Artificial Intelligence-Aided Endoscopy and Colorectal Cancer Screening
Abstract
:1. Introduction
2. Principles of Artificial Intelligence
3. Artificial Intelligence in Colonoscopy
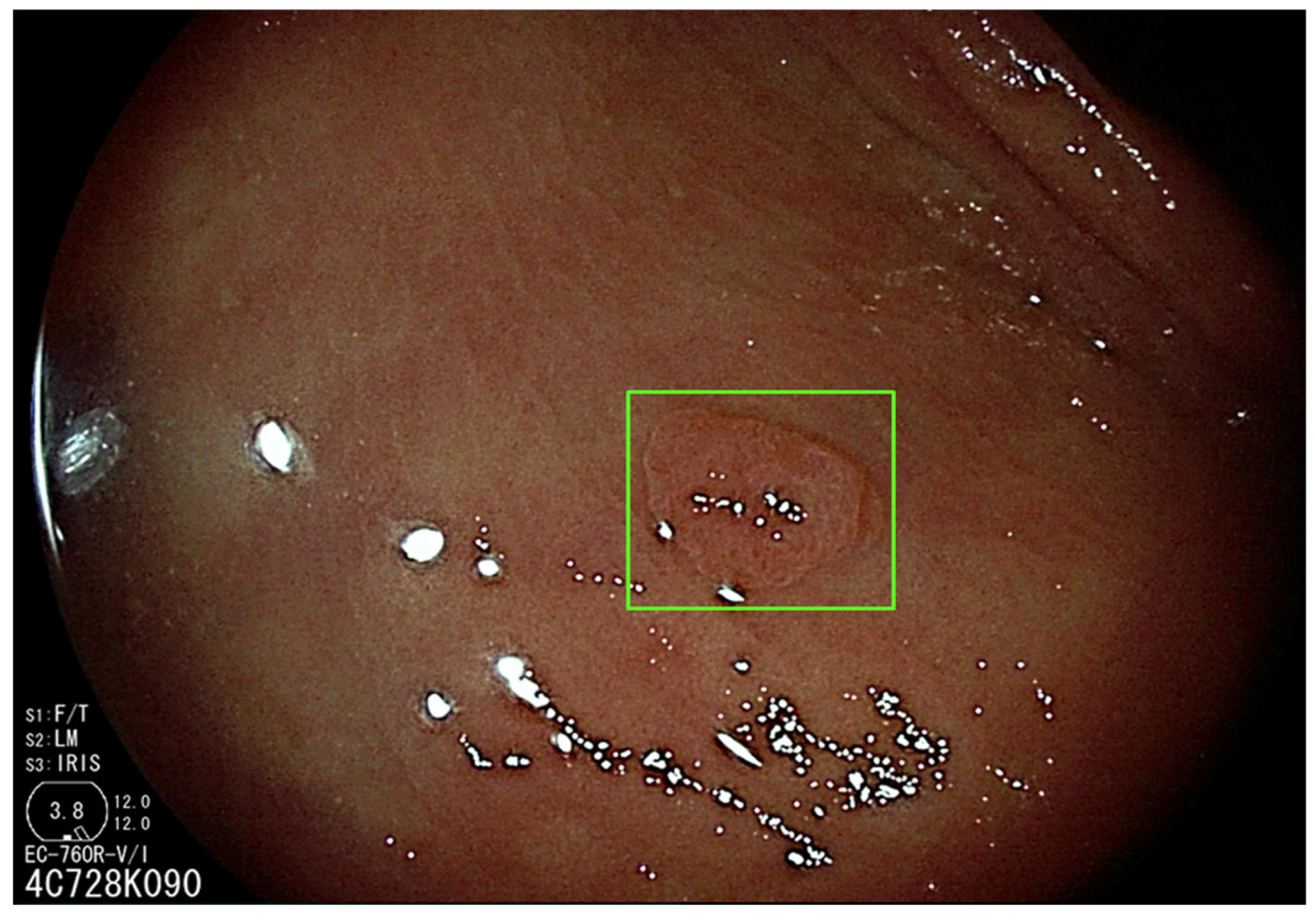
3.1. Artificial Intelligence-Assisted Detection (CADe)
3.2. Artificial Intelligence-Assisted Characterization (CADx)
3.3. Artificial Intelligence-Assisted Quality Control
4. Limitations
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer Statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Arnold, M.; Sierra, M.S.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Patterns and Trends in Colorectal Cancer Incidence and Mortality. Gut 2017, 66, 683–691. [Google Scholar] [CrossRef] [Green Version]
- Brenner, H.; Kloor, M.; Pox, C.P. Colorectal Cancer. Lancet 2014, 383, 1490–1502. [Google Scholar] [CrossRef]
- Nishihara, R.; Wu, K.; Lochhead, P.; Morikawa, T.; Liao, X.; Qian, Z.R.; Inamura, K.; Kim, S.A.; Kuchiba, A.; Yamauchi, M.; et al. Long-Term Colorectal-Cancer Incidence and Mortality after Lower Endoscopy. N. Engl. J. Med. 2013, 369, 1095–1105. [Google Scholar] [CrossRef] [Green Version]
- Schoen, R.E.; Pinsky, P.F.; Weissfeld, J.L.; Yokochi, L.A.; Church, T.; Laiyemo, A.O.; Bresalier, R.; Andriole, G.L.; Buys, S.S.; Crawford, E.D.; et al. Colorectal-Cancer Incidence and Mortality with Screening Flexible Sigmoidoscopy. N. Engl. J. Med. 2012, 366, 2345–2357. [Google Scholar] [CrossRef] [Green Version]
- Brenner, H.; Bouvier, A.M.; Foschi, R.; Hackl, M.; Larsen, I.K.; Lemmens, V.; Mangone, L.; Francisci, S.; EUROCARE Working Group. Progress in Colorectal Cancer Survival in Europe from the Late 1980s to the Early 21st Century: The EUROCARE Study. Int. J. Cancer 2012, 131, 1649–1658. [Google Scholar] [CrossRef]
- Kaminski, M.F.; Regula, J.; Kraszewska, E.; Polkowski, M.; Wojciechowska, U.; Didkowska, J.; Zwierko, M.; Rupinski, M.; Nowacki, M.P.; Butruk, E. Quality Indicators for Colonoscopy and the Risk of Interval Cancer. N. Engl. J. Med. 2010, 362, 1795–1803. [Google Scholar] [CrossRef] [Green Version]
- Corley, D.A.; Levin, T.R.; Doubeni, C.A. Adenoma Detection Rate and Risk of Colorectal Cancer and Death. N. Engl. J. Med. 2014, 370, 2541. [Google Scholar] [CrossRef] [Green Version]
- Robertson, D.J.; Lieberman, D.A.; Winawer, S.J.; Ahnen, D.J.; Baron, J.A.; Schatzkin, A.; Cross, A.J.; Zauber, A.G.; Church, T.R.; Lance, P.; et al. Colorectal Cancers Soon after Colonoscopy: A Pooled Multicohort Analysis. Gut 2014, 63, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.; Burr, N.E.; Valori, R. Causes of Post-Colonoscopy Colorectal Cancers Based on World Endoscopy Organization System of Analysis. Gastroenterology 2020, 158, 1287–1299.e2. [Google Scholar] [CrossRef]
- Hassan, C.; Piovani, D.; Spadaccini, M.; Parigi, T.; Khalaf, K.; Facciorusso, A.; Fugazza, A.; Rösch, T.; Bretthauer, M.; Mori, Y.; et al. Variability in Adenoma Detection Rate in Control Groups of Randomized Colonoscopy Trials. Gastrointest. Endosc. 2022, 97, 212–225.e7. [Google Scholar] [CrossRef]
- Zhao, S.; Wang, S.; Pan, P.; Xia, T.; Chang, X.; Yang, X.; Guo, L.; Meng, Q.; Yang, F.; Qian, W.; et al. Magnitude, Risk Factors, and Factors Associated With Adenoma Miss Rate of Tandem Colonoscopy: A Systematic Review and Meta-Analysis. Gastroenterology 2019, 156, 1661–1674.e11. [Google Scholar] [CrossRef]
- Aslanian, H.R.; Shieh, F.K.; Chan, F.W.; Ciarleglio, M.M.; Deng, Y.; Rogart, J.N.; Jamidar, P.A.; Siddiqui, U.D. Nurse Observation during Colonoscopy Increases Polyp Detection: A Randomized Prospective Study. Am. J. Gastroenterol. 2013, 108, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.K.; Park, D.I.; Lee, S.-H.; Hwangbo, Y.; Eun, C.S.; Han, D.S.; Cha, J.M.; Lee, B.-I.; Shin, J.E. Participation by Experienced Endoscopy Nurses Increases the Detection Rate of Colon Polyps during a Screening Colonoscopy: A Multicenter, Prospective, Randomized Study. Gastrointest. Endosc. 2011, 74, 1094–1102. [Google Scholar] [CrossRef]
