Optimized 18F-FDG PET-CT Method to Improve Accuracy of Diagnosis of Metastatic Cancer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
- (1)
- Mediastinal and/or hilar lymph nodes (MHLNs) in patients with known lung carcinoma at staging or restaging of the disease (n = 154, 105 initial staging and 49 restaging).
- (2)
- Extra-thoracic lymph nodes (ETLNs) (n = 143) for patients with malignant lymphoma (n = 48 patients), head and neck cancer (n = 21), breast cancer (n = 18), esophageal cancer (n = 11), colorectal carcinoma (n = 24), ovarian-uterine cancer (n = 10), and pancreatic cancer (n = 11) (all patients initial staging).
- (3)
- Hepatic parenchyma in patients with known colorectal cancer (n = 96 patients, 73 initial staging and 23 restaging).
2.2. ConSUVmax Calculation
2.3. OptSUVmax Calculation
2.4. Statistical Methods
- MHLN thresholds: SUV ≥ 4.0 for defining the presence of cancer, 3.2–3.9 for indeterminate classification, and ≤3.1 for a negative classification (not fulfilling criteria for cancer).
- ETLN thresholds: SUV ≥ 2.5 for defining the presence of cancer, 2.1–2.4 for indeterminate classification, and ≤2.0 as negative for cancer.
- Hepatic parenchymal thresholds: SUV ≥ 4.0 and a TLR (target lesion to normal liver SUVmax ratio) > 2.0 defining the presence of cancer; a value of 3.2–3.94 and a TLR of 1.7–1.9 for indeterminate classification, and an SUV in the target lesion of ≤3.1 and TLR ≤ 1.6 for excluding the presence of cancer.
3. Results
3.1. Patient Characteristics
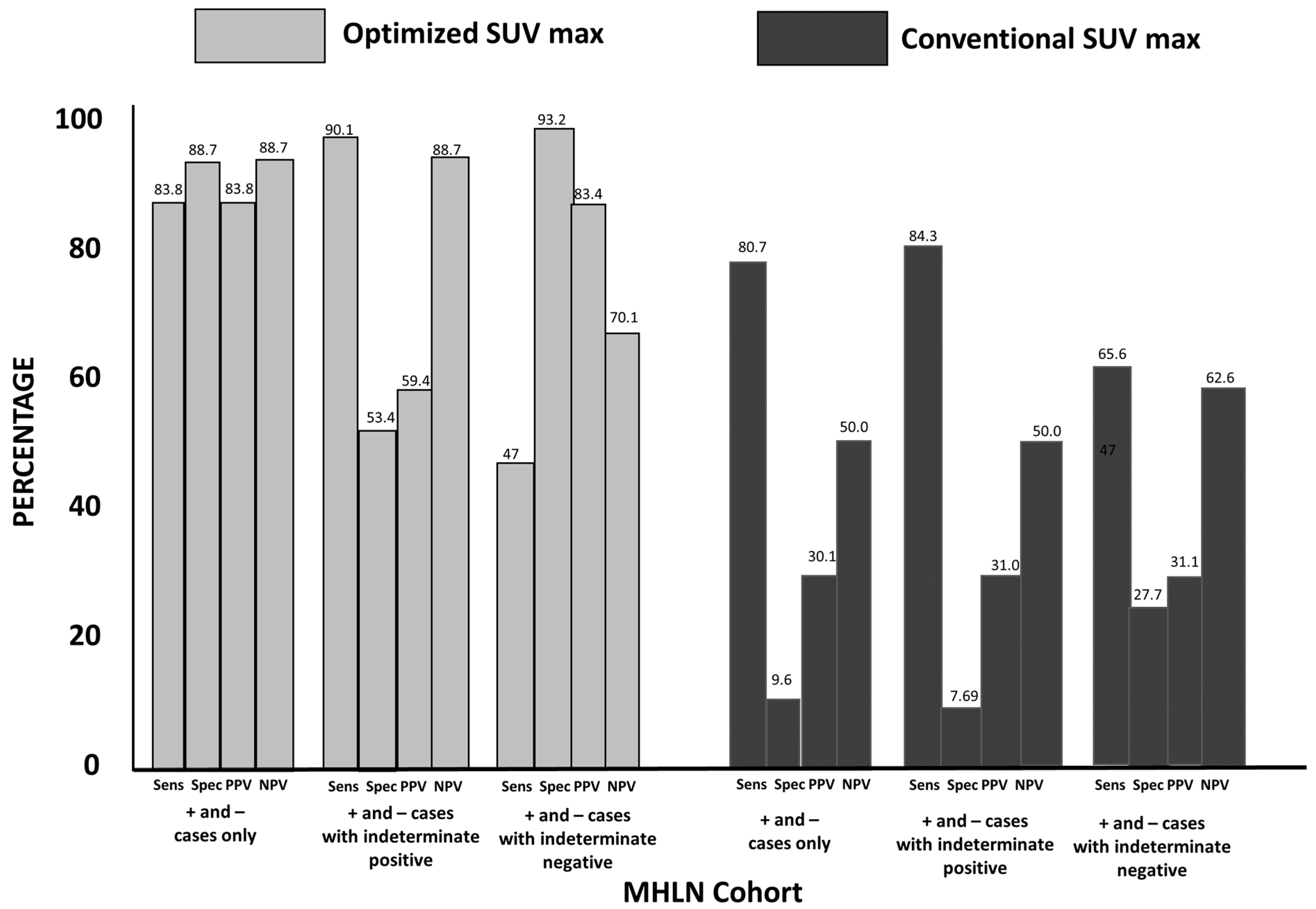
3.2. Group 1: MHLN Lung Cancer Cohort
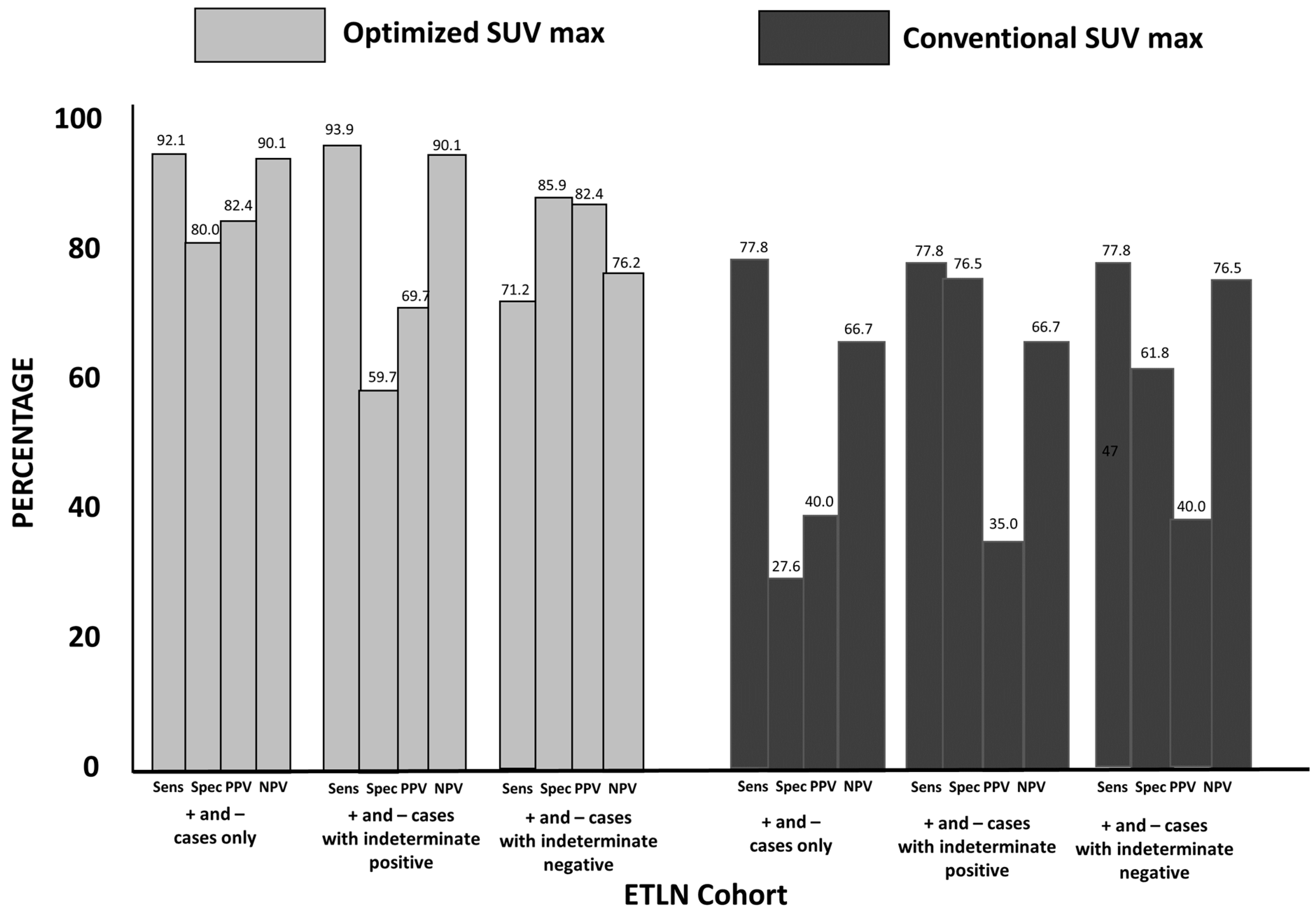
3.3. Group 2: ETLN Cohort
3.4. Group 3: Hepatic Parenchymal Colorectal Carcinoma Cohort
3.5. Total Cohort
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Bui, T.; Thompson, C.B. Cancer’s sweet tooth. Cancer Cell 2006, 9, 419–420. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.W.; Djulbegovic, B.; Soares, H.P.; Siegel, B.A.; Lowe, V.J.; Lyman, G.H.; Coleman, R.E.; Wahl, R.; Paschold, J.C.; Avril, N.; et al. Recommendations on the use of 18F-FDG PET in oncology. J. Nucl. Med. 2008, 49, 480–508. [Google Scholar] [CrossRef]
