as a Potential Biomechanical Parameter in the Recovery Process of Patients with Long COVID
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Questionnaires
2.3. Handheld Device to Measure the Adaptive Force
2.4. Manual Muscle Test to Assess the Adaptive Force: Procedure and Setting
2.5. Procedure
2.6. Data Processing and Statistical Analyses
- Maximal Adaptive Force (AFmax):
- 2.
- Maximal isometric Adaptive Force (AFisomax):
- 3.
- Adaptive Force at the moment of onset of oscillations (AFosc):
- 4.
- Slope of force rise:
3. Results
3.1. Number of Trials and Subjective MMT Ratings by the Testers
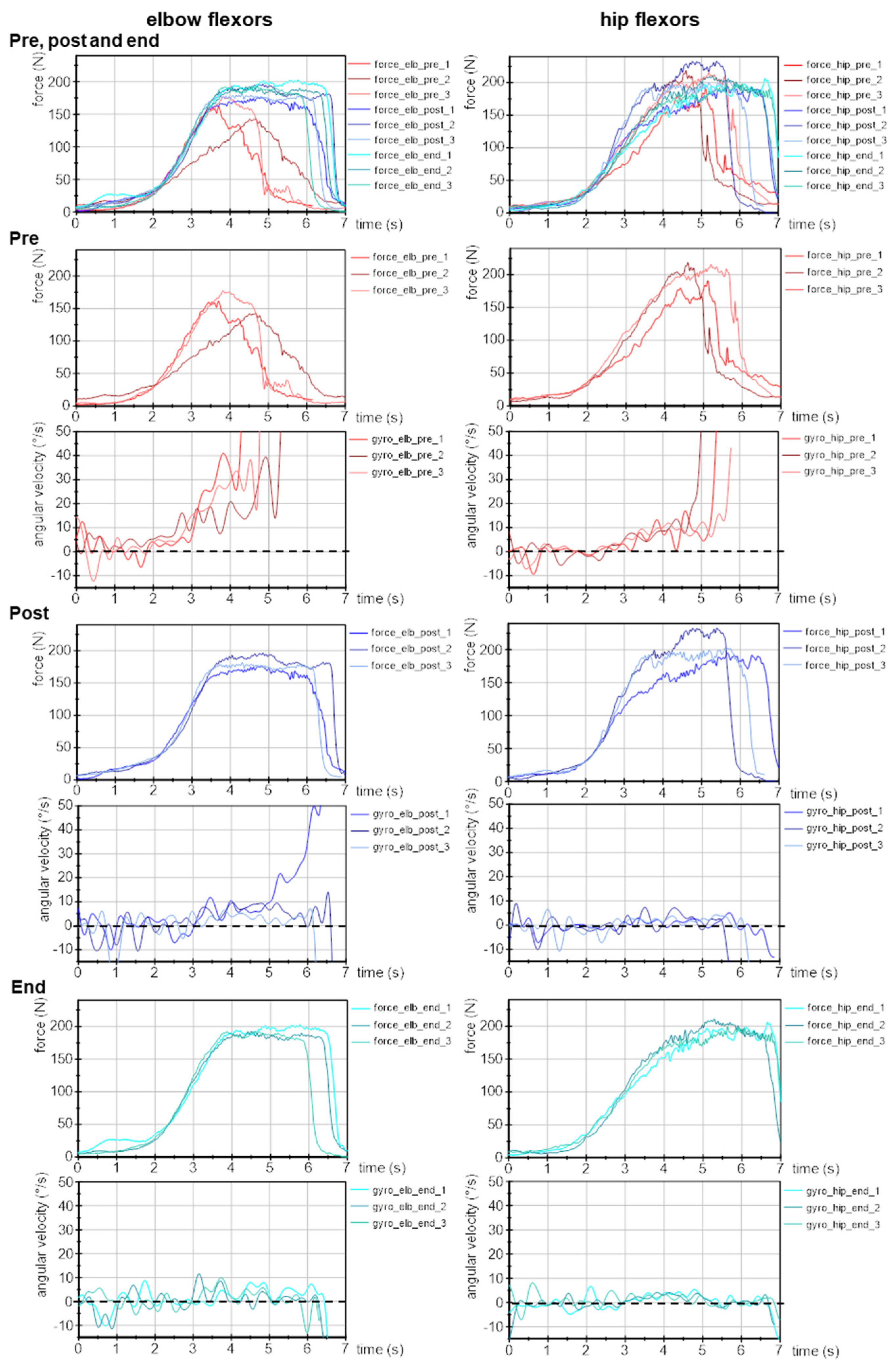
3.2. Parameters of Adaptive Force in the Course of Long COVID
3.2.1. Slope of Force Increase
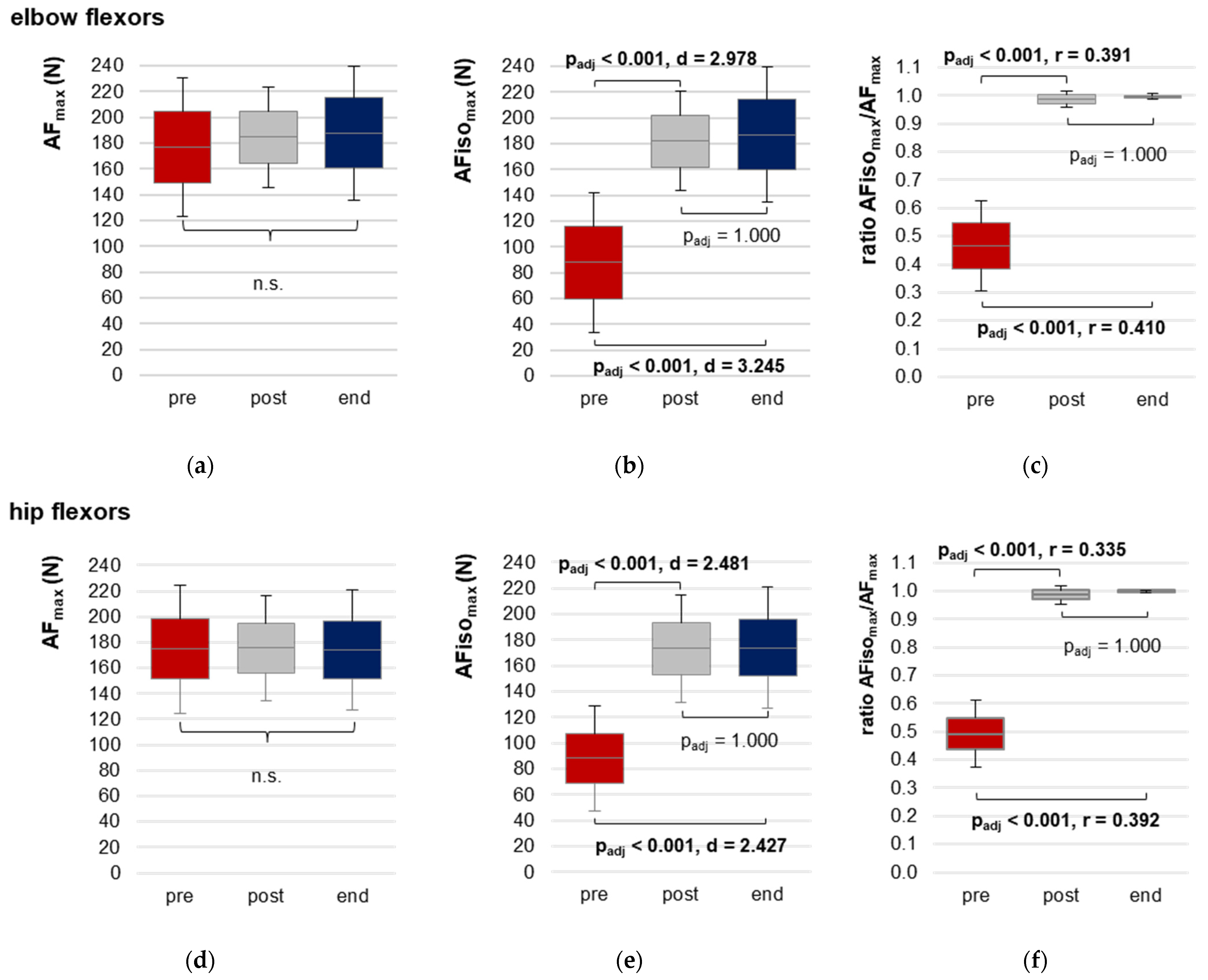
3.2.2. Maximal Adaptive Force and Maximal Isometric Adaptive Force
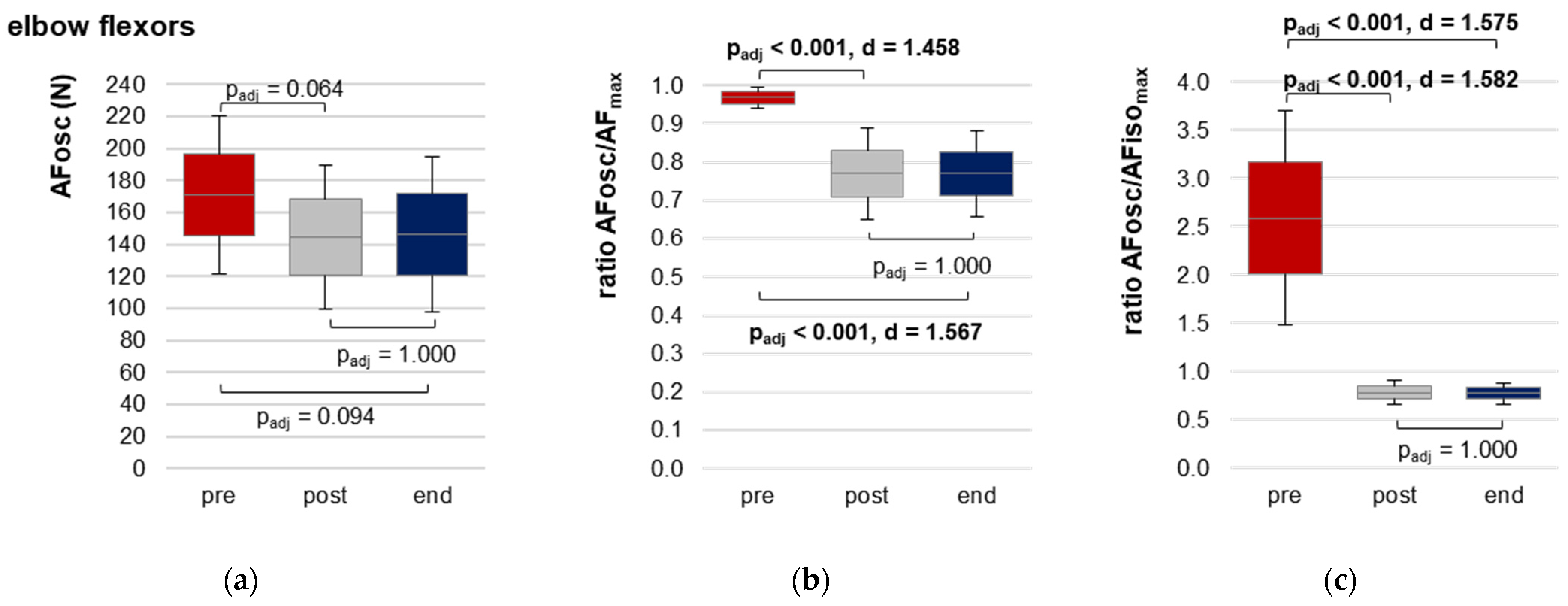
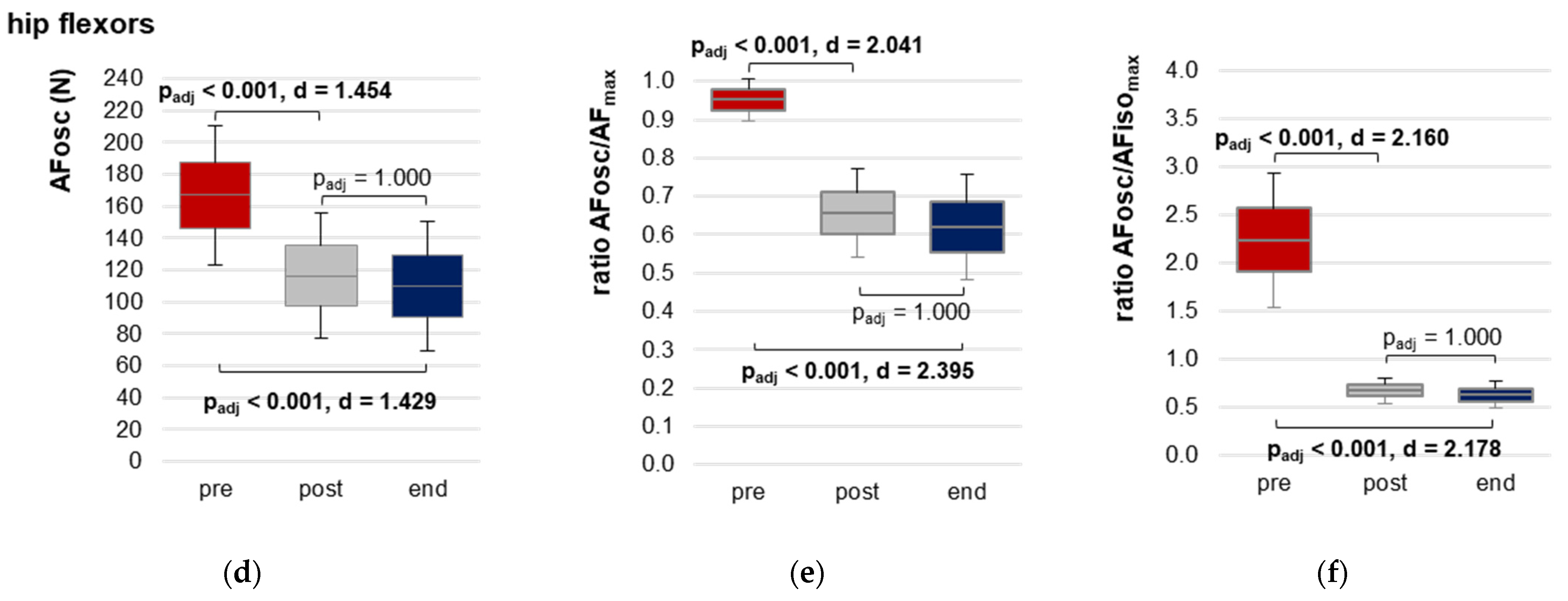
3.2.3. Onset of Oscillations during Force Increase
3.3. Patients Characteristics Regarding Long COVID
4. Discussion
4.1. Comparison of the Subjective Ratings of the Manual Muscle Test and Measured AF
4.2. Adaptive Force in the Recovery Process of Long COVID
4.3. Neurophysiological Considerations with Respect to the Reaction of AF in Long COVID
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Cristanziano, V.D.; Osebold, L.; et al. Post-COVID Syndrome in Non-Hospitalised Patients with COVID-19: A Longitudinal Prospective Cohort Study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Greenhalgh, T.; Knight, M.; A’Court, C.; Buxton, M.; Husain, L. Management of Post-Acute Covid-19 in Primary Care. BMJ 2020, 370, m3026. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Billig Rose, E.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network—United States, March–June 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Pavli, A.; Theodoridou, M.; Maltezou, H.C. Post-COVID Syndrome: Incidence, Clinical Spectrum, and Challenges for Primary Healthcare Professionals. Arch. Med. Res. 2021, 52, 575–581. [Google Scholar] [CrossRef] [PubMed]