- Buchner, A.M.; Shahid, M.W.; Heckman, M.G.; Diehl, N.N.; McNeil, R.B.; Cleveland, P.; Gill, K.R.; Schore, A.; Ghabril, M.; Raimondo, M.; et al. Trainee Participation Is Associated with Increased Small Adenoma Detection. Gastrointest. Endosc. 2011, 73, 1223–1231. [Google Scholar] [CrossRef]
- Attardo, S.; Chandrasekar, V.T.; Spadaccini, M.; Maselli, R.; Patel, H.K.; Desai, M.; Capogreco, A.; Badalamenti, M.; Galtieri, P.A.; Pellegatta, G.; et al. Artificial Intelligence Technologies for the Detection of Colorectal Lesions: The Future Is Now. World J. Gastroenterol. 2020, 26, 5606–5616. [Google Scholar] [CrossRef]
- Sinagra, E.; Badalamenti, M.; Maida, M.; Spadaccini, M.; Maselli, R.; Rossi, F.; Conoscenti, G.; Raimondo, D.; Pallio, S.; Repici, A.; et al. Use of Artificial Intelligence in Improving Adenoma Detection Rate during Colonoscopy: Might Both Endoscopists and Pathologists Be Further Helped. World J. Gastroenterol. 2020, 26, 5911–5918. [Google Scholar] [CrossRef]
- Spadaccini, M.; Koleth, G.; Emmanuel, J.; Khalaf, K.; Facciorusso, A.; Grizzi, F.; Hassan, C.; Colombo, M.; Mangiavillano, B.; Fugazza, A.; et al. Enhanced Endoscopic Ultrasound Imaging for Pancreatic Lesions: The Road to Artificial Intelligence. World J. Gastroenterol. 2022, 28, 3814–3824. [Google Scholar] [CrossRef]
- Wang, P.; Liu, P.; Glissen Brown, J.R.; Berzin, T.M.; Zhou, G.; Lei, S.; Liu, X.; Li, L.; Xiao, X. Lower Adenoma Miss Rate of Computer-Aided Detection-Assisted Colonoscopy vs Routine White-Light Colonoscopy in a Prospective Tandem Study. Gastroenterology 2020, 159, 1252–1261.e5. [Google Scholar] [CrossRef]
- Hassan, C.; Spadaccini, M.; Iannone, A.; Maselli, R.; Jovani, M.; Chandrasekar, V.T.; Antonelli, G.; Yu, H.; Areia, M.; Dinis-Ribeiro, M.; et al. Performance of Artificial Intelligence in Colonoscopy for Adenoma and Polyp Detection: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2021, 93, 77–85.e6. [Google Scholar] [CrossRef]
- Neumann, H.; Kreft, A.; Sivanathan, V.; Rahman, F.; Galle, P.R. Evaluation of Novel LCI CAD EYE System for Real Time Detection of Colon Polyps. PLoS ONE 2021, 16, e0255955. [Google Scholar] [CrossRef] [PubMed]
- Karkanis, S.A.; Iakovidis, D.K.; Maroulis, D.E.; Karras, D.A.; Tzivras, M. Computer-Aided Tumor Detection in Endoscopic Video Using Color Wavelet Features. IEEE Trans. Inf. Technol. Biomed. 2003, 7, 141–152. [Google Scholar] [CrossRef] [Green Version]
- Maroulis, D.E.; Iakovidis, D.K.; Karkanis, S.A.; Karras, D.A. CoLD: A Versatile Detection System for Colorectal Lesions in Endoscopy Video-Frames. Comput. Methods Programs Biomed. 2003, 70, 151–166. [Google Scholar] [CrossRef] [PubMed]
- Jerebko, A.; Lakare, S.; Cathier, P.; Periaswamy, S.; Bogoni, L. Symmetric Curvature Patterns for Colonic Polyp Detection. Med. Image Comput. Comput. Assist. Interv. 2006, 9, 169–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misawa, M.; Kudo, S.-E.; Mori, Y.; Cho, T.; Kataoka, S.; Yamauchi, A.; Ogawa, Y.; Maeda, Y.; Takeda, K.; Ichimasa, K.; et al. Artificial Intelligence-Assisted Polyp Detection for Colonoscopy: Initial Experience. Gastroenterology 2018, 154, 2027–2029.e3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, G.; Tripathi, P.; Alkayali, T.; Mittal, M.; Jalali, F.; Karnes, W.; Baldi, P. Deep Learning Localizes and Identifies Polyps in Real Time With 96% Accuracy in Screening Colonoscopy. Gastroenterology 2018, 155, 1069–1078.e8. [Google Scholar] [CrossRef]
- Hassan, C.; Wallace, M.B.; Sharma, P.; Maselli, R.; Craviotto, V.; Spadaccini, M.; Repici, A. New Artificial Intelligence System: First Validation Study versus Experienced Endoscopists for Colorectal Polyp Detection. Gut 2020, 69, 799–800. [Google Scholar] [CrossRef]