- Hillner, B.E.; Siegel, B.A.; Liu, D.; Shields, A.F.; Gareen, I.F.; Hanna, L.; Stine, S.H.; Coleman, R.E. Impact of positron emission tomography/computed tomography and positron emission tomography (PET) alone on expected management of patients with cancer: Initial results from the National Oncologic PET Registry. J. Clin. Oncol. 2008, 26, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Bunyaviroch, T.; Turkington, T.G.; Wong, T.Z.; Wilson, J.W.; Colsher, J.G.; Coleman, R.E. Quantitative effects of contrast enhanced CT attenuation correction on PET SUV measurements. Mol. Imaging Biol. 2008, 10, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, D.; Martin, W.H.; Patton, J.A.; Sandler, M.P. Value of iterative reconstruction, attenuation correction, and image fusion in the interpretation of FDG PET images with an integrated dual-head coincidence camera and X-ray-based attenuation maps. Radiology 2001, 218, 163–171. [Google Scholar] [CrossRef]
- Ramos, C.D.; Erdi, Y.E.; Gonen, M.; Riedel, E.; Yeung, H.W.D.; Macapinlac, H.A.; Chisin, R.; Larson, S.M. FDG-PET standardized uptake values in normal anatomical structures using iterative reconstruction segmented attenuation correction and filtered back-projection. Eur. J. Nucl. Med. 2001, 28, 155–164. [Google Scholar] [CrossRef]
- Voltin, C.A.; Mettler, J.; Boellaard, R.; Kuhnert, G.; Dietlein, M.; Borchmann, P.; Drzezga, A.; Kobe, C. Quantitative assessment of 18F-FDG PET in patients with Hodgkin lymphoma: Is it significantly affected by contrast-enhanced computed tomography attenuation correction? Nucl. Med. Commun. 2019, 40, 249–257. [Google Scholar] [CrossRef]
- Lowe, V.J.; Fletcher, J.W.; Gobar, L.; Lawson, M.; Kirchner, P.; Valk, P.; Karis, J.; Hubner, K.; Delbeke, D.; Heiberg, E.V.; et al. Prospective investigation of positron emission tomography in lung nodules. J. Clin. Oncol. 1998, 16, 1075–1084. [Google Scholar] [CrossRef]