- Maltezou, H.C.; Pavli, A.; Tsakris, A. Post-COVID Syndrome: An Insight on Its Pathogenesis. Vaccines 2021, 9, 497. [Google Scholar] [CrossRef]
- Taquet, M.; Dercon, Q.; Luciano, S.; Geddes, J.R.; Husain, M.; Harrison, P.J. Incidence, Co-Occurrence, and Evolution of Long-COVID Features: A 6-Month Retrospective Cohort Study of 273,618 Survivors of COVID-19. PLoS Med. 2021, 18, e1003773. [Google Scholar] [CrossRef]
- Huang, L.; Yao, Q.; Gu, X.; Wang, Q.; Ren, L.; Wang, Y.; Hu, P.; Guo, L.; Liu, M.; Xu, J.; et al. 1-Year Outcomes in Hospital Survivors with COVID-19: A Longitudinal Cohort Study. Lancet 2021, 398, 747–758. [Google Scholar] [CrossRef]
- Stavem, K.; Ghanima, W.; Olsen, M.K.; Gilboe, H.M.; Einvik, G. Persistent Symptoms 1.5–6 Months after COVID-19 in Non-Hospitalised Subjects: A Population-Based Cohort Study. Thorax 2021, 76, 405–407. [Google Scholar] [CrossRef]
- Carfì, A.; Bernabei, R.; Landi, F.; Gemelli Against COVID-19 Post-Acute Care Study Group. Persistent Symptoms in Patients after Acute COVID-19. JAMA 2020, 324, 603. [Google Scholar] [CrossRef]
- Raveendran, A.V.; Jayadevan, R.; Sashidharan, S. Long COVID: An Overview. Diabetes Metab. Syndr. Clin. Res. Rev. 2021, 15, 869–875. [Google Scholar] [CrossRef]
- Crook, H.; Raza, S.; Nowell, J.; Young, M.; Edison, P. Long Covid—Mechanisms, Risk Factors, and Management. BMJ 2021, 374, n1648. [Google Scholar] [CrossRef] [PubMed]
- Venkatesan, P. NICE Guideline on Long COVID. Lancet Respir. Med. 2021, 9, 129. [Google Scholar] [CrossRef] [PubMed]
- Ceban, F.; Ling, S.; Lui, L.M.W.; Lee, Y.; Gill, H.; Teopiz, K.M.; Rodrigues, N.B.; Subramaniapillai, M.; Di Vincenzo, J.D.; Cao, B.; et al. Fatigue and Cognitive Impairment in Post-COVID-19 Syndrome: A Systematic Review and Meta-Analysis. Brain Behav. Immun. 2022, 101, 93–135. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Zheng, B.; Daines, L.; Sheikh, A. Long-Term Sequelae of COVID-19: A Systematic Review and Meta-Analysis of One-Year Follow-Up Studies on Post-COVID Symptoms. Pathogens 2022, 11, 269. [Google Scholar] [CrossRef] [PubMed]