- Su, J.-R.; Li, Z.; Shao, X.-J.; Ji, C.-R.; Ji, R.; Zhou, R.-C.; Li, G.-C.; Liu, G.-Q.; He, Y.-S.; Zuo, X.-L.; et al. Impact of a Real-Time Automatic Quality Control System on Colorectal Polyp and Adenoma Detection: A Prospective Randomized Controlled Study (with Videos). Gastrointest. Endosc. 2020, 91, 415–424.e4. [Google Scholar] [CrossRef]
- Repici, A.; Badalamenti, M.; Maselli, R.; Correale, L.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; Fugazza, A.; et al. Efficacy of Real-Time Computer-Aided Detection of Colorectal Neoplasia in a Randomized Trial. Gastroenterology 2020, 159, 512–520.e7. [Google Scholar] [CrossRef]
- Wang, P.; Berzin, T.M.; Glissen Brown, J.R.; Bharadwaj, S.; Becq, A.; Xiao, X.; Liu, P.; Li, L.; Song, Y.; Zhang, D.; et al. Real-Time Automatic Detection System Increases Colonoscopic Polyp and Adenoma Detection Rates: A Prospective Randomised Controlled Study. Gut 2019, 68, 1813–1819. [Google Scholar] [CrossRef] [Green Version]
- Gong, D.; Wu, L.; Zhang, J.; Mu, G.; Shen, L.; Liu, J.; Wang, Z.; Zhou, W.; An, P.; Huang, X.; et al. Detection of Colorectal Adenomas with a Real-Time Computer-Aided System (ENDOANGEL): A Randomised Controlled Study. Lancet Gastroenterol. Hepatol. 2020, 5, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, X.; Berzin, T.M.; Glissen Brown, J.R.; Liu, P.; Zhou, C.; Lei, L.; Li, L.; Guo, Z.; Lei, S.; et al. Effect of a Deep-Learning Computer-Aided Detection System on Adenoma Detection during Colonoscopy (CADe-DB Trial): A Double-Blind Randomised Study. Lancet Gastroenterol. Hepatol. 2020, 5, 343–351. [Google Scholar] [CrossRef]
- Liu, W.-N.; Zhang, Y.-Y.; Bian, X.-Q.; Wang, L.-J.; Yang, Q.; Zhang, X.-D.; Huang, J. Study on Detection Rate of Polyps and Adenomas in Artificial-Intelligence-Aided Colonoscopy. Saudi J. Gastroenterol. 2020, 26, 13–19. [Google Scholar] [CrossRef]
- Repici, A.; Spadaccini, M.; Antonelli, G.; Correale, L.; Maselli, R.; Galtieri, P.A.; Pellegatta, G.; Capogreco, A.; Milluzzo, S.M.; Lollo, G.; et al. Artificial Intelligence and Colonoscopy Experience: Lessons from Two Randomised Trials. Gut 2022, 71, 757–765. [Google Scholar] [CrossRef]
- Yao, L.; Zhang, L.; Liu, J.; Zhou, W.; He, C.; Zhang, J.; Wu, L.; Wang, H.; Xu, Y.; Gong, D.; et al. Effect of an Artificial Intelligence-Based Quality Improvement System on Efficacy of a Computer-Aided Detection System in Colonoscopy: A Four-Group Parallel Study. Endoscopy 2022, 54, 757–768. [Google Scholar] [CrossRef]
- Liu, P.; Wang, P.; Glissen Brown, J.R.; Berzin, T.M.; Zhou, G.; Liu, W.; Xiao, X.; Chen, Z.; Zhang, Z.; Zhou, C.; et al. The Single-Monitor Trial: An Embedded CADe System Increased Adenoma Detection during Colonoscopy: A Prospective Randomized Study. Therap. Adv. Gastroenterol. 2020, 13, 1756284820979165. [Google Scholar] [CrossRef]
- Rondonotti, E.; Di Paolo, D.; Rizzotto, E.R.; Alvisi, C.; Buscarini, E.; Spadaccini, M.; Tamanini, G.; Paggi, S.; Amato, A.; Scardino, G.; et al. Efficacy of a Computer-Aided Detection System in a Fecal Immunochemical Test-Based Organized Colorectal Cancer Screening Program: A Randomized Controlled Trial (AIFIT Study). Endoscopy 2022, 54, 1171–1179. [Google Scholar] [CrossRef]
- Shaukat, A.; Lichtenstein, D.R.; Somers, S.C.; Chung, D.C.; Perdue, D.G.; Gopal, M.; Colucci, D.R.; Phillips, S.A.; Marka, N.A.; Church, T.R.; et al. Computer-Aided Detection Improves Adenomas per Colonoscopy for Screening and Surveillance Colonoscopy: A Randomized Trial. Gastroenterology 2022, 163, 732–741. [Google Scholar] [CrossRef]
- Aniwan, S.; Mekritthikrai, K.; Kerr, S.J.; Tiankanon, K.; Vandaungden, K.; Sritunyarat, Y.; Piyachaturawat, P.; Luangsukrerk, T.; Kulpatcharapong, S.; Wisedopas, N.; et al. Computer-Aided Detection, Mucosal Exposure Device, Their Combination, and Standard Colonoscopy for Adenoma Detection: A Randomized Controlled Trial. Gastrointest. Endosc. 2023, 97, 507–516. [Google Scholar] [CrossRef]