- Keyes, J.W., Jr. SUV: Standard uptake or silly useless value? J. Nucl. Med. 1995, 36, 1836–1839. [Google Scholar]
- Erasmus, J.J.; McAdams, H.P.; Patz, E.F., Jr.; Goodman, P.C.; E Coleman, R. Thoracic FDG PET: State of the art. Radiographics 1998, 18, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Delbeke, D.; Martin, W.H.; Sandler, M.P.; Chapman, W.C.; Wright, J.K., Jr.; Pinson, C.W. Evaluation of benign vs malignant hepatic lesions with positron emission tomography. Arch. Surg. 1998, 133, 510–515; discussion 515–516. [Google Scholar] [CrossRef]
- Gupta, N.C.; Rogers, J.S.; Graeber, G.M.; Gregory, J.L.; Waheed, U.; Mullet, D.; Atkins, M. Clinical role of F-18 fluorodeoxyglucose positron emission tomography imaging in patients with lung cancer and suspected malignant pleural effusion. Chest 2002, 122, 1918–1924. [Google Scholar] [CrossRef] [PubMed]
- Vansteenkiste, J.F.; Stroobants, S.G.; De Leyn, P.R.; Dupont, P.; Bogaert, J.; Maes, A.; Deneffe, G.J.; Nackaerts, K.L.; A Verschakelen, J.; E Lerut, T.; et al. Lymph node staging in non-small-cell lung cancer with FDG-PET scan: A prospective study on 690 lymph node stations from 68 patients. J. Clin. Oncol. 1998, 16, 2142–2149. [Google Scholar] [CrossRef] [PubMed]
- Boellaard, R. Need for standardization of 18F-FDG PET/CT for treatment response assessments. J. Nucl. Med. 2011, 52 (Suppl. S2), 93S–100S. [Google Scholar] [CrossRef]
- Boellaard, R.; O’Doherty, M.J.; Weber, W.A.; Mottaghy, F.M.; Lonsdale, M.N.; Stroobants, S.G.; Oyen, W.J.; Kotzerke, J.; Hoekstra, O.S.; Pruim, J.; et al. FDG PET and PET/CT: EANM procedure guidelines for tumour PET imaging: Version 1.0. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 181–200. [Google Scholar] [CrossRef]
- Tomasi, G.; Turkheimer, F.; Aboagye, E. Importance of quantification for the analysis of PET data in oncology: Review of current methods and trends for the future. Mol. Imaging Biol. 2012, 14, 131–146. [Google Scholar] [CrossRef]
- Boellaard, R. Standards for PET image acquisition and quantitative data analysis. J. Nucl. Med. 2009, 50 (Suppl. S1), 11S–20S. [Google Scholar] [CrossRef]