- Kunal, S.; Madan, M.; Tarke, C.; Gautam, D.K.; Kinkar, J.S.; Gupta, K.; Agarwal, R.; Mittal, S.; Sharma, S.M. Emerging Spectrum of Post-COVID-19 Syndrome. Postgrad Med. J. 2022, 98, 633–643. [Google Scholar] [CrossRef]
- Townsend, L.; Dyer, A.H.; Jones, K.; Dunne, J.; Mooney, A.; Gaffney, F.; O’Connor, L.; Leavy, D.; O’Brien, K.; Dowds, J.; et al. Persistent Fatigue Following SARS-CoV-2 Infection Is Common and Independent of Severity of Initial Infection. PLoS ONE 2020, 15, e0240784. [Google Scholar] [CrossRef]
- Sugiyama, A.; Miwata, K.; Kitahara, Y.; Okimoto, M.; Abe, K.; E, B.; Ouoba, S.; Akita, T.; Tanimine, N.; Ohdan, H.; et al. Long COVID Occurrence in COVID-19 Survivors. Sci. Rep. 2022, 12, 6039. [Google Scholar] [CrossRef]
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms and Functional Impairment Assessed 8 Months After Mild COVID-19 Among Health Care Workers. JAMA 2021, 325, 2015. [Google Scholar] [CrossRef]
- Nasserie, T.; Hittle, M.; Goodman, S.N. Assessment of the Frequency and Variety of Persistent Symptoms among Patients with COVID-19: A Systematic Review. JAMA Netw. Open 2021, 4, e2111417. [Google Scholar] [CrossRef]
- Salzberger, B.; Buder, F.; Lampl, B.; Ehrenstein, B.; Hitzenbichler, F.; Holzmann, T.; Schmidt, B.; Hanses, F. SARS-CoV-2/COVID-19–Epidemiologie und Prävention. Nephrologe 2021, 16, 3–9. [Google Scholar] [CrossRef]
- Antonelli, M.; Pujol, J.C.; Spector, T.D.; Ourselin, S.; Steves, C.J. Risk of Long COVID Associated with Delta versus Omicron Variants of SARS-CoV-2. Lancet 2022, 399, 2263–2264. [Google Scholar] [CrossRef] [PubMed]
- Solve ME/CFS Initiative Long COVID Alliance (LCA). Long Covid Alliance 2021. Available online: https://solvecfs.org/me-cfs-long-covid/long-covid-alliance/ (accessed on 22 February 2023).