- Gimeno-García, A.Z.; Negrin, D.H.; Hernández, A.; Nicolás-Pérez, D.; Rodríguez, E.; Montesdeoca, C.; Alarcon, O.; Romero, R.; Baute Dorta, J.L.; Cedrés, Y.; et al. Usefulness of a Novel Computer-Aided Detection System for Colorectal Neoplasia: A Randomized Controlled Trial. Gastrointest. Endosc. 2022. [Google Scholar] [CrossRef]
- Spadaccini, M.; Iannone, A.; Maselli, R.; Badalamenti, M.; Desai, M.; Chandrasekar, V.T.; Patel, H.K.; Fugazza, A.; Pellegatta, G.; Galtieri, P.A.; et al. Computer-Aided Detection versus Advanced Imaging for Detection of Colorectal Neoplasia: A Systematic Review and Network Meta-Analysis. Lancet Gastroenterol. Hepatol. 2021, 6, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Kamba, S.; Tamai, N.; Saitoh, I.; Matsui, H.; Horiuchi, H.; Kobayashi, M.; Sakamoto, T.; Ego, M.; Fukuda, A.; Tonouchi, A.; et al. Reducing Adenoma Miss Rate of Colonoscopy Assisted by Artificial Intelligence: A Multicenter Randomized Controlled Trial. J. Gastroenterol. 2021, 56, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Glissen Brown, J.R.; Mansour, N.M.; Wang, P.; Chuchuca, M.A.; Minchenberg, S.B.; Chandnani, M.; Liu, L.; Gross, S.A.; Sengupta, N.; Berzin, T.M. Deep Learning Computer-Aided Polyp Detection Reduces Adenoma Miss Rate: A United States Multi-Center Randomized Tandem Colonoscopy Study (CADeT-CS Trial). Clin. Gastroenterol. Hepatol. 2022, 20, 1499–1507.e4. [Google Scholar] [CrossRef]
- Wallace, M.B.; Sharma, P.; Bhandari, P.; East, J.; Antonelli, G.; Lorenzetti, R.; Vieth, M.; Speranza, I.; Spadaccini, M.; Desai, M.; et al. Impact of Artificial Intelligence on Miss Rate of Colorectal Neoplasia. Gastroenterology 2022, 163, 295–304.e5. [Google Scholar] [CrossRef] [PubMed]
- Gubbiotti, A.; Spadaccini, M.; Badalamenti, M.; Hassan, C.; Repici, A. Key Factors for Improving Adenoma Detection Rate. Expert Rev. Gastroenterol. Hepatol. 2022, 16, 819–833. [Google Scholar] [CrossRef] [PubMed]
- Istituto Clinico Humanitas. The CERTAIN Study: Combining Endo-Cuff in a Randomized Trial for Artificial Intelligence Navigation; U.S. National Library of Medicine: Rozzano, Italy, 2022. [Google Scholar]
- Spadaccini, M.; Mönkemüller, K. Commentary. Endoscopy 2022, 54, 1124. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Mori, Y.; Sharma, P.; Spadaccini, M.; Repici, A. Detrimental Detection of Advanced Lesions With AI: False Confidence or Prevalence Bias? Am. J. Gastroenterol. 2022, 117, 2088–2089. [Google Scholar] [CrossRef]
- Areia, M.; Mori, Y.; Correale, L.; Repici, A.; Bretthauer, M.; Sharma, P.; Taveira, F.; Spadaccini, M.; Antonelli, G.; Ebigbo, A.; et al. Cost-Effectiveness of Artificial Intelligence for Screening Colonoscopy: A Modelling Study. Lancet Digit. Health 2022, 4, e436–e444. [Google Scholar] [CrossRef]
- Wisse, P.H.A.; Erler, N.S.; de Boer, S.Y.; den Hartog, B.; Oudkerk Pool, M.; Terhaar Sive Droste, J.S.; Verveer, C.; Meijer, G.A.; Lansdorp-Vogelaar, I.; Kuipers, E.J.; et al. Adenoma Detection Rate and Risk for Interval Postcolonoscopy Colorectal Cancer in Fecal Immunochemical Test-Based Screening: A Population-Based Cohort Study. Ann. Intern. Med. 2022, 175, 1366–1373. [Google Scholar] [CrossRef]
- Van Toledo, D.E.F.W.M.; IJspeert, J.E.G.; Dekker, E. Current Approaches in Managing Colonic Serrated Polyps and Serrated Polyposis. Annu. Rev. Med. 2022, 73, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Van Toledo, D.E.F.W.M.; Breekveldt, E.C.H.; IJspeert, J.E.G.; van Vuuren, A.J.; van Kemenade, F.J.; Ramakers, C.; Nagtegaal, I.D.; van Leerdam, M.E.; Spaander, M.C.W.; Lansdorp-Vogelaar, I.; et al. Advanced Serrated Polyps as a Target of Screening: Detection Rate and Positive Predictive Value within a Fecal Immunochemical Test-Based Colorectal Cancer Screening Population. Endoscopy 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hetzel, J.T.; Huang, C.S.; Coukos, J.A.; Omstead, K.; Cerda, S.R.; Yang, S.; O’Brien, M.J.; Farraye, F.A. Variation in the Detection of Serrated Polyps in an Average Risk Colorectal Cancer Screening Cohort. Am. J. Gastroenterol. 2010, 105, 2656–2664. [Google Scholar] [CrossRef] [PubMed]