- Laffon, E.; Adhoute, X.; de Clermont, H.; Marthan, R. Is liver SUV stable over time in (1)(8)F-FDG PET imaging? J. Nucl. Med. Technol 2011, 39, 258–263. [Google Scholar] [CrossRef]
- Wahl, R.L.; Jacene, H.; Kasamon, Y.; Lodge, M.A. From RECIST to PERCIST: Evolving Considerations for PET response criteria in solid tumors. J. Nucl. Med. 2009, 50 (Suppl. S1), 122S–150S. [Google Scholar] [CrossRef]
- Delbeke, D.; Coleman, R.E.; Guiberteau, M.J.; Brown, M.L.; Royal, H.D.; Siegel, B.A.; Townsend, D.W.; Berland, L.L.; Parker, J.A.; Hubner, K.; et al. Procedure Guideline for Tumor Imaging with 18F-FDG PET/CT 1.0. J. Nucl. Med. 2006, 47, 885. [Google Scholar] [PubMed]
- Otsuka, H.; Graham, M.M.; Kubo, A.; Nishitani, H. The effect of oral contrast on large bowel activity in FDG-PET/CT. Ann. Nucl. Med. 2005, 19, 101–108. [Google Scholar] [CrossRef]
- Boktor, R.R.; Walker, G.; Stacey, R.; Gledhill, S.; Pitman, A.G. Reference Range for Intrapatient Variability in Blood-Pool and Liver SUV for 18F-FDG PET. J. Nucl. Med. 2013, 54, 677–682. [Google Scholar] [CrossRef]
- González Espinoza, P.; Massardo Vega, T.; Alavi, A. F 18-FDG heart uptake in oncologic pet studies. Rev. Med. Nucl. Alasbimn J. 2001, 3, lil-284732. [Google Scholar]
- Itti, E.; Juweid, M.E.; Haioun, C.; Yeddes, I.; Hamza-Maaloul, F.; El Bez, I.; Evangelista, E.; Lin, C.; Dupuis, J.; Meignan, M. Improvement of early 18F-FDG PET interpretation in diffuse large B-cell lymphoma: Importance of the reference background. J. Nucl. Med. 2010, 51, 1857–1862. [Google Scholar] [CrossRef] [PubMed]
- Kuruva, M.; Mittal, B.R.; Abrar, M.L.; Kashyap, R.; Bhattacharya, A. Multivariate analysis of various factors affecting background liver and mediastinal standardized uptake values. Indian J. Nucl. Med. 2012, 27, 20–23. [Google Scholar] [CrossRef]
- Paquet, N.; Albert, A.; Foidart, J.; Hustinx, R. Within-patient variability of (18)F-FDG: Standardized uptake values in normal tissues. J. Nucl. Med. 2004, 45, 784–788. [Google Scholar]