- Morris, G.; Maes, M.; Berk, M.; Puri, B.K. Myalgic Encephalomyelitis or Chronic Fatigue Syndrome: How Could the Illness Develop? Metab. Brain Dis. 2019, 34, 385–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sisó-Almirall, A.; Brito-Zerón, P.; Conangla Ferrín, L.; Kostov, B.; Moragas Moreno, A.; Mestres, J.; Sellarès, J.; Galindo, G.; Morera, R.; Basora, J.; et al. Long Covid-19: Proposed Primary Care Clinical Guidelines for Diagnosis and Disease Management. Int. J. Environ. Res. Public Health 2021, 18, 4350. [Google Scholar] [CrossRef] [PubMed]
- Wirth, K.J.; Scheibenbogen, C.; Paul, F. An Attempt to Explain the Neurological Symptoms of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Transl. Med. 2021, 19, 471. [Google Scholar] [CrossRef]
- Petracek, L.S.; Suskauer, S.J.; Vickers, R.F.; Patel, N.R.; Violand, R.L.; Swope, R.L.; Rowe, P.C. Adolescent and Young Adult ME/CFS After Confirmed or Probable COVID-19. Front. Med. 2021, 8, 668944. [Google Scholar] [CrossRef]
- Hickie, I.; Davenport, T.; Wakefield, D.; Vollmer-Conna, U.; Cameron, B.; Vernon, S.D.; Reeves, W.C.; Lloyd, A. Post-Infective and Chronic Fatigue Syndromes Precipitated by Viral and Non-Viral Pathogens: Prospective Cohort Study. BMJ 2006, 333, 575. [Google Scholar] [CrossRef] [Green Version]
- Shikova, E.; Reshkova, V.; Kumanova, A.; Raleva, S.; Alexandrova, D.; Capo, N.; Murovska, M.; on behalf of the European Network on ME/CFS (EUROMENE). Cytomegalovirus, Epstein-Barr Virus, and Human Herpesvirus-6 Infections in Patients with Myalgic Encephalomyelitis/Chronic Fatigue Syndrome. J. Med. Virol. 2020, 92, 3682–3688. [Google Scholar] [CrossRef] [Green Version]
- Estévez-López, F.; Mudie, K.; Wang-Steverding, X.; Bakken, I.J.; Ivanovs, A.; Castro-Marrero, J.; Nacul, L.; Alegre, J.; Zalewski, P.; Słomko, J.; et al. Systematic Review of the Epidemiological Burden of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome Across Europe: Current Evidence and EUROMENE Research Recommendations for Epidemiology. J. Clin. Med. 2020, 9, 1557. [Google Scholar] [CrossRef]
- Wirth, K.J.; Scheibenbogen, C. Pathophysiology of Skeletal Muscle Disturbances in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). J. Transl. Med 2021, 19, 162. [Google Scholar] [CrossRef]
- Liu, L.D.; Duricka, D.L. Stellate Ganglion Block Reduces Symptoms of Long COVID: A Case Series. J. Neuroimmunol. 2022, 362, 577784. [Google Scholar] [CrossRef]
- Jason, L.A.; Islam, M.F.; Conroy, K.; Cotler, J.; Torres, C.; Johnson, M.; Mabie, B. COVID-19 Symptoms over Time: Comparing Long-Haulers to ME/CFS. Fatigue Biomed. Health Behav. 2021, 9, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, E.; Mariotto, S.; Gabbiani, D.; Dorelli, G.; Bozzetti, S.; Federico, A.; Zanzoni, S.; Girelli, D.; Crisafulli, E.; Ferrari, S.; et al. Chronic Fatigue Syndrome: An Emerging Sequela in COVID-19 Survivors? J. Neurovirol. 2021, 27, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R. As Their Numbers Grow, COVID-19 “Long Haulers” Stump Experts. JAMA 2020, 324, 1381. [Google Scholar] [CrossRef] [PubMed]
- Fernández-de-las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Cuadrado, M.L.; Florencio, L.L. Defining Post-COVID Symptoms (Post-Acute COVID, Long COVID, Persistent Post-COVID): An Integrative Classification. Int. J. Environ. Res. Public Health 2021, 18, 2621. [Google Scholar] [CrossRef] [PubMed]
- Yong, S.J. Persistent Brainstem Dysfunction in Long-COVID: A Hypothesis. ACS Chem. Neurosci. 2021, 12, 573–580. [Google Scholar] [CrossRef] [PubMed]
- Kanjwal, K.; Jamal, S.; Kichloo, A.; Grubb, B. New-Onset Postural Orthostatic Tachycardia Syndrome Following Coronavirus Disease 2019 Infection. J. Innov. Cardiac. Rhythm. Manag. 2020, 11, 4302–4304. [Google Scholar] [CrossRef]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic Dysfunction in ‘Long COVID’: Rationale, Physiology and Management Strategies. Clin. Med. 2021, 21, e63–e67. [Google Scholar] [CrossRef] [PubMed]
- Hassani, M.; Fathi Jouzdani, A.; Motarjem, S.; Ranjbar, A.; Khansari, N. How COVID-19 Can Cause Autonomic Dysfunctions and Postural Orthostatic Syndrome? A Review of Mechanisms and Evidence. Neurol. Clin. Neurosci. 2021, 9, 434–442. [Google Scholar] [CrossRef]
- COVID-19 Rapid Guideline: Managing the Long-Term Effects of COVID-19; NICE: 2022. p. 106. Available online: https://www.nice.org.uk/guidance/ng188/resources/covid19-rapid-guideline-managing-the-longterm-effects-of-covid19-pdf-51035515742 (accessed on 22 February 2023).