- Zessner-Spitzenberg, J.; Waldmann, E.; Jiricka, L.; Rockenbauer, L.-M.; Hinterberger, A.; Cook, J.; Asaturi, A.; Szymanska, A.; Majcher, B.; Trauner, M.; et al. Comparison of Adenoma Detection Rate and Proximal Serrated Polyp Detection Rate and Their Effect on Post-Colonoscopy Colorectal Cancer Mortality in Screening Patients. Endoscopy 2022. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Wang, P.; Løberg, M.; Misawa, M.; Repici, A.; Spadaccini, M.; Correale, L.; Antonelli, G.; Yu, H.; Gong, D.; et al. Impact of Artificial Intelligence on Colonoscopy Surveillance After Polyp Removal: A Pooled Analysis of Randomized Trials. Clin. Gastroenterol. Hepatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Duvvuri, A.; Chandrasekar, V.T.; Srinivasan, S.; Narimiti, A.; Dasari, C.; Nutalapati, V.; Kennedy, K.F.; Spadaccini, M.; Antonelli, G.; Desai, M.; et al. Risk of Colorectal Cancer and Cancer Related Mortality After Detection of Low-Risk or High-Risk Adenomas, Compared With No Adenoma, at Index Colonoscopy: A Systematic Review and Meta-Analysis. Gastroenterology 2021, 160, 1986–1996.e3. [Google Scholar] [CrossRef] [PubMed]
- Abu-Freha, N.; Katz, L.H.; Kariv, R.; Vainer, E.; Laish, I.; Gluck, N.; Half, E.E.; Levi, Z. Post-Polypectomy Surveillance Colonoscopy: Comparison of the Updated Guidelines. United Eur. Gastroenterol. J. 2021, 9, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Antonelli, G.; Dumonceau, J.-M.; Regula, J.; Bretthauer, M.; Chaussade, S.; Dekker, E.; Ferlitsch, M.; Gimeno-Garcia, A.; Jover, R.; et al. Post-Polypectomy Colonoscopy Surveillance: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2020. Endoscopy 2020, 52, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Rees, C.J.; Rajasekhar, P.T.; Wilson, A.; Close, H.; Rutter, M.D.; Saunders, B.P.; East, J.E.; Maier, R.; Moorghen, M.; Muhammad, U.; et al. Narrow Band Imaging Optical Diagnosis of Small Colorectal Polyps in Routine Clinical Practice: The Detect Inspect Characterise Resect and Discard 2 (DISCARD 2) Study. Gut 2017, 66, 887–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rex, D.K.; Kahi, C.; O’Brien, M.; Levin, T.R.; Pohl, H.; Rastogi, A.; Burgart, L.; Imperiale, T.; Ladabaum, U.; Cohen, J.; et al. The American Society for Gastrointestinal Endoscopy PIVI (Preservation and Incorporation of Valuable Endoscopic Innovations) on Real-Time Endoscopic Assessment of the Histology of Diminutive Colorectal Polyps. Gastrointest. Endosc. 2011, 73, 419–422. [Google Scholar] [CrossRef] [PubMed]
- Houwen, B.B.S.L.; Hassan, C.; Coupé, V.M.H.; Greuter, M.J.E.; Hazewinkel, Y.; Vleugels, J.L.A.; Antonelli, G.; Bustamante-Balén, M.; Coron, E.; Cortas, G.A.; et al. Definition of Competence Standards for Optical Diagnosis of Diminutive Colorectal Polyps: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2022, 54, 88–99. [Google Scholar] [CrossRef]
- Tischendorf, J.J.W.; Gross, S.; Winograd, R.; Hecker, H.; Auer, R.; Behrens, A.; Trautwein, C.; Aach, T.; Stehle, T. Computer-Aided Classification of Colorectal Polyps Based on Vascular Patterns: A Pilot Study. Endoscopy 2010, 42, 203–207. [Google Scholar] [CrossRef]
- Gross, S.; Trautwein, C.; Behrens, A.; Winograd, R.; Palm, S.; Lutz, H.H.; Schirin-Sokhan, R.; Hecker, H.; Aach, T.; Tischendorf, J.J.W. Computer-Based Classification of Small Colorectal Polyps by Using Narrow-Band Imaging with Optical Magnification. Gastrointest. Endosc. 2011, 74, 1354–1359. [Google Scholar] [CrossRef]