- Scheuermann, J.S.; Saffer, J.R.; Karp, J.S.; Levering, A.M.; Siegel, B.A. Qualification of PET scanners for use in multicenter cancer clinical trials: The American College of Radiology Imaging Network experience. J. Nucl. Med. 2009, 50, 1187–1193. [Google Scholar] [CrossRef] [PubMed]
- Thie, J.A.; Hubner, K.F.; Smith, G.T. The diagnostic utility of the lognormal behavior of PET standardized uptake values in tumors. J. Nucl. Med. 2000, 41, 1664–1672. [Google Scholar]
- Vansteenkiste, J.F.; Stroobants, S.G.; Dupont, P.J.; De Leyn, P.R.; Verbeken, E.K.; Deneffe, G.J.; Mortelmans, L.A.; Demedts, M.G. Prognostic importance of the standardized uptake value on (18)F-fluoro-2-deoxy-glucose-positron emission tomography scan in non-small-cell lung cancer: An analysis of 125 cases. Leuven Lung Cancer Group. J. Clin. Oncol. 1999, 17, 3201–3206. [Google Scholar] [CrossRef]
- Graham, M.M.; Badawi, R.D.; Wahl, R.L. Variations in PET/CT Methodology for Oncologic Imaging at U.S. Academic Medical Centers: An Imaging Response Assessment Team Survey. J. Nucl. Med. 2011, 52, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Patel, K.; Hadar, N.; Lee, J.; Siegel, B.A.; Hillner, B.E.; Lau, J. The lack of evidence for PET or PET/CT surveillance of patients with treated lymphoma, colorectal cancer, and head and neck cancer: A systematic review. J. Nucl. Med. 2013, 54, 1518–1527. [Google Scholar] [CrossRef] [PubMed]
- Adams, M.C.; Turkington, T.G.; Wilson, J.M.; Wong, T.Z. A systematic review of the factors affecting accuracy of SUV measurements. AJR Am. J. Roentgenol. 2010, 195, 310–320. [Google Scholar] [CrossRef]
- Boellaard, R.; Oyen, W.J.; Hoekstra, C.J.; Hoekstra, O.S.; Visser, E.P.; Willemsen, A.T.; Arends, B.; Verzijlbergen, F.J.; Zijlstra, J.; Paans, A.M.; et al. The Netherlands protocol for standardisation and quantification of FDG whole body PET studies in multi-centre trials. Eur. J. Nucl. Med. Mol. Imaging 2008, 35, 2320–2333. [Google Scholar] [CrossRef] [PubMed]