- Lloyd, A.R.; Hales, J.P.; Gandevia, S.C. Muscle Strength, Endurance and Recovery in the Post-Infection Fatigue Syndrome. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1316–1322. [Google Scholar] [CrossRef] [Green Version]
- Rutherford, O.M.; White, P.D. Human Quadriceps Strength and Fatiguability in Patients with Post Viral Fatigue. J. Neurol. Neurosurg. Psychiatry 1991, 54, 961–964. [Google Scholar] [CrossRef] [Green Version]
- Jäkel, B.; Kedor, C.; Grabowski, P.; Wittke, K.; Thiel, S.; Scherbakov, N.; Doehner, W.; Scheibenbogen, C.; Freitag, H. Hand Grip Strength and Fatigability: Correlation with Clinical Parameters and Diagnostic Suitability in ME/CFS. J. Transl. Med. 2021, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Meeus, M.; Ickmans, K.; Struyf, F.; Kos, D.; Lambrecht, L.; Willekens, B.; Cras, P.; Nijs, J. What Is in a Name? Comparing Diagnostic Criteria for Chronic Fatigue Syndrome with or without Fibromyalgia. Clin. Rheumatol. 2016, 35, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Dech, S.; Bittmann, F.N.; Schaefer, L.V. Assessment of the Adaptive Force of Elbow Extensors in Healthy Subjects Quantified by a Novel Pneumatically Driven Measurement System with Considerations of Its Quality Criteria. Diagnostics 2021, 11, 923. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.; Hoff, M.; Bittmann, F. Measuring System and Method of Determining the Adaptive Force. Eur. J. Transl. Myol. 2017, 27, 6479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoff, M.; Schaefer, L.; Heinke, N.; Bittmann, F. Report on Adaptive Force, a Specific Neuromuscular Function. Eur. J. Transl. Myol. 2015, 25, 183. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Bittmann, F.N. Muscular Pre-Activation Can Boost the Maximal Explosive Eccentric Adaptive Force. Front. Physiol. 2019, 10, 910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bittmann, F.N.; Dech, S.; Aehle, M.; Schaefer, L.V. Manual Muscle Testing—Force Profiles and Their Reproducibility. Diagnostics 2020, 10, 996. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Bittmann, F.N. Adaptive Force and Emotionally Related Imaginations—Preliminary Results Suggest a Reduction of the Maximal Holding Capacity as Reaction to Disgusting Food Imagination. Heliyon 2021, 7, e07827. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Aehle, M.; Bittmann, F.N. Disgusting Odours Affect the Characteristics of the Adaptive Force in Contrast to Neutral and Pleasant Odours. Sci. Rep. 2021, 11, 16410. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Dech, S.; Wolff, L.L.; Bittmann, F.N. Emotional Imagery Influences the Adaptive Force in Young Women: Unpleasant Imagery Reduces Instantaneously the Muscular Holding Capacity. Brain Sci. 2022, 12, 1318. [Google Scholar] [CrossRef]
- Conable, K.M.; Rosner, A.L. A Narrative Review of Manual Muscle Testing and Implications for Muscle Testing Research. J. Chiropr. Med. 2011, 10, 157–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAuley, J.H. Physiological and Pathological Tremors and Rhythmic Central Motor Control. Brain 2000, 123, 1545–1567. [Google Scholar] [CrossRef] [PubMed]
- Beck, T. Applications of Mechanomyography for Examining Muscle Function; Transworld Research Network: Kerala, India, 2010; ISBN 978-81-7895-449-3. [Google Scholar]
- Schaefer, L.V. Synchronisationsphänomene Myotendinöser Oszillationen Interagierender Neuromuskulärer Systeme–Mit Betrachtung Einer Hypothese Bezüglich Unterschiedlicher Qualitäten Isometrischer Muskelaktion. Doctoral thesis, University of Potsdam, Potsdam, Germany, 2014. [Google Scholar]
- Schaefer, L.V.; Torick, A.H.; Matuschek, H.; Holschneider, M.; Bittmann, F.N. Synchronization of Muscular Oscillations between Two Subjects During Isometric Interaction. Eur. J. Transl. Myol. 2014, 24, 2237. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Löffler, N.; Klein, J.; Bittmann, F.N. Mechanomyography and Acceleration Show Interlimb Asymmetries in Parkinson Patients without Tremor Compared to Controls during a Unilateral Motor Task. Sci. Rep. 2021, 11, 2631. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Bittmann, F.N. Are There Two Forms of Isometric Muscle Action? Results of the Experimental Study Support a Distinction between a Holding and a Pushing Isometric Muscle Function. BMC Sports Sci. Med. Rehabil. 2017, 9, 11. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, L.V.; Bittmann, F.N. Coherent Behavior of Neuromuscular Oscillations between Isometrically Interacting Subjects: Experimental Study Utilizing Wavelet Coherence Analysis of Mechanomyographic and Mechanotendographic Signals. Sci. Rep. 2018, 8, 15456. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, L.V.; Bittmann, F.N. Parkinson Patients without Tremor Show Changed Patterns of Mechanical Muscle Oscillations during a Specific Bilateral Motor Task Compared to Controls. Sci. Rep. 2020, 10, 1168. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, L.V.; Bittmann, F.N. Case Study: Intra- and Interpersonal Coherence of Muscle and Brain Activity of Two Coupled Persons during Pushing and Holding Isometric Muscle Action. Brain Sci. 2022, 12, 703. [Google Scholar] [CrossRef]