- Kominami, Y.; Yoshida, S.; Tanaka, S.; Sanomura, Y.; Hirakawa, T.; Raytchev, B.; Tamaki, T.; Koide, T.; Kaneda, K.; Chayama, K. Computer-Aided Diagnosis of Colorectal Polyp Histology by Using a Real-Time Image Recognition System and Narrow-Band Imaging Magnifying Colonoscopy. Gastrointest. Endosc. 2016, 83, 643–649. [Google Scholar] [CrossRef]
- Mori, Y.; Kudo, S.-E.; Misawa, M.; Saito, Y.; Ikematsu, H.; Hotta, K.; Ohtsuka, K.; Urushibara, F.; Kataoka, S.; Ogawa, Y.; et al. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps During Colonoscopy: A Prospective Study. Ann. Intern. Med. 2018, 169, 357–366. [Google Scholar] [CrossRef] [PubMed]
- Byrne, M.F.; Chapados, N.; Soudan, F.; Oertel, C.; Linares Pérez, M.; Kelly, R.; Iqbal, N.; Chandelier, F.; Rex, D.K. Real-Time Differentiation of Adenomatous and Hyperplastic Diminutive Colorectal Polyps during Analysis of Unaltered Videos of Standard Colonoscopy Using a Deep Learning Model. Gut 2019, 68, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Zorron Cheng Tao Pu, L.; Maicas, G.; Tian, Y.; Yamamura, T.; Nakamura, M.; Suzuki, H.; Singh, G.; Rana, K.; Hirooka, Y.; Burt, A.D.; et al. Computer-Aided Diagnosis for Characterization of Colorectal Lesions: Comprehensive Software That Includes Differentiation of Serrated Lesions. Gastrointest. Endosc. 2020, 92, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Hassan, C.; Balsamo, G.; Lorenzetti, R.; Zullo, A.; Antonelli, G. Artificial Intelligence Allows Leaving-In-Situ Colorectal Polyps. Clin. Gastroenterol. Hepatol. 2022, 20, 2505–2513.e4. [Google Scholar] [CrossRef] [PubMed]
- Rondonotti, E.; Hassan, C.; Tamanini, G.; Antonelli, G.; Andrisani, G.; Leonetti, G.; Paggi, S.; Amato, A.; Scardino, G.; Di Paolo, D.; et al. Artificial Intelligence-Assisted Optical Diagnosis for the Resect-and-Discard Strategy in Clinical Practice: The Artificial Intelligence BLI Characterization (ABC) Study. Endoscopy 2023, 55, 14–22. [Google Scholar] [CrossRef]
- Hassan, C.; Sharma, P.; Mori, Y.; Bretthauer, M.; Rex, D.K.; EUROCARE Working Group; Repici, A. Comparative Performance of Artificial Intelligence Optical Diagnosis Systems for Leaving in Situ Colorectal Polyps. Gastroenterology 2022, 164, 467–469. [Google Scholar] [CrossRef]
- Song, E.M.; Park, B.; Ha, C.-A.; Hwang, S.W.; Park, S.H.; Yang, D.-H.; Ye, B.D.; Myung, S.-J.; Yang, S.-K.; Kim, N.; et al. Endoscopic Diagnosis and Treatment Planning for Colorectal Polyps Using a Deep-Learning Model. Sci. Rep. 2020, 10, 30. [Google Scholar] [CrossRef] [Green Version]
- Jin, E.H.; Lee, D.; Bae, J.H.; Kang, H.Y.; Kwak, M.-S.; Seo, J.Y.; Yang, J.I.; Yang, S.Y.; Lim, S.H.; Yim, J.Y.; et al. Improved Accuracy in Optical Diagnosis of Colorectal Polyps Using Convolutional Neural Networks with Visual Explanations. Gastroenterology 2020, 158, 2169–2179.e8. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Kudo, S.-E.; East, J.E.; Rastogi, A.; Bretthauer, M.; Misawa, M.; Sekiguchi, M.; Matsuda, T.; Saito, Y.; Ikematsu, H.; et al. Cost Savings in Colonoscopy with Artificial Intelligence-Aided Polyp Diagnosis: An Add-on Analysis of a Clinical Trial (with Video). Gastrointest. Endosc. 2020, 92, 905–911.e1. [Google Scholar] [CrossRef]
- Mori, Y.; Neumann, H.; Misawa, M.; Kudo, S.-E.; Bretthauer, M. Artificial Intelligence in Colonoscopy—Now on the Market. What’s Next? J. Gastroenterol. Hepatol. 2021, 36, 7–11. [Google Scholar] [CrossRef]
- Zhou, J.; Wu, L.; Wan, X.; Shen, L.; Liu, J.; Zhang, J.; Jiang, X.; Wang, Z.; Yu, S.; Kang, J.; et al. A Novel Artificial Intelligence System for the Assessment of Bowel Preparation (with Video). Gastrointest. Endosc. 2020, 91, 428–435.e2. [Google Scholar] [CrossRef]