- Kanstrup, I.L.; Klausen, T.L.; Bojsen-Moller, J.; Magnusson, P.; Zerahn, B. Variability and reproducibility of hepatic FDG uptake measured as SUV as well as tissue-to-blood background ratio using positron emission tomography in healthy humans. Clin. Physiol. Funct. Imaging 2009, 29, 108–113. [Google Scholar] [CrossRef]
- Young, H.; Baum, R.; Cremerius, U.; Herholz, K.; Hoekstra, O.; Lammertsma, A.; Pruim, J.; Price, P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: Review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur. J. Cancer 1999, 35, 1773–1782. [Google Scholar] [CrossRef]
- Skougaard, K.; Nielsen, D.; Jensen, B.V.; Hendel, H.W. Comparison of EORTC criteria and PERCIST for PET/CT response evaluation of patients with metastatic colorectal cancer treated with irinotecan and cetuximab. J. Nucl. Med. 2013, 54, 1026–1031. [Google Scholar] [CrossRef]
- Wade, A. Derivation versus validation. Arch. Dis. Child. 2000, 83, 459–460. [Google Scholar] [CrossRef]
- Vanderhoek, M.; Perlman, S.B.; Jeraj, R. Impact of the definition of peak standardized uptake on quantification of treatment response. J. Nucl. Med. 2012, 53, 4–11. [Google Scholar] [CrossRef]
- Abele, J.T.; Fong, C.I. Effect of hepatic steatosis on liver FDG uptake measured in mean standard uptake values. Radiology 2010, 254, 917–924. [Google Scholar] [CrossRef]
- Anan, N.; Zainon, R.; Tamal, M. A review on advances in (18)F-FDG PET/CT radiomics standardisation and application in lung disease management. Insights Imaging 2022, 13, 22. [Google Scholar] [CrossRef] [PubMed]
- Helsen, N.; Van den Wungaert, T.; Carp, L.; De Bree, R.; VanderVeken, O.M.; De Geeter, F.; Maes, A.; Cambier, J.P.; Spaepen, K.; Martens, M.; et al. Quantification of 18F-fluorodeoxyglucose uptake to detect residual nodal disease in locally advanced head and neck squamous cell carcinoma after chemoradiotherapy: Results from the ECLYPS study. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1075–1082. [Google Scholar] [CrossRef] [PubMed]


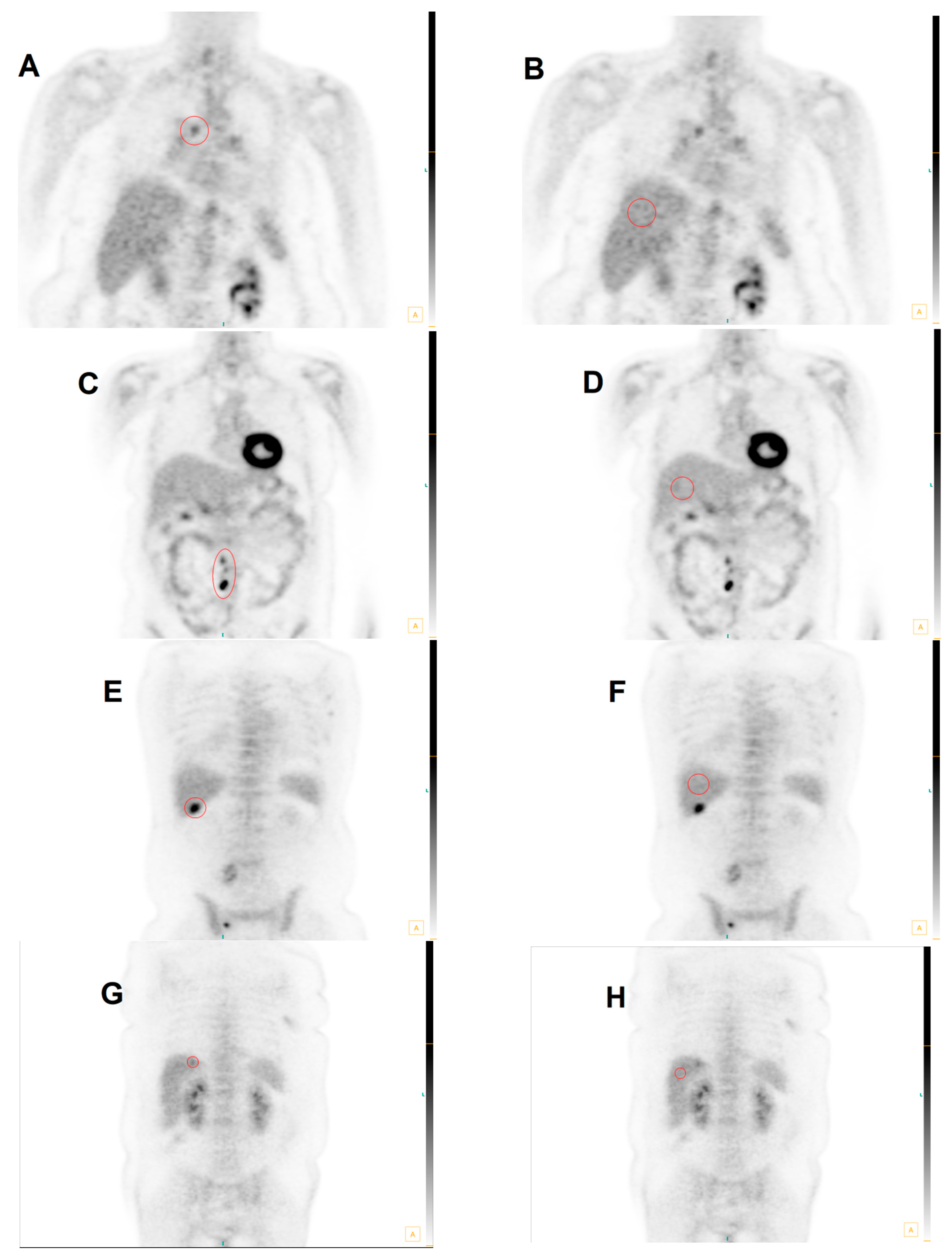
| Anatomic Site | MHLN | ETLN | LIVER | Total Group |