- Schaefer, L.V.; Bittmann, F.N. Paired Personal Interaction Reveals Objective Differences between Pushing and Holding Isometric Muscle Action. PLoS ONE 2021, 16, e0238331. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Sullivan, G.M.; Feinn, R. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, A.; Rayner, G.D. Robustness to Non-Normality of Common Tests for the Many-Sample Location Problem. J. Appl. Math. Decis. Sci. 2003, 7, 187–206. [Google Scholar] [CrossRef]
- Blanca, M.J.; Alarcón, R.; Arnau, J. Non-Normal Data: Is ANOVA Still a Valid Option? Psicothema 2017, 29, 552–557. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, L.V.; Bittmann, F.N. Case Report: Individualized Pulsed Electromagnetic Field Therapy in a Long COVID Patient Using the Adaptive Force as Biomarker. Front. Med. 2023, 9, 879971. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhou, X.; Huang, B.; Wang, Z.; Zhou, L.; Wang, M.; Yu, L.; Jiang, H. Noninvasive Low-Frequency Electromagnetic Stimulation of the Left Stellate Ganglion Reduces Myocardial Infarction-Induced Ventricular Arrhythmia. Sci. Rep. 2016, 6, 30783. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, S.; Ogiue-Ikeda, M.; Sekino, M.; Ueno, S. Effects of Pulsed Magnetic Stimulation on Tumor Development and Immune Functions in Mice. Bioelectromagnetics 2006, 27, 64–72. [Google Scholar] [CrossRef]
- Bolotova, N.V.; Raigorodsky, Y.M.; Dronova, E.G.; Posokhova, N.V. The Use of Magnetic Sympathocor-Rection for the Treatment of Vegetative Disorders in the Children with Obesity. Russ. J. Physiother. Balneol. Rehabil. 2013, 12, 30–34. [Google Scholar]
- Lee, J.-W.; Kim, J.-Y.; Hyun, J.-H.; Lee, Y.-H. Analysis of HRV and Body Temperature Variation for Manual Acupuncture and PEMF (Pulsed Electro-Magnetic Field) Acupuncture Stimulation. Acupunct. Electrother. Res. 2021, 47, 91–99. [Google Scholar] [CrossRef]
- Deer, R.R.; Rock, M.A.; Vasilevsky, N.; Carmody, L.; Rando, H.; Anzalone, A.J.; Basson, M.D.; Bennett, T.D.; Bergquist, T.; Boudreau, E.A.; et al. Characterizing Long COVID: Deep Phenotype of a Complex Condition. eBioMedicine 2021, 74, 103722. [Google Scholar] [CrossRef]
- Lopez-Leon, S.; Wegman-Ostrosky, T.; Perelman, C.; Sepulveda, R.; Rebolledo, P.A.; Cuapio, A.; Villapol, S. More than 50 Long-Term Effects of COVID-19: A Systematic Review and Meta-Analysis. Sci. Rep. 2021, 11, 16144. [Google Scholar] [CrossRef]
- Fleischer, M.; Szepanowski, F.; Tovar, M.; Herchert, K.; Dinse, H.; Schweda, A.; Mausberg, A.K.; Holle-Lee, D.; Köhrmann, M.; Stögbauer, J.; et al. Post-COVID-19 Syndrome Is Rarely Associated with Damage of the Nervous System: Findings from a Prospective Observational Cohort Study in 171 Patients. Neurol. Ther. 2022, 11, 1637–1657. [Google Scholar] [CrossRef] [PubMed]
- Ferrandi, P.J.; Alway, S.E.; Mohamed, J.S. The Interaction between SARS-CoV-2 and ACE2 May Have Consequences for Skeletal Muscle Viral Susceptibility and Myopathies. J. Appl. Physiol. 2020, 129, 864–867. [Google Scholar] [CrossRef] [PubMed]
- Arnold, P.; Njemini, R.; Vantieghem, S.; Duchateau, J.; Mets, T.; Beyer, I.; Bautmans, I. Peripheral Muscle Fatigue in Hospitalised Geriatric Patients Is Associated with Circulating Markers of Inflammation. Exp. Gerontol. 2017, 95, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Baj, J.; Karakuła-Juchnowicz, H.; Teresiński, G.; Buszewicz, G.; Ciesielka, M.; Sitarz, R.; Forma, A.; Karakuła, K.; Flieger, W.; Portincasa, P.; et al. COVID-19: Specific and Non-Specific Clinical Manifestations and Symptoms: The Current State of Knowledge. J. Clin. Med. 2020, 9, 1753. [Google Scholar] [CrossRef]
- Silva, C.C.; Bichara, C.N.C.; Carneiro, F.R.O.; Palacios, V.R.D.C.M.; Berg, A.V.S.V.D.; Quaresma, J.A.S.; Falcão, L.F.M. Muscle Dysfunction in the Long Coronavirus Disease 2019 Syndrome: Pathogenesis and Clinical Approach. Rev. Med. Virol. 2022, 32, e2355. [Google Scholar] [CrossRef]
- Paneroni, M.; Simonelli, C.; Saleri, M.; Bertacchini, L.; Venturelli, M.; Troosters, T.; Ambrosino, N.; Vitacca, M. Muscle Strength and Physical Performance in Patients Without Previous Disabilities Recovering From COVID-19 Pneumonia. Am. J. Phys. Med. Rehabil. 2021, 100, 105–109. [Google Scholar] [CrossRef]