- Karnes, W.E.; Ninh, A.; Dao, T.; Requa, J.; Samarasena, J.B. Sa1940 Unambiguous real-time scoring of bowel preparation using artificial intelligence. Gastrointest. Endosc. 2018, 87, AB258. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Zhou, W.; An, P.; Shen, L.; Liu, J.; Jiang, X.; Huang, X.; Mu, G.; Wan, X.; et al. Randomised Controlled Trial of WISENSE, a Real-Time Quality Improving System for Monitoring Blind Spots during Esophagogastroduodenoscopy. Gut 2019, 68, 2161–2169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Wu, L.; Li, Y.; Zhang, J.; Liu, J.; Huang, L.; Jiang, X.; Huang, X.; Mu, G.; Hu, S.; et al. Comparing Blind Spots of Unsedated Ultrafine, Sedated, and Unsedated Conventional Gastroscopy with and without Artificial Intelligence: A Prospective, Single-Blind, 3-Parallel-Group, Randomized, Single-Center Trial. Gastrointest. Endosc. 2020, 91, 332–339.e3. [Google Scholar] [CrossRef] [PubMed]
- McGill, S.K.; Rosenman, J.; Wang, R.; Ma, R.; Frahm, J.-M.; Pizer, S. Artificial Intelligence Identifies and Quantifies Colonoscopy Blind Spots. Endoscopy 2021, 53, 1284–1286. [Google Scholar] [CrossRef] [PubMed]
- Requa, J.; Dao, T.; Ninh, A.; Karnes, W. Can a Convolutional Neural Network Solve the Polyp Size Dilemma? Category Award (Colorectal Cancer Prevention) Presidential Poster Award: 282. Off. J. Am. Coll. Gastroenterol.|ACG 2018, 113, S158. [Google Scholar] [CrossRef]
- Abdelrahim, M.; Saiga, H.; Maeda, N.; Hossain, E.; Ikeda, H.; Bhandari, P. Automated Sizing of Colorectal Polyps Using Computer Vision. Gut 2022, 71, 7–9. [Google Scholar] [CrossRef]
- Van der Sommen, F.; de Groof, J.; Struyvenberg, M.; van der Putten, J.; Boers, T.; Fockens, K.; Schoon, E.J.; Curvers, W.; de With, P.; Mori, Y.; et al. Machine Learning in GI Endoscopy: Practical Guidance in How to Interpret a Novel Field. Gut 2020, 69, 2035–2045. [Google Scholar] [CrossRef]
- Hoogenboom, S.A.; Bagci, U.; Wallace, M.B. Artificial Intelligence in Gastroenterology. The Current State of Play and the Potential. How Will It Affect Our Practice and When? Tech. Innov. Gastrointest. Endosc. 2020, 22, 42–47. [Google Scholar] [CrossRef]
- Lui, T.K.L.; Guo, C.-G.; Leung, W.K. Accuracy of Artificial Intelligence on Histology Prediction and Detection of Colorectal Polyps: A Systematic Review and Meta-Analysis. Gastrointest. Endosc. 2020, 92, 11–22.e6. [Google Scholar] [CrossRef]
- Greenhill, A.T.; Edmunds, B.R. A Primer of Artificial Intelligence in Medicine. Tech. Innov. Gastrointest. Endosc. 2020, 22, 85–89. [Google Scholar] [CrossRef]
- Vinsard, D.G.; Mori, Y.; Misawa, M.; Kudo, S.-E.; Rastogi, A.; Bagci, U.; Rex, D.K.; Wallace, M.B. Quality Assurance of Computer-Aided Detection and Diagnosis in Colonoscopy. Gastrointest. Endosc. 2019, 90, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Antonelli, G.; Gkolfakis, P.; Tziatzios, G.; Papanikolaou, I.S.; Triantafyllou, K.; Hassan, C. Artificial Intelligence-Aided Colonoscopy: Recent Developments and Future Perspectives. World J. Gastroenterol. 2020, 26, 7436–7443. [Google Scholar] [CrossRef]
- Hassan, C.; Badalamenti, M.; Maselli, R.; Correale, L.; Iannone, A.; Radaelli, F.; Rondonotti, E.; Ferrara, E.; Spadaccini, M.; Alkandari, A.; et al. Computer-Aided Detection-Assisted Colonoscopy: Classification and Relevance of False Positives. Gastrointest. Endosc. 2020, 92, 900–904.e4. [Google Scholar] [CrossRef] [PubMed]
- Spadaccini, M.; Hassan, C.; Alfarone, L.; Da Rio, L.; Maselli, R.; Carrara, S.; Galtieri, P.A.; Pellegatta, G.; Fugazza, A.; Koleth, G.; et al. Comparing the Number and Relevance of False Activations between 2 Artificial Intelligence Computer-Aided Detection Systems: The NOISE Study. Gastrointest. Endosc. 2022, 95, 975–981.e1. [Google Scholar] [CrossRef]
- Koleth, G.; Emmanue, J.; Spadaccini, M.; Mascagni, P.; Khalaf, K.; Mori, Y.; Antonelli, G.; Maselli, R.; Carrara, S.; Galtieri, P.A.; et al. Artificial Intelligence in Gastroenterology: Where Are We Heading? Endosc. Int. Open 2022, 10, E1474–E1480. [Google Scholar] [CrossRef]