|---|---|---|---|---|
| N (M/F) | 154 (86/68) | 143 (70/73) | 96 (51/45) | 393 (207/186) |
| Age Range (y) | 39–84 | 28–83 | 32–82 | 28–84 |
| Cancer Types | Lung cancer (154: 105 initial staging & 49 restaging) | Lymphoma (48) Head & Neck (10) Breast (18) Esophageal (11) Colorectal (24) Ovarian-Uterine (10) Pancreatic (11) (all initial staging) | Colorectal (96: 73 initial staging & 23 restaging) | |
| Non-disease liver parenchymal SUV | 2.72 ± 0.76 (0.9–4.9) | 2.42 ± 0.64 (1.1–4.1) | 2.50 ± 0.70 (1.2–5.1) | 2.54 ± 0.70 (0.9–5.1) |
| Within-person Coefficient of Variation for normal liver parenchymal SUVs | 0.035 (0.0–0.10) | 0.037 (0.0–0.20) | 0.046 (0.0–0.20) | 0.039 (0.0–0.20) |
| Threshold Values Based on Literature | *** | |||
| Positive | ≥4.0 | ≥2.5 | ≥4.0 & TLR >2.0 | |
| Indeterminate | 3.2–3.9 | 2.1–2.4 | 3.2–3.9 & TLR ≈2.0 | |
| Negative | ≤3.1 | ≤2.0 | ≤3.1 & TLR <2.0 | |
| conSUVmax | ⇢ | optSUVmax | Pathology + | Pathology − |
|---|---|---|---|---|
| negative | positive | 3 (100%) | 0 | |
| positive | negative | 3 (7%) | 42 (93%) | |
| negative | indeterminate | 9 (36%) | 16 (64%) | |
| positive | indeterminate | 46 (56%) | 36 (44%) | |
| indeterminate | negative | 3 (14%) | 18 (86%) | |
| indeterminate | positive | 5 (100%) | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Black, R.; Barentsz, J.; Howell, D.; Bostwick, D.G.; Strum, S.B. Optimized 18F-FDG PET-CT Method to Improve Accuracy of Diagnosis of Metastatic Cancer. Diagnostics 2023, 13, 1580. https://doi.org/10.3390/diagnostics13091580
Black R, Barentsz J, Howell D, Bostwick DG, Strum SB. Optimized 18F-FDG PET-CT Method to Improve Accuracy of Diagnosis of Metastatic Cancer. Diagnostics. 2023; 13(9):1580. https://doi.org/10.3390/diagnostics13091580
Chicago/Turabian StyleBlack, Richard, Jelle Barentsz, David Howell, David G. Bostwick, and Stephen B. Strum. 2023. "Optimized 18F-FDG PET-CT Method to Improve Accuracy of Diagnosis of Metastatic Cancer" Diagnostics 13, no. 9: 1580. https://doi.org/10.3390/diagnostics13091580