- Andrews, A.W.; Thomas, M.W.; Bohannon, R.W. Normative Values for Isometric Muscle Force Measurements Obtained With Hand-Held Dynamometers. Phys. Ther. 1996, 76, 248–259. [Google Scholar] [CrossRef] [Green Version]
- Qin, Y.; Wu, J.; Chen, T.; Li, J.; Zhang, G.; Wu, D.; Zhou, Y.; Zheng, N.; Cai, A.; Ning, Q.; et al. Long-Term Microstructure and Cerebral Blood Flow Changes in Patients Recovered from COVID-19 without Neurological Manifestations. J. Clin. Investig. 2021, 131, e147329. [Google Scholar] [CrossRef]
- Wang, S.; Quan, L.; Chavarro, J.E.; Slopen, N.; Kubzansky, L.D.; Koenen, K.C.; Kang, J.H.; Weisskopf, M.G.; Branch-Elliman, W.; Roberts, A.L. Associations of Depression, Anxiety, Worry, Perceived Stress, and Loneliness Prior to Infection With Risk of Post–COVID-19 Conditions. JAMA Psychiatry 2022, 79, 1081. [Google Scholar] [CrossRef]
- Huggenberger, S.; Moser, N.; Schröder, H.; Cozzi, B.; Granato, A.; Merighi, A. Neuroanatomie Des Menschen: Mit 202 Größtenteils Farbigen Abbildungen; Springer-Lehrbuch; Springer: Berlin, Germany, 2019; ISBN 978-3-662-56461-5. [Google Scholar]
- Vogt, B.A.; Finch, D.M.; Olson, C.R. Functional Heterogeneity in Cingulate Cortex: The Anterior Executive and Posterior Evaluative Regions. Cereb. Cortex 1992, 2, 435–443. [Google Scholar] [CrossRef]
- Morecraft, R.J.; Tanjii, J. Cingulofrontal Interactions and the Cingulate Motor Areas. In Cingulate Neurobiology and Disease; Oxford University Press: New York, NY, USA, 2009; ISBN 978-0-19-856696-0. [Google Scholar]
- Vogt, B.A.; Nimchinsky, E.A.; Vogt, L.J.; Hof, P.R. Human Cingulate Cortex: Surface Features, Flat Maps, and Cytoarchitecture. J. Comp. Neurol. 1995, 359, 490–506. [Google Scholar] [CrossRef] [PubMed]
- Vitek, J.L.; Ashe, J.; DeLong, M.R.; Alexander, G.E. Physiologic Properties and Somatotopic Organization of the Primate Motor Thalamus. J. Neurophysiol. 1994, 71, 1498–1513. [Google Scholar] [CrossRef] [PubMed]
- Rensing, L. Krank durch Stress: Molekulare Wirkmechanismen und Folgen für die Gesundheit. Biol. Unserer Zeit 2006, 36, 284–292. [Google Scholar] [CrossRef]
- Kiecolt-Glaser, J.K.; Glaser, R.; Gravenstein, S.; Malarkey, W.B.; Sheridan, J. Chronic Stress Alters the Immune Response to Influenza Virus Vaccine in Older Adults. Proc. Natl. Acad. Sci. 1996, 93, 3043–3047. [Google Scholar] [CrossRef] [Green Version]
- v.Holst, D. Machen psychische Konflikte krank?: Neue Wege der Verhaltensforschung. Biol. Unserer Zeit 2001, 31, 78–87. [Google Scholar] [CrossRef]
- Song, H.; Fang, F.; Tomasson, G.; Arnberg, F.K.; Mataix-Cols, D.; Fernández de la Cruz, L.; Almqvist, C.; Fall, K.; Valdimarsdóttir, U.A. Association of Stress-Related Disorders With Subsequent Autoimmune Disease. JAMA 2018, 319, 2388. [Google Scholar] [CrossRef] [Green Version]
- Stefanski, V.; Engler, H. Social Stress, Dominance and Blood Cellular Immunity. J. Neuroimmunol. 1999, 94, 144–152. [Google Scholar] [CrossRef]
- Siegmann, E.-M.; Müller, H.H.O.; Luecke, C.; Philipsen, A.; Kornhuber, J.; Grömer, T.W. Association of Depression and Anxiety Disorders with Autoimmune Thyroiditis: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2018, 75, 577. [Google Scholar] [CrossRef] [Green Version]
- Jarczok, M.N.; Jarczok, M.; Thayer, J.F. Work Stress and Autonomic Nervous System Activity. In Handbook of Socioeconomic Determinants of Occupational Health; Theorell, T., Ed.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–33. ISBN 978-3-030-05031-3. [Google Scholar]



| Hip Flexors (n = 144) | Elbow Flexors (n = 118) | |||||
|---|---|---|---|---|---|---|
| MMT Rating | pre | Post | End | pre | Post | End |
| unstable | 48 | 2 | 0 | 39 | 2 | 1 |
| stable | 0 | 42 | 47 | 0 | 35 | 40 |
| unclear | 0 | 3 | 2 | 0 | 1 | 0 |
| Parameter | Time Point | M ± SD | 95%-CI | F (df1,df2) or z (df) | Significance p | η2/Kendall’s W |
|---|---|---|---|---|---|---|
| elbow flexors (n = 14) | ||||||
| AFmax (N) | pre | 177.02 ± 53.47 | 149.01; 205.03 | 1.054 (1.43,18.63) a | 0.345 | - |
| post | 184.74 ± 39.02 | 164.27; 205.15 | ||||
| end | 187.87 ± 52.00 | 160.63; 215.11 | ||||
| AFisomax (N) | pre | 87.92 ± 54.41 | 59.42; 116.42 | 114.772 (2,26) | <0.0001 | 0.898 |
| post | 182.26 ± 38.58 | 162.05; 202.47 | ||||
| end | 187.22 ± 52.15 | 159.90; 214.53 | ||||
| Ratio AFisomax to AFmax (%) | pre | 46.58 ± 15.91 | 38.25; 54.91 | 25.064 (2) b | <0.0001 | 0.895 b |
| post | 98.73 ± 3.01 | 97.15; 100.31 | ||||
| end | 99.62 ± 0.96 | 99.12; 100.13 | ||||
| AFosc (N) | pre | 170.95 ± 49.17 | 145.20; 196.71 | 5.274 (2,26) | 0.012 | 0.289 |