- Messmann, H.; Bisschops, R.; Antonelli, G.; Libânio, D.; Sinonquel, P.; Abdelrahim, M.; Ahmad, O.F.; Areia, M.; Bergman, J.J.G.H.M.; Bhandari, P.; et al. Expected Value of Artificial Intelligence in Gastrointestinal Endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Position Statement. Endoscopy 2022, 54, 1211–1231. [Google Scholar] [CrossRef] [PubMed]



| Publication | Country | Study Type | Number of Patients | Gender | Mean Age | Indication | Adenoma Detection Rate (%) | AI System | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | AI-Assisted Colonoscopy | Standard Colonoscopy | Men | Women | Screening | Non-Screening | AI-Assisted Colonoscopy | Standard Colonoscopy | |||||
| Wang, P. 2019 | China | Single Center | 1058 | 522 | 536 | 512 | 546 | 50 | 84 | 974 | 29 | 20 | EndoScreener |
| Wang, P. 2020 | China | Single Center | 962 | 484 | 478 | 495 | 467 | 49 | 158 | 804 | 34 | 28 | EndoScreener |
| Liu, P. 2020 | China | Single Center | 1026 | 508 | 518 | 551 | 475 | 50 | 66 | 960 | 39 | 24 | EndoScreener |
| Gong, D. 2020 | China | Single Center | 704 | 355 | 349 | 345 | 359 | 50 | 36 | 668 | 16 | 8 | ENDOANGEL |
| Repici, A. 2020 | Italy | Multi center | 685 | 341 | 344 | 336 | 348 | 61 | 524 | 161 | 55 | 40 | GI GENIUS Medtronic |
| Su, J.-R. 2020 | China | Single Center | 623 | 315 | 308 | 307 | 316 | 51 | 216 | 407 | 29 | 17 | Self-Developed |
| Repici, A. 2021 | Italy | Multi center | 660 | 330 | 330 | 330 | 330 | 62 | 245 | 415 | 53 | 45 | GI GENIUS Medtronic |
| Yao, L. 2021 | China | Single Center | 539 | 268 | 271 | 235 | 304 | 51 | 534 | 5 | 21 | 15 | ENDOANGEL |
| Liu, P. 2021 | China | Single Center | 790 | 393 | 397 | 374 | 416 | 49 | 182 | 608 | 29 | 19 | EndoScreener |
| Rondonotti, E. 2022 | Italy | Multi center | 800 | 405 | 395 | 409 | 391 | 61 | 800 | 0 | 53 | 45 | CAD EYE FujiFilm |
| Shaukat, A. 2022 | USA | Multi center | 1359 | 682 | 677 | 723 | 636 | 60 | 1359 | 0 | 48 | 44 | SKOUT device |
| Aniwan, S. 2022 | Thailand | Single Center | 622 | 312 | 310 | 266 | 356 | 62 | \ | \ | 52 | 42 | CAD EYE FujiFilm |
| Gimeno-Garcia, A.Z. 2022 | Spain | Single Center | 312 | 155 | 157 | 165 | 147 | 64 | 206 | 106 | 57 | 45 | ENDO AID Olympus |
| Publication | Country | Study Type | Number of Patients | Gender | Mean Age | Indication | Adenoma Detection Rate (%) | AI System | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | AI-Assisted Colonoscopy | Standard Colonoscopy | Men | Women | Screening | Non-Screening | AI-Assisted Colonoscopy | Standard Colonoscopy | |||||
| Wang, P. 2020 | China | Single Center | 369 | 184 | 185 | 179 | 190 | 47 | 113 | 256 | 35 | 26 | EndoScreener |
| Kamba, S. 2021 | Japan | Multi center | 295 | 178 | 117 | 272 | 23 | 62 | 228 | 67 | 65 | 53 | Self-Developed |
| Glissen Brown, J.R. 2021 | USA | Multi Center | 223 | 113 | 110 | 122 | 101 | 61 | 133 | 90 | 50 | 44 | EndoScreener |
| Wallace, M.B. 2022 | Italy | Multi center | 230 | 116 | 114 | 157 | 73 | 64 | 230 | 0 | 62 | 61 | GI GENIUS Medtronic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spadaccini, M.; Massimi, D.; Mori, Y.; Alfarone, L.; Fugazza, A.; Maselli, R.; Sharma, P.; Facciorusso, A.; Hassan, C.; Repici, A. Artificial Intelligence-Aided Endoscopy and Colorectal Cancer Screening. Diagnostics 2023, 13, 1102. https://doi.org/10.3390/diagnostics13061102
Spadaccini M, Massimi D, Mori Y, Alfarone L, Fugazza A, Maselli R, Sharma P, Facciorusso A, Hassan C, Repici A. Artificial Intelligence-Aided Endoscopy and Colorectal Cancer Screening. Diagnostics. 2023; 13(6):1102. https://doi.org/10.3390/diagnostics13061102
Chicago/Turabian StyleSpadaccini, Marco, Davide Massimi, Yuichi Mori, Ludovico Alfarone, Alessandro Fugazza, Roberta Maselli, Prateek Sharma, Antonio Facciorusso, Cesare Hassan, and Alessandro Repici. 2023. "Artificial Intelligence-Aided Endoscopy and Colorectal Cancer Screening" Diagnostics 13, no. 6: 1102. https://doi.org/10.3390/diagnostics13061102