| post | 144.54 ± 44.83 | 121.06; 168.03 | ||||
| end | 146.51 ± 48.64 | 121.04; 171.99 | ||||
| Ratio AFosc to AFmax (%) | pre | 96.87 ± 2.85 | 95.38; 98.36 | 23.403 (2,26) | <0.0001 | 0.643 |
| post | 76.95 ± 11.89 | 70.73; 83.18 | ||||
| end | 76.98 ± 11.09 | 71.17; 82.79 | ||||
| Ratio AFosc to AFisomax (%) | pre | 258.83 ± 110.74 | 200.82; 316.84 | 34.701 (1.02,13.19) a | <0.0001 | 0.727 |
| post | 78.06 ± 12.30 | 71.62; 84.50 | ||||
| end | 77.28 ± 10.92 | 71.55; 83.00 | ||||
| Slope lg(N/s) | pre | 1.85 ± 0.23 | 1.73; 1.98 | 1.282 (2,26) | 0.294 | - |
| post | 1.87 ± 0.18 | 1.78; 1.97 | ||||
| end | 1.90 ± 0.21 | 1.79; 2.01 | ||||
| hip flexors (n = 17) | ||||||
| AFmax (N) | pre | 174.98 ± 50.03 | 148.77; 148.77 | 0.015 (2,32) a | 0.952 | - |
| post | 175.67 ± 40.95 | 154.22; 197.12 | ||||
| end | 174.21 ± 46.78 | 149.71; 198.72 | ||||
| AFisomax (N) | pre | 88.30 ± 40.67 | 66.99; 109.61 | 88.739 (1.47,23.45) a | <0.0001 | 0.847 |
| post | 173.30 ± 41.75 | 151.43; 195.18 | ||||
| end | 174.06 ± 46.80 | 149.54; 198.58 | ||||
| Ratio AFisomax to AFmax (%) | pre | 49.25 ± 12.01 | 42.96; 55.54 | 32.109 (2) b | <0.0001 | 0.944 b |
| post | 98.54 ± 3.44 | 96.74; 100.35 | ||||
| end | 99.91 ± 0.39 | 99.70; 100.11 | ||||
| AFosc (N) | pre | 167.10 ± 43.80 | 144.16; 190.04 | 27.952 (2,32) | <0.0001 | 0.636 |
| post | 116.47 ± 39.63 | 95.71; 137.23 | ||||
| end | 110.06 ± 40.81 | 88.68; 131.44 | ||||
| Ratio AFosc to AFmax (%) | pre | 95.19 ± 5.59 | 92.26; 98.12 | 53.417 (2,32) | <0.0001 | 0.77 |
| post | 65.62 ± 11.56 | 59.57; 71.68 | ||||
| end | 62.01 ± 13.74 | 54.81; 69.21 | ||||
| Ratio AFosc to AFisomax (%) | pre | 223.06 ± 69.65 | 186.57; 259.54 | 78.199 (1.07,17.11) a | <0.0001 | 0.83 |
| post | 66.88 ± 13.18 | 59.97; 73.78 | ||||
| end | 62.07 ± 13.78 | 54.85; 69.29 | ||||
| Slope lg(N/s) | pre | 1.85 ± 0.18 | 1.75; 1.94 | 3.260 (1.45,21.73) a | 0.071 | - |
| post | 1.93 ± 0.15 | 1.85; 2.00 | ||||
| end | 1.89 ± 0.14 | 1.81; 1.97 | ||||
| Stress M ± SD (Range, n) | Before COVID | Long COVID State (pre) | End | Friedman Test | Significance p | Effect Size Kendall’s W |
|---|---|---|---|---|---|---|
| Stress level job-related | 4.23 ± 2.56 (0–9, n = 11) | 5.64 ± 2.95 (0–10, n = 14 *) | 2.29 ± 3.17 (0–8, n = 12) | 0.667 | 0.717 | - |
| Stress level personal life | 3.77 ± 2.70 (2–10, n = 12) | 4.76 ± 2.75 (0–10, n = 17) | 3.29 ± 3.53 (0–9, n = 12) | 4.056 | 0.132 | - |
| Symptoms M ± SD (range) | n = 14 | n = 14 | n = 13 | |||
| Depression/anxiety | 1.43 ± 2.21 (0–8) | 3.96 ± 3.78 (0–10) | 1.58 ± 2.33 (0–7) | 9.389 | 0.009 1,2 | 0.361 |
| Fatigue | 0.43 ± 0.76 (0–2) | 7.75 ± 2.50 (1–10) | 2.23 ± 2.67 (0–7.5) | 22.217 | <0.001 1,2 | 0.855 |
| Post-exertion malaise | 0.57 ± 1.40 (0–5) | 8.14 ± 1.96 (3–10) | 2.23 ± 3.06 (0–9) | 20.311 | <0.001 1,2 | 0.781 |
| Muscle pain | 0.29 ± 0.61 (0–2) | 5.64 ± 4.27 (0–10) | 1.65 ± 2.81 (0–8) | 14.800 | 0.001 1,2 | 0.569 |
| Chest pain/tightness | 0.00 ± 0.00 | 3.43 ± 3.01 (0–9.5) | 0.88 ± 1.23 (0–4) | 18.667 | <0.001 1,2 | 0.718 |
| Breathing difficulties | 0.29 ± 0.61 (0–2) | 4.29 ± 2.37 (1–8) | 1.23 ± 1.36 (0–3) | 23.106 | <0.001 1,2 | 0.889 |
| Cough | 0.14 ± 0.36 (0–1) | 2.23 ± 3.00 (0–10) | 0.46 ± 0.97 (0–3) | 16.267 | <0.001 1,2 | 0.678 |
| Strong/fast heartbeat | 0.36 ± 1.08 (0–4) | 4.93 ± 4.03 (0–10) | 0.69 ± 1.18 (0–3) | 17.882 | <0.001 1,2 | 0.688 |
| Concentration/memory problems | 0.43 ± 0.76 (0–2) | 5.96 ± 3.20 (0–10) | 2.88 ± 2.60 (0–9) | 23.130 | <0.001 1,2 | 0.890 |
| Dizziness | 0.14 ± 0.36 (0–1) | 4.71 ± 3.81 (0–10) | 0.92 ± 1.98 (0–7) | 16.800 | <0.001 1,2 | 0.646 |
| Headache | 0.64 ± 1.28 (0–4) | 5.29 ± 4.07 (0–10) | 0.81 ± 1.28 (0–3) | 18.242 | <0.001 1,2 | 0.702 |
| Loss of smell or taste | 0.00 ± 0.00 | 4.39 ± 4.85 (0–10) | 1.35 ± 3.16 (0–10) | 13.923 | 0.001 1 | 0.536 |
| Fever | 0.00 ± 0.00 | 2.14 ± 3.66 (0–10) | 0.23 ± 0.83 (0–3) | 7.538 | 0.023 | 0.290 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schaefer, L.V.; Bittmann, F.N. The Adaptive Force as a Potential Biomechanical Parameter in the Recovery Process of Patients with Long COVID. Diagnostics 2023, 13, 882. https://doi.org/10.3390/diagnostics13050882
Schaefer LV, Bittmann FN. The Adaptive Force as a Potential Biomechanical Parameter in the Recovery Process of Patients with Long COVID. Diagnostics. 2023; 13(5):882. https://doi.org/10.3390/diagnostics13050882
Chicago/Turabian StyleSchaefer, Laura V., and Frank N. Bittmann. 2023. "The Adaptive Force as a Potential Biomechanical Parameter in the Recovery Process of Patients with Long COVID" Diagnostics 13, no. 5: 882. https://doi.org/10.3390/diagnostics13050882








